Back to Journals » Vascular Health and Risk Management » Volume 19
Pulmonary Valve Stenosis: From Diagnosis to Current Management Techniques and Future Prospects
Authors Marchini F, Meossi S, Passarini G, Campo G, Pavasini R
Received 28 March 2023
Accepted for publication 23 June 2023
Published 30 June 2023 Volume 2023:19 Pages 379—390
DOI https://doi.org/10.2147/VHRM.S380240
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Federico Marchini, Sofia Meossi, Giulia Passarini, Gianluca Campo, Rita Pavasini
Cardiology Unit, Azienda Ospedaliero Universitaria di Ferrara, Ferrara, Italy
Correspondence: Rita Pavasini, Cardiology Unit, Azienda Ospedaliero Universitaria di Ferrara, Via Aldo Moro 8, Ferrara, FE, 44124, Italy, Tel +39 0532237227, Email [email protected]
Abstract: Pulmonary stenosis (PS) is mainly a congenital defect that accounts for 7– 12% of congenital heart diseases (CHD). It can be isolated or, more frequently, associated with other congenital defects (25– 30%) involving anomalies of the pulmonary vascular tree. For the diagnosis of PS an integrated approach with echocardiography, cardiac computed tomography and cardiac magnetic resonance (CMR) is of paramount importance for the planning of the interventional treatment. In recent years, transcatheter approaches for the treatment of PS have increased however, meaning surgery is a possible option for complicated cases with anatomy not suitable for percutaneous treatment. The present review aims to summarize current knowledge regarding diagnosis and treatment of PS.
Keywords: pulmonary valve stenosis, percutaneous treatment, valvotomy, valvuloplasty
Introduction
Pulmonary stenosis (PS) is almost always congenital in origin. Isolated PS is a rare condition that occurs in about 1 per 2000 live births worldwide and it accounts for approximately 8% of all congenital heart disease (CHD).1
The prevalence seems to be steadily increasing overtime, with a slightly higher birth prevalence in Asia compared to Europe and the USA.1
The therapeutic approach is determined by the hemodynamic severity of the obstruction. Intervention should be considered in patients with severe PS, but also in non-severe stenosis in the presence of symptoms, such as otherwise unexplained congestive heart failure, cyanosis from interatrial right-to-left communication and exercise intolerance.2
The last decade was characterized by significant improvements in the diagnostic and therapeutic possibilities for diseases of the pulmonary valve, with both the development of new treatments (fetal interventions, new surgical strategies and percutaneous pulmonary valve implantation), and with the improved understanding of the long-term sequelae of pulmonary valve disease.3
The main purpose of this review is to summarize the main findings regarding the diagnosis and therefore the treatment of PS, underlining the importance of the new techniques with percutaneous approach that are being proposed as therapeutic alternatives in the patient with PS.
Methodological Considerations
We performed a review of literature including studies regarding PS. Accordingly, the following terms using medical subject heading (MeSH) strategy were searched: (pulmonary valve stenosis) AND (treatment) NOT (case report) NOT (case series) NOT (artery or vein). The databases analyzed were PubMed, BioMed Central and Google Scholar. The literature search was carried out in December 2022. Only full-text article published in English and in peer-reviewed journals were selected. The following were inclusion criteria: 1) observational or randomized studies on the treatment and diagnosis of PS; and 2) relevant reviews about treatment and diagnosis of PS. Exclusion criteria were: 1) articles not regarding humans; 2) editorials; and 3) case report or case series. Literature search and screening of the literature of selected items was performed by two independent reviewers (FM and SM). Divergences have been solved by discussion and consensus. In case of discordance a third reviewer (RP) was asked to solve the disagreement and reach consensus. Two reviewers (FM and SM) retrieved data from the included studies. Overall, 125 studies were selected. After evaluation of records, 70 studies published between 1982 and 2022 were included in the review.
Etiology
In the classical form of PS the valve is dome shaped, characterized by a narrow central opening with preserved valve motion. There are generally two to four rudimentary raphes without a real separation into valve leaflets. Valve calcification is rare but it is seen in some elderly patients.4,5 Less commonly the valve may be uni, bi or tricuspid with various degrees of commissural fusion and thickened cusps. Approximately 10–20% of cases involve pulmonic valve dysplasia. Dysplastic valves are trileaflet with thickened cusps composed of myxomatous tissue and little or no fusions, with relative valve immobility. This type of valve defect is a common component of Noonan syndrome.6–8 PS may also occur as part of complex congenital lesions (i.e., tetralogy of Fallot, complete atrioventricular canal, double outlet RV, univentricular heart).8 Most patients develop dilation of the pulmonary trunk (post stenotic dilation), with a degree of dilation not always proportional to the severity of obstruction. One exception to this finding is peripheral pulmonary artery stenosis usually found in Noonan’s and Williams syndrome.4
Only a small percentage of PS is acquired and caused by rheumatic disease, carcinoid disease and neoplastic lesions or may occur after surgical reconstruction for other complex congenital cardiac disorders: reconstruction often entails the placement of a pulmonary valve prosthesis, right ventricle-to-pulmonary artery homograft, or valved conduit, which degenerates over time, manifesting as stenosis, regurgitation, or both.9,10
The natural history of PS leads to secondary changes in other cardiac structures. In particular, the right ventricular systolic pressures needed to override the outlet stenosis can be even higher than systemic left ventricular pressures. Pressure overload increases wall stress and, in order to maintain a normal cardiac output, there is an increase in contractility and a compensatory right ventricular hypertrophy, an increase of end-systolic volume and end-diastolic volume and high right ventricular end-diastolic pressures. At first, these compensatory adaptations enable the right ventricle (RV) to maintain stroke volume in presence of increased afterload. Over time, progressive right ventricular hypertrophy and stiffness can determine right ventricular diastolic and systolic dysfunction and this leads to fibrosis of the endocardium and of the tricuspid valve apparatus, right ventricular ischemia, hypertension and hypertrophy of the right atrium and even arrhythmias.11 Therefore, elevated levels of NT-proBNP in neonates might be used as a biomarker for diagnosis of severe PS.12,13
Diagnosis
For the diagnosis of PS and planning of treatment it is of paramount importance the application of multimodality imaging (Table 1), with the integrated use of echocardiography, cardiac magnetic resonance (CMR) and of cardiac computer tomography (CCT).
 |
Table 1 Main Available Diagnostic Methods for Evaluation of Pulmonary Valve Stenosis |
Transthoracic Echocardiography
The primary method for determining PS is the echocardiogram: in fact, it can assess severity, anatomy of the stenosis, etiology, associated lesions, and impact on the RV. The conventional and modified views of two-dimensional transthoracic echocardiography used to evaluate PS are parasternal short axis view (PSAX – great vessels plane), subcostal view, modified parasternal long axis (PLAX) and five chamber views (5 Ch) for RVOT and pulmonary arteries (PA)14 (Table 1).
First, qualitative examination of PS is necessary to determinate the mechanism of valve dysfunction (e.g., abnormal cuspids number, dysplastic valve, prolapse, atresia or agenesia) and associated lesions, like RV hypertrophy or dilatation and right atria dilatation.6,14
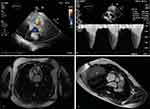
The quantitative assessment of PS, instead, is mostly based on transvalvular pressure gradient because the calculation of planimetric PV area is not possible and continuity equation or proximal isovelocity surface area method are rarely performed (Figure 1). Through the simplified Bernoulli equation (ΔP= 4V2) the systolic pressure gradient is estimated using the transpulmonary velocity flow curve calculated by continuous wave (CW) doppler from PSAX view (Figure 1).
According to the 2020 ESC guidelines for the management of adult CHD, PS can be classified as follow:5
- Severe stenosis: peak gradient ≥ 64 mmHg;
- Moderate stenosis: peak gradient between 36 and 64 mmHg;
- Mild stenosis: peak gradient ≤ 36 mmHg.
Finally, in individuals with PS, determining the RV systolic pressure from the tricuspid regurgitant velocity and adding an estimate of right atrial pressure might be a helpful measure of severity.14
Transesophageal Echocardiography
Pulmonary valve may also be evaluated through transesophageal echocardiography (TOE). The mid-esophageal (ME) RV inflow-outflow view (45°–60°) explores the long axis of the RVOT and od pulmonary valve, anterior to the aortic valve. The sub-pulmonary region is examined from this projection to assess muscle bundles or constriction at the infundibular outflow septum, which may indicate a double-chambered RV. Although there may be color Doppler turbulence, the ultrasound beam angle is insufficient to generate a valid peak Doppler gradient for sub valvular or valvular stenosis.15 From ME RV inflow-outflow view, withdrawing the probe up to the upper esophageal (UE) view may optimize imaging of the main and proximal branch PA. Doppler evaluation of valvular and supravalvular PS is optimum from this angle; however, supravalvular stenosis can be visualized only approximately 50% of the time because this probe position is particularly uncomfortable for the patients. The best window to visualize the role of the infundibulum and moderator band in dynamic sub-valvular stenosis is from the transgastric view (TG): TG inflow-outflow view is obtained at 0° to 20°, while a long-axis view of the entire RVOT is best visualized at 40° to 70°. 3D rendering of the valve is quite difficult since the PV appears in the far field at all imaging levels: because the annular plane is more perpendicular to the ultrasound beam, the UE view often offers the best imaging of the leaflets to improve 3D capture15 (Table 1).
Cardiac Magnetic Resonance
In most cases, echocardiography remains the first-line imaging method for PS, however CMR allows the reconstruction of unlimited image planes and views of PV and RVOT, even when cardiac anatomy or heart position in the chest are challenging16 (Figure 1). In fact, the optimal visualization of the RVOT with CMR allows identification of site and severity of PS. Compared to the evaluation of the left valves, the pulmonary valve requires specific image views for the RVOT, which can be used to provide a qualitative assessment of severity by observing the valve motion and stenotic jet. Quantitative evaluation of valve dysfunction can be assessed by the combination of high image quality and phase contrast flow mapping sequences.17,18 CMR guarantees direct planimetry of the valve orifice from an image acquired through the valve tips at peak opening during systole. It may provide ideal assessment of valve anatomy and quantifies the degree of stenosis. When precise echo beam alignment with the jet is not possible, phase-contrast velocity mapping can be used to quantify transvalvular velocity. This is especially useful in angulated roots or when the stenotic jet is not parallel to the RVOT. From the outflow tract planes, identifying sub-valvular and supravalvular stenosis is easy and the site of maximal velocity can be identified by in-plane velocity mapping. Finally, RV anatomy and function can be correctly assessed with cine imaging and any concomitant pulmonary trunk or branch artery stenosis can also be recognized through angiography sequences of the PA19 (Table 1).
Cardiac Computed Tomography
Thanks to its high spatial resolution, CCT scan is a useful diagnostic tool which can provide detailed anatomical information regarding the pulmonary valve and surrounding anatomical structures such as the RVOT, distal PAs, and coronary arteries. Moreover, it gives detailed information about prosthetic valves, and it is also essential for surgical and transcatheter intervention planning.20 To correctly visualize the pulmonary valve and its surrounding structures, the right heart chambers must be appropriately opacified using optimal imaging methodology and ECG-gated acquisitions: in particular, the split-bolus injection offers adequate attenuation for right heart chambers avoiding streak artifacts from high-attenuation contrast medium.21
In PS, CT typically shows a thickened and fused pulmonary valve with reduced opening area.22,23 With developments in CT scan technology, axial data may now be rebuilt into sagittal and coronal images using multiplanar reformation (MPR). Furthermore, maximum intensity projection (MIP) and volumetric rendering (VR) have increased diagnostic sensibility and specificity of post-processing software.24 Finally, accurate three-dimensional views produced by CCT scan can aid in determining the degree of stenosis and the effects of flow abnormalities (Table 1).
Treatment
According to the American Heart Association/American College of Cardiology (AHA/ACC) guidelines, in case of domed pulmonic valve with moderate or severe valvular stenosis and less than moderate pulmonic valve regurgitation, balloon valvotomy is recommended25 (Figure 2).
 |
Figure 2 Flow chart for the management of patients with PS. Abbreviation: PS, pulmonary stenosis. |
Otherwise, surgical repair is recommended in case of symptomatic patients with moderate or severe valvular pulmonic stenosis who are ineligible for balloon valvotomy or who have failed it. This includes patients with severe PS and an associated hypoplastic pulmonary annulus, severe pulmonary regurgitation (PR), sub-valvular PS or supravalvular pulmonic stenosis. Surgery is also preferred for most dysplastic valves and when there is associated severe tricuspid regurgitation or other cardiopathy that warrants operative intervention25, 26 (Figure 2).
At last, pulmonary valve replacement may be necessary when there is a failure with repair or severe residual symptomatic PR, in particular in cases with marked dysplasia of the pulmonary valve or significant hypoplasia of the annulus.25
Balloon Pulmonary Valvuloplasty (BPV)
Balloon pulmonary valvuloplasty represents the first line of treatment for PS and should be performed as soon as the diagnosis is made, without waiting for the development of symptoms or the patients to reach a certain size. It has been described for the first time in 1982 by Kan et al, who used the approach of Gruntzing to alleviate PS using the radial forces of balloon inflation of a balloon catheter positioned across the pulmonary valve.27 BPV is usually indicated when peak-to-peak gradient is more then 50 mmHg with normal cardiac index. It is currently the preferred therapeutic strategy for “doming” valvular PS.28 However, it has also a palliative function in some diseases like tetralogy of Fallot.29 Nowadays it has replaced surgical intervention as the initial treatment for moderate and severe PS thank to its safety and excellent early and intermediate outcome.30 In fact, it is associated with low PR than surgery, but the same relief from restenosis. The procedure is usually performed through percutaneous femoral access and consists of introducing one or more balloon catheters across the stenotic valve, commonly over an extra-stiff guide wire, and inflating the balloons with diluted contrast material.
Procedural complications are rare, like transient bradycardia, premature beats and drop in systemic pressure, which usually regress after balloon deflation. Other complications reported are blood loss requiring transfusions, complete right bundle branch block, permanent or transient heart block, cerebrovascular accident, and cardiac arrest.31 Moreover, in nearly 30% of patients it’s possible to develop a severe infundibular obstruction, which usually decreases during follow-up and rarely require surgical intervention.32 Complications are generally low in children and adults but more prevalent in infants and neonates. Immediately after the dilatation, a rapid reduction in gradient, increase in jet width, and free mobility of the pulmonary valve leaflets with reduced doming are usually seen with an improvement in RV and tricuspid valve function. Immediate and mid-term results (<2 years) are usually good: in the study of Hong et al,31 residual transvalvular pressure gradients of 67.31 ± 15.19 mmHg (range: 50–92 mmHg) were found in only 8.2% of patients, 6.4% of patients had variable‐staged restenosis at follow‐up and 3.8% underwent reintervention by balloon dilatation or surgical repairs.33 The long-term outcome has historically considered excellent, but the most of available data comes from small, single center studies. The study of Hansen et al34 aimed to evaluate long‐term outcomes (25 years) following BPV for PS. Among 254 patients, 69 (29%) had ≥ moderate PR, 41 (17%) had residual PS with peak gradient > 40 mmHg, and 31 (13%) had re‐intervention.
Predictor of worst outcome after BPV are a dysplastic valve, a small annular size, an higher gradient immediately after the procedure and being neonates (< 1 year).4
Surgical Valvotomy
Surgical relief of PS was one of the first congenital heart operation and was described independently by Sellors and Brock in 1948.35,36 This first surgical procedure consists in a closed transventricular pulmonary valvotomy via the RV outflow tract. The Brock technique dilates and cuts the stenotic pulmonary valve and likely the pulmonary infundibulum using a valvulotome and dilators through a purse string in the RV below the native valves.37,38
Surgical treatment was the preferred procedure for PS until the late 1980s, when the less invasive balloon valvuloplasty replaced surgery.27,31 Indeed, relieving the stenosis by splitting the valve leaflets is efficient but this relief occurs at the cost of PR, leading to right ventricular dilatation and tricuspid regurgitation. Nowadays surgical valvuloplasty is reserved for those patients with a hypoplastic pulmonary annulus, diminutive main pulmonary artery, or dysplastic pulmonary valves. In these patients, surgical repair is required to excise thickened and obstructive valve leaflets and place a transannular patch. Surgery is also the intervention of choice for patients with sub-valvular and supravalvular PS.26,39,40
Current surgery is associated with low early mortality and excellent long-term survival that is nearly as good as survival in the general population. Less favorable outcome is found only in patients operated at older age. An explanation for this may be the longer period of right ventricular pressure overload with subsequent right ventricular failure.41,42
The most important sequel after successful valvotomy is residual PR requiring reintervention in one-quarter of the patients. The need for reoperation is not correlated with the pre-operative severity of stenosis, but rather with the surgical technique used and the amount of pulmonary regurgitation, which occurred after surgery.43
Between possible complications, the incidence of supraventricular arrhythmias after repair of PS is very low and they are mainly present in patients with severe PR needing of reoperation and they generally disappear after reintervention.44
Surgical Valve Replacement
A wide spectrum of CHDs requires pulmonary valve replacement with or without reconstruction of the right ventricular outflow tract.45 Pulmonary valve replacement is also the main reintervention in patients with PS who have previously undergone valvotomy with subsequent PR and/or residual PS. Pulmonary valve replacement, if appropriately implemented, effectively alleviates right ventricular dilatation and dysfunction and resolves the related symptoms.46–49
There are several options for pulmonary valve replacement, including:45, 50
- Homograft conduits;
- Bovine jugular vein grafts;
- Mechanical prosthetic valves;
- Biological prosthetic valves.
Pulmonary Homograft
Homografts for pulmonary valve replacement have some advantages (low associate gradients, no need of anticoagulation) and some disadvantages (calcifications, valve insufficiency necessitating replacement).33 However, compared to biological valves34, 35 no differences has been seen in terms of durability, rate of valve disfunction, reintervention, acute complications. Finally, homografts as well as bovine jugular vein conduits have limited availability and sizes, therefore in most cases heterograft are preferred.27, 36
Bioprosthetic Valves
Bioprosthesis are predominantly used for pulmonary valve replacement as they are associated with the minimal risk of a thromboembolic event and the lower rate of prosthesis failure in the pulmonary position.51 Furthermore, bioprosthetic valves have occupied a solid position with the advent of percutaneous pulmonary valve replacement, owing to the possibility of valve-in-valve implantation of a transcatheter prosthesis.52
Because of the structural similarities between the pulmonary and aortic valves, the commercial valves that were originally developed for the aortic valve position have been used in the pulmonic position in patients with CHDs, especially those patients with RVOT disfunction.53
The most implanted bioprosthetic valves in the pulmonary position are the stented bovine pericardial valves. Originally designed for left-sided valvular heart disease, they have shown excellent results in the aortic and mitral valve position, and later in the pulmonary valve position and have become a leading choice for pulmonary valve replacement.45, 50
Stented porcine valves represent another option for pulmonary valve replacement. The published experience with this type of bioprosthetic valves is significant, and most studies have reported favorable performance and durability with no relevant differences in freedom from replacement compared to bovine pericardial valves.50, 54–56
Mechanical Valves
There are limited experiences about implanting mechanical valve in the pulmonary position.57, 58 However, while the age at pulmonary valve replacement is getting lower and life expectancy of the patients with CHDs increases, durability of the prosthesis has become a relevant issue, particularly in younger patients under high risk of repeated surgery. Consequently, mechanical prosthesis has received attention as an alternative for pulmonary valve replacement to minimize the risk of reoperation, because it does not need to be replaced again theoretically, if adult-sized prosthesis could be implanted.46, 57, 59
The main disadvantage of mechanical valves is the higher rates of valve thrombosis, requiring lifelong use of anticoagulation medication.60 Therefore, currently they are mainly used in patients with multiple prior operations or another need for anticoagulation, such as presence of a mechanical valve in other positions.61
Percutaneous Treatment
Symptomatic PS is frequently treated in childhood. However, even if severe PS is less prevalent in adults, sometimes it is possible to come across unrepaired PS or residual pulmonary valve stenosis after a previous intervention. Over the years, clinicians have focused on postponing surgical treatments for as long as feasible to reduce the number of surgical operations: in fact, long-term durability of conduits, bioprosthetic valves, and trans-anular patch (TAP) may be affected by the materials used, the age of the patient and the type of surgery.62 Significant PR after RVOT correction with TAP is seen in 48% of individuals immediately after the operation and in 85% after 2 years;62 moreover, following an implantation of valved homograft, RVOT degenerative dysfunction, PR, and/or PS are detected in half of the patients during the first 10 years after the first operation and after 5–6 years after the second operation.63,64 This is associated to multiple surgical interventions which can increase morbidity and mortality. The management of these diseases has been radically changed by the introduction of the transcatheter pulmonary valve replacement (tPVR), leading to a reduction in the number of surgical interventions over lifetime.65 The main available valves for transcatheter replacement are the Melody Valve and the Edwards SAPIEN XT and S3 Valves.
The Melody Valve
The Melody transcatheter pulmonary valve is a percutaneous valve system designed to treat obstruction or regurgitation of prosthetic conduits positioned between the RV and PAs.66 The device consists of an 18 mm valved bovine jugular venous conduit sutured into a Cheatham platinum Stent. The bovine derived tissue and the valve morphology allows a good adaptation to several conduit diameter. The valve is implanted through femoral access using a Medtronic 22F Ensemble® Transcatheter Delivery System, which is a balloon-in-balloon system that allows the valve to be replaced after the inner balloon has been inflated, if necessary.66 The main indications for Melody Valve use (from the US Melody Valve Investigational Device Exemption trial) are the following:9,67–69
- Age ≥ 5 years or weight ≥ 30 kg;
- Original conduit diameter ≥ 16 cm;
- Echocardiographic signs of RVOT conduit dysfunction;
- Patients in NYHA class > I;
- Doppler mean gradient ≥ 35 mmHg or ≥ moderate PR;
- Patients in NYHA class I with Doppler mean gradient ≥ 40 mmHg or severe PR associated with TV annulus Z-score ≥ 2 RVEF < 40%.
Procedural success is typically high, with a mean valve deployment success rate of 95%, an acute reduction of RV pressure to a median of 42 mmHg and a reduction in peak gradient across the RVOT to a median of 12 mmHg.9
Moreover, short and long-term outcomes are generally good. In the updated report from the multicenter US Melody trial from Mc Elhinney et al, freedom from diagnosis of stent fracture was 77.8 ± 4.3% at 14 months.7 Freedom from Melody valve dysfunction or reintervention was 93.5 ± 2.4% at 1 year.9 Furthermore, valvular replacement can reduce RV volumes and improve left ventricular diastolic function. In patients with significant RVOT obstruction, the Melody valve implantation improves exercise, cardiopulmonary function, ventricular systolic function, and early left ventricular diastolic filling pressure. Finally, transcatheter replacement with the Melody valve provides good hemodynamic and clinical outcomes up to 7 years after the intervention. In the work of Cheatman et al, the five-year freedom from reintervention and explanation was 76 ± 4% and 92 ± 3% respectively, and almost all patients were in NYHA class ≤ II.66
The main complication remains the stent fracture, which is associated with restenosis and need for reintervention. Other rare complications are conduit fracture, valve migration or embolization and, in the follow-up, endocarditis.62
The Edwards SAPIEN XT and SAPIEN 3 Percutaneous Pulmonary Valve
If the Melody Valve was originally used only for stenotic RVOT conducts, the introduction of larger Edwards SAPIEN valves (SAPIEN XT valve and its next generation SAPIEN 3 valve) allowed tPVR to be performed also in native and wider RVOTs.70, 71 The SAPIEN XT valve is made of three bovine pericardial leaflets stitched to a cobalt chromium balloon expandable stent; SAPIEN 3 valve, on the other side, shows an improved frame geometry thank to its low frame height and outer skirt made of polyethylene terephthalate which minimize paravalvular leak. Both valves are available in several diameters (20 mm only for SAPIEN XT, 23, 26, and 29 mm for both valves) which allow implantation in larger RVOT than the Melody.62 The 29 mm valves, in fact, suit with native or patched RVOT larger then 26 mm. Recently, the Altera stent has been released by Edwards Lifesciences: it is a single-size (40 × 45 mm) nitinol self-expanding covered stent (hourglass) with a firm landing zone for SAPIEN S3 29 mm, suited for RVOT sizes up to 38 mm.72, 73
The implantation procedure is often carried out through percutaneous femoral access using the NovaFlex catheter (Edwards Lifesciences Inc.) but, as a result of the engineering development in valve prosthesis, two recent technical changes in tPVR were introduced: the use of a 26-F larger Gore DrySeal sheath (Gore Medical, Flagstaff, Arizona)74 and direct valve insertion without pre-stenting.75 This more effective approach allows the valve to be directly implanted in the precise available landing zone, using angiography to deliver the valve in the correct position. Complications are similar to the ones reported for the Melody valve, with the exception of stent fracture which has not been reported for SAPIEN valves.
The short outcomes show excellent results with all types of RVOT: in a large cohort of patients who underwent tPVR with either a Sapien XT or S3 valve, the implant was technically successful in 754 (97.4%) patients, serious adverse events were described in 67 patients (10%), without difference between RVOT anatomy groups. Valve function at discharge was excellent in most patients, and only 58 (8.5%) had moderate or greater PR or maximum Doppler gradients >40 mm Hg. During the follow-up (median 12 months), 9 patients were diagnosed with endocarditis, and 17 additional patients underwent surgical valve replacement or valve-in-valve.76 About the long-term outcomes of the XT valve, in the study of Le Ruz et al, the primary efficacy outcome (freedom from valve-reintervention) was met for 87.1% patients after a mean follow-up of 4.6 ± 1.8 years, corresponding to a freedom of reintervention at 5 years of 89% (95% CI 74.8–95.6%).77 The ongoing COMPASSION S3 (ClinicalTrials.gov Identifier: NCT02744677) aims to demonstrate the safety and effectiveness of the Edwards Lifesciences SAPIEN 3 Transcatheter Heart Valve System in subjects with a dysfunctional right ventricular outflow tract (RVOT) conduit or previously implanted valve in the pulmonic position with a clinical indication for intervention.
New Prospective and Future Directions of tPVR
There is worldwide great interest in developing new devices (valves or RVOT reducers) and technologies to make more patients suitable for tPVR. In fact, even if indications for tPVR are limited to patients with RVOT diameter up to 22 mm for the Melody valve and up to 27 mm for the SAPIEN valve,78 more than 80% of patients who are potential candidates for tPVR do not satisfy these criteria.79, 80 To face this problem, several RVOT reducing techniques have been proposed, as well as self-expanding valve systems. RVOT reducing techniques usually consist on the implantation of several stents before the insertion of the valve or anchoring multiple overlapping stents in one of the branch of the PA to allow the implantation of the valve into the protruding frame.62, 81 The first small experiences with self-expanding valve systems were in 2010 with the Valve Harmony ® and in 2014 with the Venus P Valve (Venus Medtech, Shanghai, China): short term outcomes of first case series seem encouraging but larger studies are needed to evaluate long term efficacy and safety.82, 83
Conclusions
PS is mostly a congenital disease, but it can also develop later in life due to other medical conditions. The severity of the stenosis can vary widely, ranging from mild to severe and potentially life-threatening. Although it is primarily a disease of childhood, its sequelae have become increasingly prevalent in the adult population due to improvements in diagnosis and treatment. One of the most significant advances in the treatment of PS is the development of percutaneous procedures and devices that can correct the condition without surgical intervention. This approach has proven to be effective and less invasive, offering patients a safe and efficient option for managing their condition.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Van der linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(21):2241–2247. doi:10.1016/j.jacc.2011.08.025
2. Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;139(14):e637–e97. doi:10.1161/CIR.0000000000000602
3. Odenwald T, Taylor AM. Pulmonary valve interventions. Expert Rev Cardiovasc Ther. 2011;9(11):1445–1457. doi:10.1586/erc.11.150
4. Shaddy REP, Feltes DJ, Cetta TF. Moss and Adams’ heart disease in infants, children, and adolescents: including the fetus and young adult. JAMA. 2022;2022:1.
5. Baumgartner H, De Backer J, Babu-Narayan SV, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42(6):563–645. doi:10.1093/eurheartj/ehaa554
6. Lancellotti Pao. The EACVI Textbook of Echocardiography. The European Society of Cardiology Textbooks. Oxford University Press; 2016.
7. Schicchi N, Secinaro A, Muscogiuri G, et al. Multicenter review: role of cardiovascular magnetic resonance in diagnostic evaluation, pre-procedural planning and follow-up for patients with congenital heart disease. Radiol Med. 2016;121(5):342–351. doi:10.1007/s11547-015-0608-z
8. Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22(1):
9. McElhinney DB, Hellenbrand WE, Zahn EM, et al. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010;122(5):507–516. doi:10.1161/CIRCULATIONAHA.109.921692
10. Khambadkone S, Coats L, Taylor A, et al. Percutaneous pulmonary valve implantation in humans: results in 59 consecutive patients. Circulation. 2005;112(8):1189–1197. doi:10.1161/CIRCULATIONAHA.104.523266
11. Ruckdeschel E, Kim YY. Pulmonary valve stenosis in the adult patient: pathophysiology, diagnosis and management. Heart. 2019;105(5):414–422. doi:10.1136/heartjnl-2017-312743
12. Lin Z, Chen Y, Zhou L, Chen S, Xia H. Serum N-Terminal Pro-B-Type natriuretic peptide as a biomarker of critical pulmonary stenosis in neonates. Front Pediatr. 2021;9:788715. doi:10.3389/fped.2021.788715
13. Duchnowski P. N-Terminal of the prohormone brain natriuretic peptide predicts postoperative cardiogenic shock requiring extracorporeal membrane oxygenation. J Clin Med. 2022;11:19.
14. Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32(1):1–64. doi:10.1016/j.echo.2018.06.004
15. Hahn RT, Saric M, Faletra FF, et al. Recommended standards for the performance of transesophageal echocardiographic screening for structural heart intervention: from the American Society of echocardiography. J Am Soc Echocardiogr. 2022;35(1):1–76. doi:10.1016/j.echo.2021.07.006
16. Lombardi Mao. The EACVI Textbook of Cardiovascular Magnetic Resonance. Lombardi Mao; 2018.
17. Kilner PJ, Manzara CC, Mohiaddin RH, et al. Magnetic resonance jet velocity mapping in mitral and aortic valve stenosis. Circulation. 1993;87(4):1239–1248. doi:10.1161/01.CIR.87.4.1239
18. Sondergaard L, Hildebrandt P, Lindvig K, et al. Valve area and cardiac output in aortic stenosis: quantification by magnetic resonance velocity mapping. Am Heart J. 1993;126(5):1156–1164. doi:10.1016/0002-8703(93)90669-Z
19. Myerson SG. CMR in evaluating valvular heart disease: diagnosis, severity, and outcomes. JACC Cardiovasc Imaging. 2021;14(10):2020–2032. doi:10.1016/j.jcmg.2020.09.029
20. Costantini P, Perone F, Siani A, et al. Multimodality imaging of the neglected valve: role of echocardiography, cardiac magnetic resonance and cardiac computed tomography in pulmonary stenosis and regurgitation. J Imaging. 2022;8(10):278. doi:10.3390/jimaging8100278
21. Kerl JM, Ravenel JG, Nguyen SA, et al. Right heart: split-bolus injection of diluted contrast medium for visualization at coronary CT angiography. Radiology. 2008;247(2):356–364. doi:10.1148/radiol.2472070856
22. Jonas SN, Kligerman SJ, Burke AP, Frazier AA, White CS. Pulmonary valve anatomy and abnormalities: a pictorial essay of radiography, Computed Tomography (CT), and Magnetic Resonance Imaging (MRI). J Thorac Imaging. 2016;31(1):W4–12. doi:10.1097/RTI.0000000000000182
23. Rajiah P, Nazarian J, Vogelius E, Gilkeson RC. CT and MRI of pulmonary valvular abnormalities. Clin Radiol. 2014;69(6):630–638. doi:10.1016/j.crad.2014.01.019
24. Tops LF, Wood DA, Delgado V, et al. Noninvasive evaluation of the aortic root with multislice computed tomography implications for transcatheter aortic valve replacement. JACC Cardiovasc Imaging. 2008;1(3):321–330. doi:10.1016/j.jcmg.2007.12.006
25. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143(5):e35–e71. doi:10.1161/CIR.0000000000000932
26. Nielsen EA, Hjortdal VE. Surgically treated pulmonary stenosis: over 50 years of follow-up. Cardiol Young. 2016;26(5):860–866. doi:10.1017/S1047951115001158
27. Kan JS, White RI Jr, Mitchell SE, Gardner TJ. Percutaneous balloon valvuloplasty: a new method for treating congenital pulmonary-valve stenosis. N Engl J Med. 1982;307(9):540–542. doi:10.1056/NEJM198208263070907
28. Rao PS. Indications for balloon pulmonary valvuloplasty. Am Heart J. 1988;116(6 Pt 1):1661–1662. doi:10.1016/0002-8703(88)90790-9
29. Rao PS. Role of palliative balloon pulmonary valvuloplasty in babies with tetralogy of Fallot. Heart Vessels. 2020;35(11):1629–1630. doi:10.1007/s00380-020-01628-7
30. Merino-Ingelmo R, Santos-de Soto J, Coserria-Sanchez F, Descalzo-Senoran A, Valverde-Perez I. Long-term results of percutaneous balloon valvuloplasty in pulmonary valve stenosis in the pediatric population. Rev Esp Cardiol. 2014;67(5):374–379. doi:10.1016/j.recesp.2013.08.020
31. Rao PS. Percutaneous balloon pulmonary valvuloplasty: state of the art. Catheter Cardiovasc Interv. 2007;69(5):747–763. doi:10.1002/ccd.20982
32. Thapar MK, Rao PS. Significance of infundibular obstruction following balloon valvuloplasty for valvar pulmonic stenosis. Am Heart J. 1989;118(1):99–103. doi:10.1016/0002-8703(89)90078-1
33. Hong D, Qian MY, Zhang ZW, et al. Immediate therapeutic outcomes and medium-term follow-up of percutaneous balloon pulmonary valvuloplasty in infants with pulmonary valve stenosis: a single-center retrospective study. Chin Med J. 2017;130(23):2785–2792. doi:10.4103/0366-6999.219155
34. Hansen RL, Naimi I, Wang H, et al. Long-term outcomes up to 25 years following balloon pulmonary valvuloplasty: a multicenter study. Congenit Heart Dis. 2019;14(6):1037–1045. doi:10.1111/chd.12788
35. Sellors TH. Surgery of pulmonary stenosis; a case in which the pulmonary valve was successfully divided. Lancet. 1948;1(6513):988.
36. Brock SR. The Surgical Treatment of Pulmonary Stenosis. Br Heart J. 1961;23(4):337–356. doi:10.1136/hrt.23.4.337
37. Brock RC, Campbell M. Valvulotomy for pulmonary valvular stenosis. Br Heart J. 1950;12(4):377–402. doi:10.1136/hrt.12.4.377
38. S S, B PM, A AM. Outcomes 60 years after surgical valvotomy for isolated congenital pulmonary valve stenosis. J Card Surg. 2021;36(4):1531–1533. doi:10.1111/jocs.15276
39. Stanger P, Cassidy SC, Girod DA, Kan JS, Lababidi Z, Shapiro SR. Balloon pulmonary valvuloplasty: results of the Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am J Cardiol. 1990;65(11):775–783. doi:10.1016/0002-9149(90)91387-L
40. DiSessa TG, Alpert BS, Chase NA, Birnbaum SE, Watson DC. Balloon valvuloplasty in children with dysplastic pulmonary valves. Am J Cardiol. 1987;60(4):405–407. doi:10.1016/0002-9149(87)90266-9
41. Kopecky SL, Gersh BJ, McGoon MD, et al. Long-term outcome of patients undergoing surgical repair of isolated pulmonary valve stenosis. Follow-up at 20–30 years. Circulation. 1988;78(5 Pt 1):1150–1156. doi:10.1161/01.CIR.78.5.1150
42. McNamara DG, Latson LA. Long-term follow-up of patients with malformations for which definitive surgical repair has been available for 25 years or more. Am J Cardiol. 1982;50(3):560–568. doi:10.1016/0002-9149(82)90325-3
43. Cuypers JA, Menting ME, Opić P, et al. The unnatural history of pulmonary stenosis up to 40 years after surgical repair. Heart. 2017;103(4):273–279. doi:10.1136/heartjnl-2015-309159
44. Roos-Hesselink JW, Meijboom FJ, Spitaels SE, et al. Long-term outcome after surgery for pulmonary stenosis (a longitudinal study of 22–33 years). Eur Heart J. 2006;27(4):482–488. doi:10.1093/eurheartj/ehi685
45. P H, S MH, A RE, et al. A stented bovine pericardial prosthesis in the pulmonary position. J Thorac Cardiovasc Surg. 2020;159(3):1063–71.e1. doi:10.1016/j.jtcvs.2019.05.086
46. Kim DH, Choi ES, Kwon BS, et al. Pulmonary valve replacement following repair of tetralogy of Fallot: comparison of outcomes between bio- and mechanical prostheses. Europ J Cardio Thor Surg. 2021;60(4):947–954. doi:10.1093/ejcts/ezab099
47. Discigil B, Dearani JA, Puga FJ, et al. Late pulmonary valve replacement after repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2001;121(2):344–351. doi:10.1067/mtc.2001.111209
48. Nomoto R, Sleeper LA, Borisuk MJ, et al. Outcome and performance of bioprosthetic pulmonary valve replacement in patients with congenital heart disease. J Thorac Cardiovasc Surg. 2016;152(5):1333–42 e3. doi:10.1016/j.jtcvs.2016.06.064
49. Ferraz Cavalcanti PE, Sa MP, Santos CA, et al. Pulmonary valve replacement after operative repair of tetralogy of Fallot: meta-analysis and meta-regression of 3118 patients from 48 studies. J Am Coll Cardiol. 2013;62(23):2227–2243. doi:10.1016/j.jacc.2013.04.107
50. Schubert SA, Myers JL, Kunselman AR, Clark JB. Early outcomes of pulmonary valve replacement with the mitroflow bovine pericardial bioprosthesis. Ann Thorac Surg. 2015;99(5):1692–1698. doi:10.1016/j.athoracsur.2014.12.081
51. Freling HG, van Slooten YJ, van Melle JP, et al. Pulmonary valve replacement: twenty-six years of experience with mechanical valvar prostheses. Ann Thorac Surg. 2015;99(3):905–910. doi:10.1016/j.athoracsur.2014.10.034
52. Asoh K, Walsh M, Hickey E, et al. Percutaneous pulmonary valve implantation within bioprosthetic valves. Eur Heart J. 2010;31(11):1404–1409. doi:10.1093/eurheartj/ehq056
53. Kwak JG, Bang JH, Cho S, et al. Long-term durability of bioprosthetic valves in pulmonary position: pericardial versus porcine valves. J Thorac Cardiovasc Surg. 2020;160(2):476–484. doi:10.1016/j.jtcvs.2019.11.134
54. Lee C, Park CS, Lee CH, et al. Durability of bioprosthetic valves in the pulmonary position: long-term follow-up of 181 implants in patients with congenital heart disease. J Thorac Cardiovasc Surg. 2011;142(2):351–358. doi:10.1016/j.jtcvs.2010.12.020
55. Sabate Rotes A, Eidem BW, Connolly HM, et al. Long-term follow-up after pulmonary valve replacement in repaired tetralogy of Fallot. Am J Cardiol. 2014;114(6):901–908. doi:10.1016/j.amjcard.2014.06.023
56. Chen XJ, Smith PB, Jaggers J, Lodge AJ. Bioprosthetic pulmonary valve replacement: contemporary analysis of a large, single-center series of 170 cases. J Thorac Cardiovasc Surg. 2013;146(6):1461–1466. doi:10.1016/j.jtcvs.2012.09.081
57. Stulak JM, Dearani JA, Burkhart HM, et al. The increasing use of mechanical pulmonary valve replacement over a 40-year period. Ann Thorac Surg. 2010;90(6):2009–2014. doi:10.1016/j.athoracsur.2010.07.023
58. Shin HJ, Kim YH, Ko JK, Park IS, Seo DM. Outcomes of mechanical valves in the pulmonic position in patients with congenital heart disease over a 20-year period. Ann Thorac Surg. 2013;95(4):1367–1371. doi:10.1016/j.athoracsur.2012.07.008
59. Abbas JR, Hoschtitzky JA. Is there a role for mechanical valve prostheses in pulmonary valve replacement late after tetralogy of Fallot repair? Interact Cardiovasc Thorac Surg. 2014;18(5):661–666. doi:10.1093/icvts/ivt541
60. Pragt H, van Melle JP, Javadikasgari H, et al. Mechanical valves in the pulmonary position: an international retrospective analysis. J Thorac Cardiovasc Surg. 2017;154(4):1371–8 e1. doi:10.1016/j.jtcvs.2017.04.072
61. Lee C, Jacobs JP, Lee CH, Kwak JG, Chai PJ, Quintessenza JA. Surgical pulmonary valve insertion--when, how, and why. Cardiol Young. 2012;22(6):702–707. doi:10.1017/S1047951112001722
62. Alkashkari W, Alsubei A, Hijazi ZM. Transcatheter pulmonary valve replacement: current state of Art. Curr Cardiol Rep. 2018;20(4):27. doi:10.1007/s11886-018-0966-y
63. Ong K, Boone R, Gao M, et al. Right ventricle to pulmonary artery conduit reoperations in patients with tetralogy of fallot or pulmonary atresia associated with ventricular septal defect. Am J Cardiol. 2013;111(11):1638–1643. doi:10.1016/j.amjcard.2013.01.337
64. Boethig D, Thies WR, Hecker H, Breymann T. Mid term course after pediatric right ventricular outflow tract reconstruction: a comparison of homografts, porcine xenografts and Contegras. Eur J Cardiothorac Surg. 2005;27(1):58–66. doi:10.1016/j.ejcts.2004.09.009
65. Coats L, Tsang V, Khambadkone S, et al. The potential impact of percutaneous pulmonary valve stent implantation on right ventricular outflow tract re-intervention. Eur J Cardiothorac Surg. 2005;27(4):536–543. doi:10.1016/j.ejcts.2004.12.053
66. McElhinney DB, Hennesen JT. The Melody(R) valve and Ensemble(R) delivery system for transcatheter pulmonary valve replacement. Ann N Y Acad Sci. 2013;1291(1):77–85. doi:10.1111/nyas.12194
67. Zahn EM, Hellenbrand WE, Lock JE, McElhinney DB. Implantation of the melody transcatheter pulmonary valve in patients with a dysfunctional right ventricular outflow tract conduit early results from the u.s. Clinical trial. J Am Coll Cardiol. 2009;54(18):1722–1729. doi:10.1016/j.jacc.2009.06.034
68. Borik S, Crean A, Horlick E, et al. Percutaneous pulmonary valve implantation: 5 years of follow-up: does age influence outcomes? Circ Cardiovasc Interv. 2015;8(2):e001745. doi:10.1161/CIRCINTERVENTIONS.114.001745
69. Aboulhosn J, Levi DS. Percutaneous pulmonary valve implantation: is earlier valve implantation better? Circ Cardiovasc Interv. 2015;8(2):e002260. doi:10.1161/CIRCINTERVENTIONS.115.002260
70. Kenny D, Rhodes JF, Fleming GA, et al. 3-Year outcomes of the edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position from the COMPASSION multicenter clinical trial. JACC Cardiovasc Interv. 2018;11(19):1920–1929. doi:10.1016/j.jcin.2018.06.001
71. Faccini A, Giugno L, Piazza L, et al. Evolving technique for SAPIEN pulmonary valve implantation: a single-center experience. JACC Cardiovasc Interv. 2020;13(12):1500–1502. doi:10.1016/j.jcin.2020.02.039
72. Levi DS, Sinha S, Salem MM, Aboulhosn JA. Transcatheter native pulmonary valve and tricuspid valve replacement with the sapien XT: initial experience and development of a new delivery platform. Catheter Cardiovasc Interv. 2016;88(3):434–443. doi:10.1002/ccd.26398
73. DeGiovanni J. Transcatheter pulmonary valve replacement. The Edwards Sapien Valve. J Struct Heart Dis. 2017;3(3):62–72. doi:10.12945/j.jshd.2017.016.14
74. Hascoet S, Karsenty C, Tortigue M, et al. A modified procedure for percutaneous pulmonary valve implantation of the Edwards SAPIEN 3 valve. EuroIntervention. 2019;14(13):1386–1388. doi:10.4244/EIJ-D-18-00530
75. Morgan GJ, Sadeghi S, Salem MM, et al. SAPIEN valve for percutaneous transcatheter pulmonary valve replacement without “pre-stenting”: a multi-institutional experience. Catheter Cardiovasc Interv. 2019;93(2):324–329. doi:10.1002/ccd.27932
76. Shahanavaz S, Zahn EM, Levi DS, et al. Transcatheter pulmonary valve replacement with the sapien prosthesis. J Am Coll Cardiol. 2020;76(24):2847–2858. doi:10.1016/j.jacc.2020.10.041
77. Le Ruz R, Plessis J, Houeijeh A, et al. Edwards SAPIEN XT transcatheter pulmonary valve implantation: 5-year follow-up in a French Registry. Catheter Cardiovasc Interv. 2021;98(5):990–999. doi:10.1002/ccd.29862
78. Feltes TF, Bacha E, Beekman RH, et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation. 2011;123(22):2607–2652. doi:10.1161/CIR.0b013e31821b1f10
79. Boshoff DE, Cools BL, Heying R, et al. Off-label use of percutaneous pulmonary valved stents in the right ventricular outflow tract: time to rewrite the label? Catheter Cardiovasc Interv. 2013;81(6):987–995. doi:10.1002/ccd.24594
80. Schievano S, Coats L, Migliavacca F, et al. Variations in right ventricular outflow tract morphology following repair of congenital heart disease: implications for percutaneous pulmonary valve implantation. J Cardiovasc Magn Reson. 2007;9(4):687–695. doi:10.1080/10976640601187596
81. Boudjemline Y, Brugada G, Van-Aerschot I, et al. Outcomes and safety of transcatheter pulmonary valve replacement in patients with large patched right ventricular outflow tracts. Arch Cardiovasc Dis. 2012;105(8–9):404–413. doi:10.1016/j.acvd.2012.05.002
82. Schievano S, Taylor AM, Capelli C, et al. First-in-man implantation of a novel percutaneous valve: a new approach to medical device development. EuroIntervention. 2010;5(6):745–750. doi:10.4244/EIJV5I6A122
83. Cao QL, Kenny D, Zhou D, et al. Early clinical experience with a novel self-expanding percutaneous stent-valve in the native right ventricular outflow tract. Catheter Cardiovasc Interv. 2014;84(7):1131–1137. doi:10.1002/ccd.25544
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.