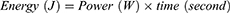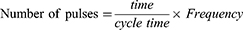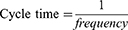Back to Journals » Journal of Pain Research » Volume 16
Pulsed Radiofrequency 2 Hz Preserves the Dorsal Root Ganglion Neuron Physiological Ca2+ Influx, Cytosolic ATP Level, Δψm, and pERK Compared to 4 Hz: An Insight on the Safety of Pulsed Radiofrequency in Pain Management
Authors Laksono RM , Siswagama TA, Nery FRP, van der Weegen W , Halim W
Received 11 July 2023
Accepted for publication 4 October 2023
Published 31 October 2023 Volume 2023:16 Pages 3643—3653
DOI https://doi.org/10.2147/JPR.S424489
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Ristiawan Muji Laksono,1 Taufiq Agus Siswagama,1 Fa’urinda Riam Prabu Nery,1 Walter van der Weegen,2 Willy Halim3
1Department of Anesthesiology and Intensive Therapy, Faculty of Medicine, Brawijaya University, Dr. Saiful Anwar General Hospital, Malang, Indonesia; 2Sports & Orthopedics Research Centre., St. Anna Hospital, Geldrop, the Netherlands; 3Medical Department, Faculty of Medicine, Brawijaya University, Malang, Indonesia
Correspondence: Ristiawan Muji Laksono, Department of Anesthesiology and Intensive Therapy, Faculty of Medicine, Brawijaya University, Jl. Jaksa Agung Suprapto No. 2, Malang, East Java, Indonesia, Tel +62 812-3377-3593, Email [email protected]
Background: Pulsed radiofrequency (PRF) is beneficial for radicular pain and is commonly administered at pulse frequencies of 2 or 4 Hz. However, its effects on healthy neurons have not yet been widely studied. This study aims to determine the effect of PRF at 2 Hz and 4 Hz on the physiology of healthy dorsal root ganglion (DRG) neurons.
Methods: An in vitro experimental study was conducted using DRG neuron cultures divided into three groups. Control cells received no treatment, one cell group received 20 ms 2 Hz PRF for 360 s, and one cell group received a 4 Hz PRF 10 ms pulse for 360 s with similar energy. Ca2+ influx, mitochondrial membrane potential (Δψm), cytosolic Adenosine triphosphate (ATP), and phosphorylated extracellular signal-regulated kinase (pERK) levels were measured. The data were analyzed using the One-Way ANOVA variance with α=5%.
Results: DRG neurons exposed to PRF 2 Hz did not experience a significant change in Ca2+ influx, whereas PRF 4 Hz caused a significant decrease in Ca2+ influx compared to the basal level. PRF at 2 Hz did not cause a change in Δψm, whereas PRF at 4 Hz caused a significant decrease in Δψm (p< 0.05). Both 2 and 4 Hz PRF resulted in a significant elevation in cytosolic ATP concentration, but the 2 Hz PRF had a higher cytosolic ATP than the 4 Hz group (p< 0.05). Both 2 and 4 Hz did not show a significant difference in pERK intensity with respect to the control (p> 0.05), indicating that there was no significant neuron activation.
Conclusion: Both frequencies did not significantly activate DRG neurons, but with similar energy delivery, PRF 2 Hz preserved the physiological properties of healthy neurons better than PRF 4 Hz did. A 2 Hz PRF is the preferred frequency in clinical applications for neuron-targeted therapy.
Keywords: pulsed radiofrequency, neurons, physiology, dorsal root ganglion neuron
A Letter to the Editor has been published for this article.
A Response to Letter by Dr Russo has been published for this article.
Introduction
Neuropathic pain is a type of chronic pain caused by damage to the peripheral nerves or the central nervous system. Radicular pain occurs due to the stimulation of the dorsal root ganglion (DRG) or sensory nerve roots of the spinal nerves.1 Continuous radiofrequency (CRF) has been used since 1981 to relieve several types of pain.2 However, because CRF is ablative, Pulsed Radiofrequency (PRF) was first developed in 1996.3
PRF has a smaller impact on tissue damage than CRF4 and can treat various types of pain.5 CRF uses an alternating electric current with temperatures reaching 60–80 °C, which can cause tissue injury, and PRF uses temperatures below 42 °C3 with a shorter exposure time to avoid tissue damage.3,5 PRF works by delivering a series of short-wave radiofrequency pulses from the generator to the nerves, which are sources of pain. It is suspected that the effect of PRF on pain management is due to changes in the cell membrane potential caused by electrical fields. Disruption of the cell transmembrane potential causes electroporation,6 a process of deformation and pore formation. If the membrane potential disturbance is sufficiently high, cell rupture can occur.7
Several studies have reported that PRF treatment for acute pain changes the structure of the smooth endoplasmic reticulum, myelin, and mitochondrial morphology.8 The use of PRF in experimental animal studies of chronic constriction injury (CCI) reduced the number of pro-inflammatory cytokines, β-catenin expression, and neuropathic pain behavior.9 Other studies have reported that PRF regulates several types of proteins in microglia.10
Dorsal root ganglion (DRG) neurons are the most frequently targeted point of PRF therapy in patients with radicular pain, with better results than those in other parts of the peripheral nerve. DRG neurons located in the spinal cord and play a key role in the transmission of peripheral pain signals to the central nervous system.11 In DRG, many primary sensory neuronal cell bodies function in the delivery of pain stimuli.12 A previous study demonstrated that spinal cord stimulation targeting DRG neurons was effective in reducing chronic low back pain.13
In some clinical cases, the electrical stimulus given by PRF to the targeted dorsal root ganglion reduces pain and provides positive outcomes, including a reduction in the degree of pain in lumbosacral radicular pain,14 reduction of microglia hyperactivity in neuropathic pain,15 and modulation of neuroimmunity related to chronic radicular pain.16 However, electrical stimulation of PRF can also expose neighboring healthy neurons. Although PRF is recognized for its minimal tissue damage, it is essential to comprehend the effects of PRF on healthy neurons to gain a comprehensive understanding of the role of PRF in the management of pain.
For excitable cells, several physiological parameters can be analyzed to determine the effect of PRF on healthy neurons. Ca2+ ions are the most important ions for neuronal excitability.17 Changes in Ca2+ concentrations affect cellular processes in neurons.18 Furthermore, the role of mitochondria as the primary source of adenosine triphosphate (ATP) production greatly affects the function and survival rate of neurons. Neurons are excitable cells that require substantial amounts of ATP to maintain membrane-ion gradients, neurotransmission, and neuronal plasticity.19
Clinically, PRF is available at 1–10 Hz, but is mostly performed at frequencies of 2 or 4 Hz.4,5,7,20 Currently, there is a lack of comparative research regarding the effects of 2 and 4 Hz PRF on the physiology of healthy neurons. Consequently, this study was undertaken to investigate the differences between the effects of 2 and 4 Hz PRF on healthy dorsal root ganglion (DRG) neurons by analyzing multiple parameters related to excitable cells, namely, calcium ions (Ca2+), mitochondrial membrane potential, cytosolic adenosine triphosphate (ATP) concentration, and phosphorylated extracellular signal-regulated kinase (pERK).
Materials and Methods
Cell Culture
This study uses DRG neurons that are differentiated from F11 cell line (08062601; Sigma, USA). Cell culture method according to Hashemian et al21 with modifications described in a previous study by Laksono et al.22 We use DRG neurons that differentiated from F11 cell line on day 5. In this study, there were three treatment groups with nine replicates in each group (Figure 1), including the control, 2 Hz PRF, and 4 Hz groups. To understand the safety of PRF 2 and 4 Hz to the healthy DRG neuron, we assess the effect of both frequencies to calcium influx, mitochondrial membrane mitochondria, cytosolic ATP, and phosphorylated extracellular regulated kinase (p-ERK) level.
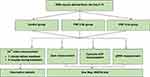 |
Figure 1 Study design and grouping. |
Pulsed Radiofrequency Treatment
PRF treatment was carried out by transmitting pulsed frequencies of 2 Hz and 20 ms for 360 s (2 Hz group) and 4 Hz and 10 ms for 360 s (4 Hz group). PRF treatment is based on Laksono et al22 study. Both PRF frequencies delivered the same energy, following the formula derived from Ohm’s law:
In case of pulsed radiofrequency, with constant voltage and resistance, the formula can be derived as follows:
Calcium Influx Measurement
Calcium influx measurement was done according to the Laksono et al22 study. Calcium influx was marked using Fluo-3/AM (F4897-1MG, Sigma-Aldrich, USA) and observed using a confocal laser scanning microscope (CLSM) in 340 nm and 380 nm. The basal calcium level was measured first for 1 min. Calcium influx dynamics was observed during treatment in each group for 9 min.
Mitochondrial Membrane Potential Measurement (Δψm)
Neurons that had previously undergone treatment were obtained through trypsinization and underwent two rounds of washing with PBS. The cells were then suspended in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 25 mM DMEM medium, and subjected to staining using 40 nM MitoTracker Green FM (M7514, Thermo Fisher Scientific, USA) for a duration of 30 min at a temperature of 37 °C. Any excess, unbound dye was removed by washing with DMEM containing 10% FBS at 37 °C. The luminescent properties of the dye were examined using confocal laser scanning microscopy (CLSM) with an excitation wavelength of 579 nm.
Cytosolic ATP Measurement
ATP levels were assessed using the ATP Assay Kit (Colorimetric/Fluorometric) (ab83355, Abcam, USA). A total of 1×106 cells that had undergone previous treatment were collected and utilized for analysis. The cells were rinsed with PBS and subsequently resuspended in 100 mL of ATP Assay Buffer. To homogenize the cells, they were pipetted several times, followed by centrifugation at 13,000 g for 5 min at 4 °C. The resulting supernatant was transferred to a fresh tube and kept on ice. To prevent interference from enzymes present in the supernatant, the cells were deproteinized. Cold 4 M perchloric acid (PCA) was added to the homogenate until a final concentration of 1 M was achieved, and the mixture was thoroughly mixed. After incubating the samples for 5 min on ice, they were centrifuged at 13,000 g (10,000 rpm) for 2 min at 4 °C, and the supernatant was transferred to a new tube. The volume of the supernatant was measured, and excess PCA was precipitated through neutralization. This was achieved by adding 2 M cold KOH equivalent to 20–35% of the sample volume (sample + PCA). The neutralization involved adjusting the pH using either 0.1 M KOH or PCA with a pH range of 6.5 to 8.0. The sample was then centrifuged at 13,000 g for 15 min at 4 °C, and the resulting supernatant was collected. The sample was used for testing, and the dilution factor after the deproteination process was calculated using an appropriate equation:
For each reaction, 50 µL of the ATP reaction mix and background control mix were prepared. The 50 µL reaction mixture was then added to each well of a 96-well plate, which already contained the standard and sample. The background control sample received an additional 50 µL of the background reaction mix. The samples were thoroughly mixed and incubated in the dark at room temperature for 30 min. After the incubation period, the absorbance of the samples was measured using a microplate reader at a wavelength of 570 nm. ATP levels were determined using a formula provided by the manufacturer (Abcam, USA) and a standard curve that was also provided.
B: represent the ATP amount in the sample on a standard curve.
V: represent the sample volume of each well l
D: represent sample dilution factor
DDF: deproteinization dilution factor
Measurement of Phosphorylated Extracellular Signal-Regulated Kinase (p-ERK)
Measurement of pERK using immunocytochemical methods and fluorescence microscopy. The cells used for the pERK measurements were differentiated into 24 well plates. The treated cells were fixed by adding 4% formaldehyde in 1X PBS to the cell surface, approximately 2–3 mm above the cells. The fixation process lasted for 15 min at room temperature. Afterward, the fixative solution was removed, and the cells were washed three times with 1X PBS for 5 min per wash.
The subsequent step involved immunostaining. The cells were supplemented with a blocking buffer consisting of 1X PBS, 5% normal serum, and 0.3% Triton™ X-100. After aspirating the blocking buffer, a polyclonal antibody, specifically Phospho (Erk1/2) Thr202/Tyr204 (36–8800, Thermo Fisher Scientific, USA), was added at a 1:200 dilution in an antibody dilution buffer (1X PBS/1% BSA/0.3% Triton X-100). The cells were then incubated overnight at 4 °C. Following the incubation period, the cells were washed three times with 1X PBS for 5 min each.
Next, the cells were incubated with a goat anti-rabbit IgG secondary antibody FITC (31,635, Thermo Fisher Scientific, USA), which had been diluted in the antibody dilution buffer. This incubation lasted for 1–2 hr at room temperature in a dark environment. Subsequently, the cells were washed three times with 1X PBS for 5 min per wash. Finally, slides containing the cells were observed using fluorescence microscopy, employing appropriate excitation wavelengths for visualization purposes.
Statistical Analysis
The effects of PRF at 2 and 4 Hz on Δψm, cytosolic ATP, pERK were tested using One-Way ANOVA. The post-hoc test was done using Duncan’s test. The calcium influx data are displayed descriptively. Data analysis was performed using SPSS (version 20.0; IBM Statistics, USA). This study use α= 5% and confidence intervals (CI) 95%.
Results
Effect of PRF on Ca2+ Influx
Based on the measurements of Ca2+ influx, DRG neurons that received PRF 2 Hz did not experience a significant change in Ca2+ influx compared to the basal concentration (red line). However, administration of 4 Hz PRF resulted in a gradual decrease in Ca2+ influx compared to the basal level (blue line). In the first few seconds of observation, there was no decrease in Ca2+ influx in the 2 Hz PRF group or the control group (Figure 2).
Effect of PRF on Mitochondrial Membrane Potential (Δψm)
Administration of 2 Hz PRF to DRG neurons did not show a significant difference in the mitochondrial membrane potential compared to control. Neurons that received 2 Hz PRF exhibited an increase in mitochondrial membrane potential, rising from 76.191 to 79.003 (AU). However, this increase was not statistically significant when compared to the control group (p>0.05). However, the administration of 4 Hz PRF led to a notable reduction in mitochondrial membrane potential when compared to the control group (p<0.05) (Figure 3A). Fluorescent imaging shows a lower intensity of Δψm in the DRG neurons exposed to 4 Hz PRF (Figure 3B).
Effect of PRF on Cytosolic ATP Concentrations
Administration of PRF at 2 and 4 Hz resulted in a significant (p<0.05) increase in the cytosolic ATP concentration. PRF at 2 Hz caused an increase in cytosolic ATP concentration from 0.0228 nM (control) to 0.0516 nM. Administration of 4 Hz PRF also increased the cytosolic ATP concentration (0.0433 nM) but at a lower concentration than the 2 Hz PRF group (Figure 4).
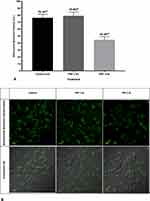
Effect of PRF on pERK
Administration of PRF at both 2 and 4 Hz did not result in a significant difference in pERK intensity when compared to normal/control neurons (p>0.05). Administration of 2 Hz PRF caused an increase in pERK from 42.108 AU to 48.40 AU, with no significant difference from control (p>0.05). Administration of 4 Hz PRF also showed no significant difference from the control group (p>0.05) (Figure 5A). The fluorescent imaging also shows no significant difference in green fluorescent illuminating pERK (Figure 5B).
Discussion
PRF, an electromagnetic wave-based therapy, is clinically used to treat several types of pain.23 PRF therapy delivers short-duration electrical stimulation that minimizes tissue damage24 compared with CRF. Possible mechanisms of PRF include long-term depression (LTD),25 changes in the resting membrane potential of neurons, and changes in epigenetic neurons.26 In clinical practice, there are two common PRF frequency modes that are often used to target the nerves from which the pain originates, namely, 2 and 4 Hz.4,20,27 Since they are excitable cells, the physiology of neurons is strongly influenced by electrical stimulation, depolarization or hyperpolarization of neurons.28 Although literature states that PRF results in minimal tissue/cell damaging effects,29 no studies have compared the effects of 2 and 4 Hz PRF on the physiology of healthy DRG neurons, especially on parameters related to the physiology of neurons such as excitable cells. Our study demonstrated the use of PRF at 2 and 4 Hz with the same energy delivered to healthy DRG neuron cultures.
We demonstrated that 2 Hz PRF showed better results in preserving the physiology of healthy neurons than did 4 Hz PRF. Healthy DRG neurons that received 2 Hz PRF did not experience a significant change in Ca2+ influx compared to the basal concentration, whereas healthy DRG neurons that received 4 Hz PRF showed a gradual decrease in Ca2+ influx compared to the basal levels. Calcium ions in neurons play an important role in biological mechanisms such as gene transcription and neuronal plasticity.27,30 The homeostasis of calcium signaling maintains the integrity and survival rate of neurons.31 In addition, an increased Ca2+ influx plays a role in the transmission of neuronal depolarization signals that help conduct impulses.29,32 The only other study related to PRF exposure in healthy cells showed that the administration of 2 Hz PRF to kidney embryonic cells resulted in a moderate Ca2+ influx, which was thought to be due to the electroporation process.30,33 Differences in cell type might explain why this was observed in kidney cells, but not in our study with neurons since neurons have a minimum limit of stimulus strength to trigger an action potential (AP) due to Ca2+ influx.34
In addition to Ca2+ influx, this study also elaborates on the effect of PRF on the Δψm since it is related to Ca2+ influx and energy generation in neurons.35 Mitochondrial membrane potential is induced by ion pumps and is an important component of ATP synthesis and cell viability.36 PRF 2 Hz exposure of DRG neurons causes an increase in Δψm, but it is not statistically significantly different from normal conditions. In contrast, 4 Hz PRF treatment resulted in a significant decrease in Δψm compared with the controls. Normally, cells maintain their Δψm and ATP concentrations under stable conditions in order to support normal cell physiology.36 Under physiological conditions, neurons maintain transient Δψm to achieve cell viability.37 A significant decrease in Δψm also causes an increase in the mitochondrial inner membrane permeability to ions and small molecules, which can cause osmotic swelling and damage to the mitochondrial membrane.38 The decrease in Δψm may be related to decreased mitochondrial oxidative stress and an increased susceptibility to apoptosis.37 However, this study has not yet compared the threshold/basal levels of Δψm; therefore, the effect of PRF at 4 Hz on osmotic swelling and oxidative stress requires further investigation.
During cytosolic ATP measurement, both 2 and 4 Hz PRF caused an increase in cytosolic ATP concentration. However, 2 Hz PRF resulted in a higher cytosolic ATP concentration than 4 Hz PRF. It is possible to increase this by Δψm, where the 2 Hz PRF has a higher Δψm, although it is not statistically different from the control. Δψm is associated with neuronal activity in energy synthesis;36 therefore, decreased Δψm upon PRF 4 Hz exposure might contribute to a lower level of cytosolic ATP compared to PRF 2 Hz. The increase in cytosolic ATP can be due to an accumulation of increased ATP production in the mitochondria, as indicated by an increase in Δψm. No studies have measured ATP concentration after PRF administration. However, a previous study reported that electrical stimulation can increase the release of ATP from neurons, which functions as a trigger for active molecular cascade reactions in neurons and axons.39 Thus, it can be assumed that an increase in cytosolic ATP is a cellular response to the increased need for ATP after electrical stimulation. Neuronal activity following PRF stimulation was also assessed by measuring pERK intensity in neurons.
ERK phosphorylation is a cellular biomarker that indicates neuronal activation and central sensitization,40 making cells more responsive to nociceptive and non-nociceptive stimuli.41 Increased pERK levels are associated with chronic and persistent pain.40 Thus, it can be determined whether PRF at 2 or 4 Hz causes neuronal activation. In this study, we demonstrated that both 2 and 4 Hz PRF showed no difference in pERK intensity compared to the control. This indicates that the two frequencies used in PRF do not affect the activation or sensitization of healthy neurons. This condition can also be related to cytosolic ATP levels. In the activated state, neurons consume a larger amount of ATP, resulting in a supply-demand imbalance that decreases the level of cytosolic ATP.42 In addition, the cytosolic ATP levels were not significantly reduced. No studies have analyzed the differences in pERK intensity in healthy neurons treated with 2 or 4 Hz PRF. Previous research on the effect of PRF on pERK was carried out in experimental animals with the spared nerve injury (SNI) model using only one type of frequency,10 where there was a decrease in pERK compared to controls, indicating a decrease in pain. Experimental research on the effects of PRF in pain models and in normal cells is limited. This study demonstrated that by using the same energy, PRF 2 Hz for 20 ms is preferred to PRF 4 Hz for 10 ms because it does not alter physiological properties of healthy neurons, especially important parameters of excitable cells, including Ca2+ influx, mitochondrial membrane potential, and cytosolic ATP level. Based on clinical data on PRF efficacy in radicular pain, both 2 Hz and 4 Hz are used in pain practice with no evidence of superiority in terms of pain relief. The majority of pain physicians used a pulsed rate of 2 Hz.16,43,44 Therefore, the findings of this study could underlie the molecular mechanism of PRF 2 Hz safety in pain management by preserving healthy neurons.
Conclusion
Using identical energy quantities, PRF at a frequency of 2 Hz better preserves the physiology of healthy DRG neurons than PRF at a frequency of 4 Hz. In contrast to PFR 4 Hz, PRF 2 Hz maintained the Ca2+ levels and mitochondrial membrane potential. PRF 2 and 4 Hz both increased cytosolic ATP levels but did not cause neuron activation. This study underlies the use and safety of PRF 2 Hz in pain management by maintaining the physiological properties of healthy dorsal root neurons.
Ethical Statement
The Brawijaya University Ethical Clearance Committee approved the study protocol (No. 114-KEP-UB-2020).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dower A, Davies MA, Ghahreman A. Pathologic Basis of Lumbar Radicular Pain. World Neurosurg. 2019;128:114–121. doi:10.1016/j.wneu.2019.04.147
2. Ahadian FM. Pulsed radiofrequency neurotomy: advances in pain medicine. Curr Pain Headache Rep. 2004;8(1):34–40. doi:10.1007/s11916-004-0038-4
3. Byrd D, Mackey S. Pulsed radiofrequency for chronic pain. Curr Pain Headache Rep. 2008;12(1):37–41. doi:10.1007/s11916-008-0008-3
4. Ewertowska E, Mercadal B, Muñoz V, Ivorra A, Trujillo M, Berjano E. Effect of applied voltage, duration and repetition frequency of RF pulses for pain relief on temperature spikes and electrical field: a computer modelling study. Int J Hyperthermia. 2018;34(1):112–121. doi:10.1080/02656736.2017.1323122
5. Chua NHL, Halim W, Beems T, Vissers KCP. Pulsed radiofrequency treatment for trigeminal neuralgia. Anesth Pain Med. 2012;1(4):257–261. doi:10.5812/AAPM.3493
6. Cosman ER, Cosman ER. Electric and Thermal Field Effects in Tissue Around Radiofrequency Electrodes. Pain Medicine. 2005;6(6):1526. doi:10.1111/j.1526-4637.2005.00076.x
7. Chua NHL, Vissers KC, Sluijter ME. Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications - A review. Acta Neurochir. 2011;153(4):763–771. doi:10.1007/s00701-010-0881-5
8. Protasoni M, Reguzzoni M, Sangiorgi S, et al. Pulsed radiofrequency effects on the lumbar ganglion of the rat dorsal root: a morphological light and transmission electron microscopy study at acute stage. Eur Spine J. 2009;18(4):473–478. doi:10.1007/s00586-008-0870-z
9. Jiang R, Li P, Yao YX, et al. Pulsed radiofrequency to the dorsal root ganglion or the sciatic nerve reduces neuropathic pain behavior, decreases peripheral pro-inflammatory cytokines and spinal β-catenin in chronic constriction injury rats. Reg Anesth Pain Med. 2019;44(7):742–746. doi:10.1136/rapm-2018-100032
10. Xu X, Fu S, Shi X, Liu R. Microglial BDNF, PI3K, and p-ERK in the Spinal Cord Are Suppressed by Pulsed Radiofrequency on Dorsal Root Ganglion to Ease SNI-Induced Neuropathic Pain in Rats. Pain Res Manag. 2019;2019:1–15. doi:10.1155/2019/5948686
11. Martínez-Lavín M. Dorsal root ganglia: fibromyalgia pain factory? Clinical Rheumatology. 2021;40(2):783–787. doi:10.1007/s10067-020-05528-z/Published
12. Berta T, Qadri Y, Tan PH, Ji RR. Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin Ther Targets. 2017;21(7):695–703. doi:10.1080/14728222.2017.1328057
13. Deer TR, Levy RM, Kramer J, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017;158(4):669–681. doi:10.1097/j.pain.0000000000000814
14. Luo Q, Zhao Z, Yi D, Li S, Liu X. Dorsal root ganglion pulsed radiofrequency using bipolar technology in patients with lumbosacral radicular pain duration ≥ 2 years. Front Neurosci. 2022;16:1021374. doi:10.3389/fnins.2022.1021374
15. Liu R, Xu X, Xu Y, Fang X, Lin X. Pulsed Radiofrequency on Dorsal Root Ganglion Relieved Neuropathic Pain Associated with Downregulation of the Spinal Interferon Regulatory Factor 8, Microglia, p38MAPK Expression in a CCI Rat Model. Pain Phys. 2018;21(4):E307–E322. doi:10.36076/ppj.2018.4.E307
16. Das B, Conroy M, Moore D, Lysaght J, McCrory C. Human dorsal root ganglion pulsed radiofrequency treatment modulates cerebrospinal fluid lymphocytes and neuroinflammatory markers in chronic radicular pain. Brain Behav Immun. 2018;70:157–165. doi:10.1016/j.bbi.2018.02.010
17. Gleichmann M, Mattson MP. Neuronal Calcium Homeostasis and Dysregulation. Antioxidants & Redox Signaling. 2011;14(7):1261–1273. doi:10.1089/ars.2010.3386
18. Alessandro Formenti ADSEA and MM. Changes in Extracellular Ca2+can Affect the Pattern of discharge in Rat Thalamic Neurons. 2014.
19. Kann O, Kovács R, Kann O. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007;292:641–657. doi:10.1152/ajpcell.00222.2006.-Mitochondria
20. Wang CL, Song T. The Clinical Efficacy of High-Voltage Long-Duration Pulsed Radiofrequency Treatment in Pudendal Neuralgia: a Retrospective Study. Neuromodulation. 2022;25(8):1372–1377. doi:10.1111/ner.13401
21. Hashemian S, Alhouayek M, Fowler CJ. TLR4 receptor expression and function in F11 dorsal root ganglion × neuroblastoma hybrid cells. Innate Immun. 2017;23(8):687–696. doi:10.1177/1753425917732824
22. Laksono RM, Kalim H, Rohman MS, et al. Pulsed Radiofrequency Decreases pERK and Affects Intracellular Ca2+ Influx, Cytosolic ATP Level, and Mitochondrial Membrane Potential in the Sensitized Dorsal Root Ganglion Neuron Induced by N-Methyl D-Aspartate. J Pain Res. 2023;16:1697–1711. doi:10.2147/JPR.S409658
23. Facchini G, Spinnato P, Guglielmi G, Albisinni U, Bazzocchi A. A comprehensive review of pulsed radiofrequency in the treatment of pain associated with different spinal conditions. Br J Radiol. 2017;90(1073):20150406. doi:10.1259/bjr.20150406
24. Lee JS, Yoon KB, Kim IK, Yoon DM. Pulsed radiofrequency treatment of pain relieving point in a soft tissue. Korean J Pain. 2011;24(1):57–59. doi:10.3344/kjp.2011.24.1.57
25. Park D, Chang MC. The mechanism of action of pulsed radiofrequency in reducing pain: a narrative review. J Yeungnam Med Sci. 2022;39(3):200–205. doi:10.12701/jyms.2022.00101
26. Guo L, Kubat NJ, Isenberg RA. Pulsed radio frequency energy (PRFE) use in human medical applications. Electromagn Biol Med. 2011;30(1):21–45. doi:10.3109/15368378.2011.566775
27. Gofeld M, Restrepo-Garces CE, Theodore BR, Faclier G. Pulsed Radiofrequency of Suprascapular Nerve for Chronic Shoulder Pain: a Randomized Double-Blind Active Placebo-Controlled Study. Pain Practice. 2013;13(2):96–103. doi:10.1111/j.1533-2500.2012.00560.x
28. Ye H, Steiger A. Neuron matters: electric activation of neuronal tissue is dependent on the interaction between the neuron and the electric field. J Neuroeng Rehabil. 2015;12(1). doi:10.1186/s12984-015-0061-1
29. Hagiwara S, Iwasaka H, Takeshima N, Noguchi T. Mechanisms of analgesic action of pulsed radiofrequency on adjuvant-induced pain in the rat: roles of descending adrenergic and serotonergic systems. Eur J Pain. 2009;13(3):249–252. doi:10.1016/j.ejpain.2008.04.013
30. West AE, Chen WG, Dalva MB, et al. Calcium Regulation of Neuronal Gene Expression. Available from: www.pnas.orgcgidoi10.1073pnas.191352298.
31. Marambaud P, Dreses-Werringloer U, Vingtdeux V. Calcium signaling in neurodegeneration. Mol Neurodegener. 2009;4(1):20. doi:10.1186/1750-1326-4-20
32. Brini M, Calì T, Ottolini D, Carafoli E. Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci. 2014;71(15):2787–2814. doi:10.1007/s00018-013-1550-7
33. Mercadal B, Vicente R, Ivorra A. Pulsed radiofrequency for chronic pain: in vitro evidence of an electroporation mediated calcium uptake. Bioelectrochemistry. 2020;136. doi:10.1016/j.bioelechem.2020.107624
34. Boinagrov D, Pangratz-Fuehrer S, Suh B, Mathieson K, Naik N, Palanker D. Upper threshold of extracellular neural stimulation. J Neurophysiol. 2012;108:3233–3238. doi:10.1152/jn.01058.2011.-It
35. Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50(2):98–115. doi:10.2144/000113610
36. Zorova LD, Popkov VA, Plotnikov EY, et al. Mitochondrial membrane potential. Anal Biochem. 2018;552:50–59. doi:10.1016/j.ab.2017.07.009
37. Izyumov DS, Domnina LV, Nepryakhina OK, et al. Mitochondria as source of reactive oxygen species under oxidative stress. Study with novel mitochondria-targeted antioxidants - The “Skulachev- Ion” derivatives. Biochemistry. 2010;75(2):123–129. doi:10.1134/S000629791002001X
38. Fiskum G, Kowaltowksi AJ, Andreyev AY, Kushnareva YE, Starkov AA. Apoptosis-related activities measured with isolated mitochondria and digitonin-permeabilized cells. Methods Enzymol. 2000;322:222–234. doi:10.1016/s0076-6879(00)22023-5
39. Osorio-Fuentealba C, Contreras-Ferrat AE, Altamirano F, et al. Electrical stimuli release ATP to increase GLUT4 translocation and glucose uptake via PI3Kγ-Akt-AS160 in skeletal muscle cells. Diabetes. 2013;62(5):1519–1526. doi:10.2337/db12-1066
40. Gao YJ, Ji RR. c-Fos or pERK, Which is a Better Marker for Neuronal Activation and Central Sensitization After Noxious Stimulation and Tissue Injury? Open Pain J. 2009;2(1):11–17. doi:10.2174/1876386300902010011
41. Ruthirago D, Julayanont P, Kim J. Translational Correlation: migraine. In: Conn’s Translational Neuroscience. Elsevier Inc.; 2017:159–165. doi:10.1016/B978-0-12-802381-5.00013-0
42. Shetty PK, Galeffi F, Turner DA. Cellular links between neuronal activity and energy homeostasis. Front Pharmacol. 2012;3. doi:10.3389/fphar.2012.00043
43. Tortora F, Negro A, Russo C, Cirillo S, Caranci F. Chronic intractable lumbosacral radicular pain, is there a remedy? Pulsed radiofrequency treatment and volumetric modifications of the lumbar dorsal root ganglia. Radiol Med. 2021;126(1):124–132. doi:10.1007/s11547-020-01212-z
44. Moore D, Galvin D, Conroy MJ, et al. Characterisation of the effects of pulsed radio frequency treatment of the dorsal root ganglion on cerebrospinal fluid cellular and peptide constituents in patients with chronic radicular pain: a randomised, triple-blinded, controlled trial. J Neuroimmunol. 2020;343:577219. doi:10.1016/j.jneuroim.2020.577219
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.