Back to Journals » Journal of Pain Research » Volume 10
Pulsed radiofrequency of brachial plexus under ultrasound guidance for refractory stump pain: a case report
Authors Zheng B, Song L, Liu H
Received 6 August 2017
Accepted for publication 10 October 2017
Published 7 November 2017 Volume 2017:10 Pages 2601—2604
DOI https://doi.org/10.2147/JPR.S148479
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Bixin Zheng, Li Song, Hui Liu
Department of Pain Management, West China Hospital of Sichuan University, Chengdu, China
Abstract: The post-amputation (pain) syndrome, including stump pain, phantom limb sensation, and phantom limb pain is common but difficult to treat. Refractory stump pain in the syndrome is an extremely challenging and troublesome clinical condition. Patients respond poorly to drugs, nerve blocks, and other effective treatments like spinal cord stimulation and surgery. Pulsed radiofrequency (PRF) technique has been shown to be effective in reducing neuropathic pain. This report describes a patient with persistent and refractory upper limb stump pain being successfully relieved with PRF of brachial plexus under ultrasound guidance after a 6-month follow-up period, suggesting that PRF may be considered as an alternative treatment for refractory stump-neuroma pain.
Keywords: ultrasound guidance, pulsed radiofrequency, brachial plexus, refractory stump pain
Introduction
The post-amputation (pain) syndrome is an extremely challenging clinical condition. About 30%–85% amputees suffer from stump pain, phantom limb sensation, and phantom limb pain after the surgery.1 In the post-amputation (pain) syndrome, stump pain is related to tissue injury or neuroma formation in the distal residual limb, and >70% of stump pain patients develop refractory stump pain. Refractory stump pain affects amputees through biological, psychological, and social pathways.2,3 Patients with refractory stump pain respond poorly to drugs, nerve blocks, and other treatments like spinal cord stimulation and surgery.4 Pulsed radiofrequency (PRF) technique has been shown to be effective in reducing neuropathic pain. This report describes a patient with persistent and refractory upper limb stump pain being successfully relieved with PRF of brachial plexus under ultrasound guidance, suggesting that PRF may be considered as an alternative treatment for refractory stump pain.
Consent for publication
The patient has provided written informed consent for the publication of this case and any accompanying images.
Case description
A 56-year-old man with a 20-year history of persistent and refractory upper limb stump pain was referred to our pain clinic. His left upper humeral was amputated after a car accident 20 years ago. About 2 weeks after the amputation operation, he felt a continuous stabbing, burning sensation like “blowing up a balloon” on the residual limb. He received pregabalin (150 mg per day; Pfizer, New York, NY, USA) and slow-release tramadol (300 mg per day; Grünenthal GmbH, Stolberg, Germany). Three years after operation, he occasionally felt mild paroxysmal, sharp, shooting phantom limb pain from his “little finger”, and phantom sensation was also reported. In recent 2 years, the pain was aggravated, especially when clothing friction and pressure were applied to the stump. The pain lasted from a few minutes to several hours. His numerical rating scale (NRS) score was consistently 6/10 all the day, responding poorly to multimodal analgesic regimen. His life and daily activities were so seriously affected by refractory stump pain that he even attempted to commit suicide. Physical Examination: stump tenderness (+), mechanically allodynia (+), hyperalgesia (+), no residual infection. Stump neuroma was diagnosed by magnetic resonance imaging (MRI) brachial plexus hydrography (Figure 1).
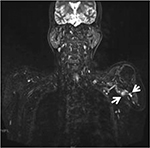  | Figure 1 MRI appearance of the suspected neuroma (arrows). Abbreviation: MRI, magnetic resonance imaging. |
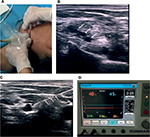
PRF with ultrasound guidance was first considered to ablate the stump neuroma but later was dismissed because it was difficult to locate neuroma among muscles, tendons, and vessels. Instead, interscalene brachial plexus PRF was used far away from the position of the neuroma and the result was satisfying. An interscalene brachial plexus block (15 mL of 0.5% lidocaine [Shanghai Zhaohui Pharmaceutical Co. Ltd., Shanghai, China] + 0.1% ropivacaine [AstraZeneca AB, Sodertalje, Sweden] + saline) was performed under ultrasound guidance. After the nerve block, ~70% stump pain was relieved immediately (NRS 9/10 to 3/10 post block), and the analgesic effect lasted for about 20 hours. The diagnostic block was effective, brachial plexus PRF was applied (Figure 2A). The patient was put in the supine position with head tilted to the right, monitored with electrocardiography, pulse oximetry, and non-invasive blood pressure. After disinfection, Sparq ultrasound system (Royal Philips, Amsterdam, the Netherlands) was used with a linear transducer (5–12 MHz) in-plane interscalene approach to obtain a transverse axial view of brachial plexus (Figure 2B). The 50-mm stimulation-guided nerve block needle (Cosman Medical, Inc., Burlington, MA, USA) was used, and stimulation in sensory mode was set at 50 Hz. When the needle was close to the nerve and patient’s reproduced pain was at 0.4 mA, it was the ideal position for PRF (Figure 2C). Before proceeding with the lesioning, 2 mL of 1% lidocaine and 3.5 mg betamethasone (Schering-Plough Labo NV, Heist-op-den-Berg, Belgium) were injected through the needle. The patient received PRF lesions at 42°C in 240 seconds for 3 times with radiofrequency generator (Cosman Medical, Inc.,) (Figure 2D). After the procedure, the patient reported complete resolution of stump pain (initial NRS 8/10 to 2/10, average from 6/10 to 1/10). There were no complications reported after all procedures. He was contacted in the following first, third and sixth months with pregabalin 150 mg taken per day as routine oral analgesics to prevent recurrent phantom limb pain, and slow-release tramadol were reduced to 100 mg per day. He also reported a significant improvement in mood, sleep, and daily activities. The baseline NRS (6/10 on average) was decreased by 90% in the first month, 70% in the third month, and 60% in the sixth month.
Discussion
Amputation is one of the leading causes of chronic pain. Multifactorial mechanisms, including supraspinal, spinal, and peripheral-level participate in this post-amputation (pain) syndrome.5 Amputees describe phantom limb pain as “burning, shooting, stabbing, and throbbing pain” on the absent limb. Stump pain is related to tissue injury or neuroma formation in the distal residual limb. Many previous studies have focused on phantom limb pain.6,7 However, persistent and refractory stump pain is a more challenging and troublesome clinical condition. Our patient responded poorly to drugs and his quality of life was seriously affected. Neuroma development is one of the leading palpable reasons for refractory stump pain, and the diagnosis could be confirmed by ultrasound8 and MRI. Under ultrasound-guidance, the neuroma can be visualized. Previous study has reported that alcohol neurolysis under ultrasound guidance is an effective management for stump-neuroma pain.9 Unlike radiofrequency (RF) nerve ablation (temperature 60°C–80°C), PRF is a non-neurodestructive procedure with temperature no higher than 42°C. This minimizes neuritis and lessens the neural damage.10 PRF has shown to give long-term pain relief in a variety of chronic pain conditions, including complex regional pain syndrome, trigeminal neuralgia, and postherpetic neuralgia.11–13 Studies have shown ultrasound-guided PRF ablation of the stump neuroma is an effective treatment,14 but it is difficult to identify neuroma from muscles, tendons, and vessels. In this case, brachial plexus PRF was used and successfully relieved refractory stump pain. After a 6-month follow-up period, significant stump pain relief after brachial plexus PRF was observed.
This finding indicates that apart from neuroma, multiple ectopic activity generators may participate in refractory stump pain. Our report suggests that PRF of brachial plexus under ultrasound guidance may be considered as an alternative treatment for refractory stump pain. To our knowledge, this is the first report using ultrasound-guided brachial plexus PRF for the management of refractory stump pain.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81500956), 1.3.5 project for excellent disciplines of West China Hospital (ZY2016101), and national key clinical specialty construction project of MOH.
Disclosure
The authors report no conflicts of interest in this work.
References
Visser EJ. Chronic post-surgical pain: epidemiology and clinical implications for acute pain management. Acute Pain. 2006;8(2):73–81. | ||
Ehde DM, Czerniecki JM, Smith DG, et al. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehabil. 2000;81(8):1039–1044. | ||
Roullet S, Nouette-Gaulain K, Brochet B, Sztark F. Phantom limb pain:from physiopathology to prevention. Ann Fr Anesth Reanim. 2009;28(5):460–472. | ||
Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237–251. | ||
Hsu E, Cohen SP. Post-amputation pain: epidemiology, mechanisms, and treatment. J Pain Res. 2013;6:121–136. | ||
Richardson C, Glenn S, Nurmikko T, Horgan M. Incidence of phantom phenomena including phantom limb pain 6 months after major lower limb amputation in patients with peripheral vascular disease. Clin J Pain. 2006;22(4):353–358. | ||
Dijkstra PU, Geertzen JH, Stewart R, van der Schans CP. Phantom pain and risk factors: a multivariate analysis. J Pain Symptom Manage. 2002;24(6):578–585. | ||
Ernberg LA, Adler RS, Lane J. Ultrasound in the detection and treatment of a painful stump neuroma. Skeletal Radiol. 2003;32(5):306–309. | ||
Zhang X, Xu Y, Zhou J, et al. Ultrasound-guided alcohol neurolysis and radiofrequency ablation of painful stump neuroma: effective treatments for post-amputation pain. J Pain Res. 2017;10:295–302. | ||
Cahana A, Van Zundert J, Macrea L, vanKleef M, Sluijter M. Pulsed radiofrequency: current clinical and biological literature available. Pain Med. 2006;7(5):411–423. | ||
Fang L, Tao W, Jingjing L, Nan J. Comparison of high-voltage-with standard-voltage pulsed radiofrequency of Gasserian ganglion in the treatment of idiopathic trigeminal neuralgia. Pain Pract. 2015;15(7):595–603. | ||
Rohof OJ. Caudal epidural of pulsed radiofrequency in Post Herpetic Neuralgia (PHN); report of three cases. Anesth Pain Med. 2014;4(3):e16369. | ||
Akkoc Y, Uyar M, Oncu J, Ozcan Z, Durmaz B. Complex regional pain syndrome in a patient with spinal cord injury: management with pulsed radiofrequency lumbar sympatholysis. Spinal Cord. 2008;46(1):82–84. | ||
Restrepo-Garces CE, Marinov A, McHardy P, Faclier G, Avila A. Pulsed radiofrequency under ultrasound guidance for persistent stump-neuroma pain. Pain Pract. 2011;11(1):98–102. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.