Back to Journals » International Journal of Women's Health » Volume 14
Pulmonary Embolism After in vitro Fertilization and Cesarean Section: Two Case Reports and Brief Review of the Literature
Authors Wu YY, Shan TT, Pan XT
Received 1 May 2022
Accepted for publication 5 October 2022
Published 25 October 2022 Volume 2022:14 Pages 1489—1497
DOI https://doi.org/10.2147/IJWH.S366355
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Yu-Yan Wu, Tian-Tian Shan, Xiang-Tao Pan
Hematology Department, Taicang Hospital of Soochow University, Medical College of Soochow University, Taicang City, People’s Republic of China
Correspondence: Xiang-Tao Pan, Hematology Department, Taicang hospital of Soochow University, Medical College of Soochow University, Taicang City, People’s Republic of China, Email [email protected]
Abstract: This paper reports two cases of postpartum pulmonary embolism in Taicang First People’s Hospital affiliated to Soochow University. They share many similarities in age, fertilization way, birthing method, incidence of pulmonary embolism, treatment and prognosis. The main purpose is to inspire the current maternal PTE risk assessment, diagnosis and treatment, as well as to explore the existing limitations and problems.
Keywords: postpartum pulmonary embolism, pulmonary embolism, in vitro fertilization, IVF, cesarean section, anticoagulation
Introduction
Venous thromboembolism (VTE) includes deep vein thrombosis (DVT) and pulmonary embolism (PE). Pulmonary embolism during pregnancy and postpartum is one of the important causes of maternal mortality.1–3 However, reliable data and guidelines guided diagnosis and treatment have not reached a definitive conclusion yet,4 and there are still more studies that need to be further improved. In the United States, PE accounts for 9.2% of pregnancy-related deaths.5 Compared with non-pregnant women of the same age, pregnant women have higher risk of developing PE, and this risk increases throughout pregnancy, peaking after delivery.6 It is well recognized that pregnancy increases the risk of thromboembolism owing to conditions involving hypercoagulability, decreased mobility, and the compression of the inferior vena cava and pelvic veins.7 Apart from that, there may be more risk factors that we can discuss in these two highly similar cases: the two cases had assisted reproductive techniques (ART) or in vitro fertilization (IVF) process in common, studies have shown that IVF is maternal risk factors for PE in pregnancy;8 Cesarean section is a traditional surgery; PE-related morbidity and mortality are particularly high in cesarean section; some studies have pointed out that the cumulative incidence of PE after cesarean section is higher than that after vaginal delivery.9 Besides, it was found that the common symptoms of PE overlap with the typical symptoms such as shortness of breath and edema in normal pregnancy,10 and plasma D-dimer levels are elevated physiologically during this period, which is not necessarily related to thromboembolic complications.11,12 The imaging examination plays a diagnostic role, which has many limitations in the special period of pregnancy, making it difficult for pregnant women to diagnose PE. The case reports followed the CARE Guidelines.13
Case Presentation
History of IVF
They were both 32-year-old female patients with a history of IVF. Case 1 chose IVF because of infertility for 2 years. The specific data could not be provided, but she was found to have fallopian tube obstruction by the preoperative examination, and the ART succeed after the first operation. In case 2, IVF was chosen because of infertility for 5 years, and the cause of infertility was not found exactly, and succeeded after the first successful operation.
Relevant Conditions During Pregnancy
Case 1 was admitted to the obstetrics department of our hospital on February 19, 2020, for “fetal distress may be found at 39th week after IVF”, presenting without abdominal pain or vaginal bleeding. She had a history of “subclinical hypothyroidism” but no medication. General physical examination on admission was normal. Color Doppler ultrasound revealed a single, cephalic position, survival fetal; fetal biparietal diameter (FBD): 95mm, abdomen Circumference (AC): 345mm, amniotic fluid index (AFI): 139mm, placenta grade II. The plasma D-dimer was 4140 ng/mL, and oxytocin challenge test (OCT) was performed negative, the blood routine, coagulation test, liver and kidney function and electrolyte were generally normal. The patient refused to try vaginal delivery and asked for termination of pregnancy. Lower uterine cesarean section + uterine B-Lynch suture was performed under combined anesthesia on February 21. A baby girl weight 3580g was delivered in left occiput anterior (LOA) position with the Apgar score 10 for both 1 minute and 5 minutes. The blood plasma levels of D-dimer decreased in three consecutive days after surgery, measured at 7470ng/mL, 4910ng/mL and 3900ng/mL, respectively. The general condition of the patient was improved later, without cough, chest tightness, shortness of breath, hemoptysis and other discomfort. She was discharged on February 26 and instructed to follow-up in the outpatient clinic one week later.
Case 2 was admitted to hospital on June 29, 2020, for “chest tightness for 1 week and IVF after 9 months”. She was suffered from repeated chest tightness and discomfort one week before the admission, slept in a high position at night, without palpitation and dyspnea. She had a history of double mammary fibroma resection. OGTT test showed as 4.12–10.15-7.44 mmol/L after 28 weeks of menopause so that “Gestational Diabetes Mellitus (GDM)” was diagnosed, which was intervened by dietary and monitoring blood glucose without medication. After 23 weeks of menopause, irregular abdominal distension occurred repeatedly, which became worse after standing up and walking, so she stayed in bed for a long time. Abdominal distension became worse half a month before admission, but without abdominal pain. General physical examination on admission was normal. Color Doppler ultrasound revealed a single, cephalic position, survival fetal; BPD: 95mm, AC: 340mm, AFI: 129mm, placenta grade I. The blood routine, coagulation test, liver and kidney function and electrolytes were almost normal, plasma D-dimer was measured at 3050ng/mL, and OCT was performed negative. Considering the history of IVF, chest tightness and discomfort that cannot be relieved, but the gestational age was small and complicated with gestational diabetes, the fetus was at risk of immature. The patient and his family insisted on caesarean section. Lower uterine cesarean section + uterine B-Lynch suture was performed under combined anesthesia on July 1. A baby girl weight 2920g was delivered in LOA position with the Apgar score 10 for 1 minute and 5 minutes. The plasma D-dimers were examined twice after operation and were 5940ng/mL and 2550ng/mL, respectively. Ultrasonography did not find lower limb vein thrombosis. Gradient tension socks were used for treatment, and appropriate activities were asked. The general condition of the patient improved later, without cough, chest tightness, shortness of breath, hemoptysis and other discomfort. She was discharged on July 25 and instructed to follow-up in the outpatient clinic one week later. The clinical features of the patients are shown in Table 1.
 |
Table 1 Main Clinical Features of 2 Patients |
The Related Situation of Postpartum PE
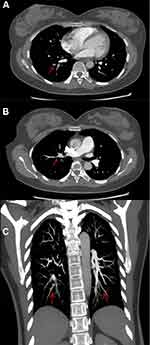
Case 1 was followed up as requested after discharge, and D-dimer was reexamined 7440ng/mL in the respiratory clinic of our hospital on March 5, 2020. The patient was presented with shortness of breath after activity. Computed tomographic pulmonary angiography (CTPA) showed pulmonary embolism in the anterior segment of the upper lobe of the right lung, the outer basal segment of the lower lobe of the right lung and the posterior basal segment (Figure 1A–C), and ultrasound demonstrated no abnormalities in lower limb vein. She was admitted to the respiratory department of our hospital. The heart rate was 124bpm. After admission, she was treated with anticoagulation immediately by subcutaneous injection of low molecular weight heparin (LWMH) calcium 4100U twice daily and instructed to stay in bed and keep defecation unobstructed. Plasma D-dimer was reexamined 2690ng/mL on March 9. On the fifth day in our hospital, as the patient’s condition stabilized, LMWH was replaced by oral Rivaroxaban 15mg once daily. She was discharged two days later, and oral Rivaroxaban was prescribed for continued anticoagulant therapy. During the outpatient follow-up, D-dimer decreased steadily. D-dimer was 910ng/mL, and the CTPA showed normal findings, which suggested completed recovery of PE on May 12, so the patient ended the treatment and follow-up and had a good long-term survival.
Case 2 was suffering from paroxysmal chest tightness, shortness of breath, discomfort, slight cough, mainly dry cough after last discharge. D-dimer was reexamined 1500ng/mL in the respiratory clinic of our hospital on July 19, 2020, and blood gas analysis, blood routine and troponin were normal. CTPA showed pulmonary embolism in right lower segment of the right lung (Figure 2), and ultrasound demonstrated no abnormalities in lower limb vein. According to the clinical manifestations, CT image and results of laboratory tests, a diagnosis of PE was established. She was admitted to the respiratory department of our hospital for “Pulmonary embolism after cesarean section”. General physical examination on admission was normal. After admission, the patients were given anticoagulation therapy immediately by subcutaneous injection of Enoxaparin 4100U twice daily, and the patient was instructed to stay in bed and keep defecation unobstructed. During her hospital stay, the patient had liver damage (ALT 59U/L) and postpartum depression, which were treated in time. Because the patient’s urine occult blood was positive, considering the risk of continued bleeding, Enoxaparin was replaced by subcutaneous injection of Fondaparinux sodium 2.5mg once daily on July 23. After re-examination, urinary occult blood decreased and urinary B-ultrasound was normal. Fondaparinux sodium was discontinued and changed to oral Rivaroxaban 15mg once daily on July 25. D-dimer reexamined 560ng/mL on July 26, ALT 72.5U/L, AST 50U/L, urine occult blood turned out negative, and she has no chest tightness, shortness of breath, cough and other discomfort. The patient required discharge with medicine and was told to continue anticoagulation therapy by oral Rivaroxaban and follow-up. During the follow-up, the liver function was improved, and the CTPA showed normal findings which suggested completed recovery of PE on September 18, so the patient ended treatment and follow-up, and had a good long-term survival.
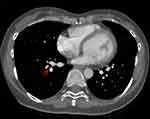 |
Figure 2 Pulmonary embolism (PE) of case 2 shown in the computed tomographic pulmonary angiography (CTPA). The red arrow indicates the pulmonary embolism in right lower segment of the right lung. |
The time from delivery to diagnosis of PTE was 14 days in case 1 and 18 days in case 2. PTE occurred in different segments: case 1 was located in the anterior segment of right upper lung, the outer basal segment and posterior basal segment of right lower lung and case 2 was located in the right lower lung. No deep venous thrombosis (DVT) was found in both patients by ultrasound. They both had shortness of breath. The symptoms of case 1 were mild, while those of case 2 lasted for 10 days, showing paroxysmal symptoms with dry cough. The main examination results of these two patients are shown in Table 2.
 |
Table 2 The Main Indicator of PE |
Discussion
Pulmonary embolism (PE) is the abbreviation of pulmonary artery embolism, and its incidence is the third most common vascular disease behind coronary heart disease and stroke.14 It refers to the clinical syndrome in which one or more pulmonary arteries are blocked by endogenous or exogenous emboli, resulting in pulmonary circulation and right heart dysfunction. It was reported that the absolute incidence of pregnancy-associated VTE was 1/1000 to 1/2000 deliveries, 5-fold higher than that in non-pregnant women of the same age, and risk-related VTE is also higher in postpartum than during pregnancy.10 According to a 30-year population-based study in the USA, the incidence of PE is 47.9 per 100,000 women-years, and the incidence of VTE in the first three months after delivery is higher than that in pregnancy, that the risk of VTE is 20 to 80 times higher in the first 6 postpartum weeks and 100-fold higher in the first postpartum week.15
These two patients had a lot in common, both of them were 32-year-old female patients and of pregnancy-age. In some ways, they were different from ordinary pregnant women and they both had a history of in vitro fertilization (IVF). The IVF procedure normally involves controlled ovarian stimulation with exogenous hormones used to induce ovulation. Then, through the incubation of egg and sperm, or by direct intracytoplasmic injection of sperm into the egg, the fertilized embryos were transferred directly to the uterus a few days later or can be cryopreserved used for future embryo transfer.8 It was recently found that there is a 7-fold increase in the incidence of PE during the first trimester of an IVF pregnancy as compared to spontaneous pregnancy.16–18 The main hypothetical reasons are as follows: ① Sex hormone increase vascular permeability mainly by the increase of ovarian renin-angiotensin system, prostaglandins, histamines, cytokines, vascular endothelial growth factor/vascular factor (VEGF/VPF) and so on, a large amount of fluid and protein were transferred to other chambers, resulting in intracavascular volume concentration. Typical hyperphysiological estrogen levels increased 10,100 folds in ART, and hyperconnectivity state may occur accordingly;19,20 ② Women who receive IVF are more prone to VTE, which means that the cause of infertility may be the risk factor of PE. The cause may exactly lead to the higher incidence of VTE in the IVF group. We do lack information on acquired thrombophilia and other comorbidities increasing the risk of thrombosis, such as the antiphospholipid syndrome or inflammatory diseases. At the same time, it can be argued that women undergoing IVF may be healthier and have a lower risk of VTE, because only those who meet the health requirements will be the candidates for IVF.20 Inspired by the theory, we can review our cases again. Both patients went through an IVF and succeeded for the first time, so compared with spontaneous pregnant women, hyperphysiological dose of estrogen stimulation must be a risk factor. However, we also need to discuss in terms of infertility etiology of the two patients. Before IVF, both patients and their partners underwent physical examination. Exactly, case 1 had a cause of tubal obstruction, but there was no evidence to support tubal patency leading to PE. However, the cause of case 2 was not found. In other words, the cause of infertility in two patients, or the cause that can be found, was not a risk factor. Beyond that, both patients met the health requirements of IVF. However, it was important to notice that case 1 had a history of subclinical hypothyroidism, which may be another cause of infertility besides tubal obstruction. Because several meta-analytical studies and randomized trials have confirmed that subclinical hypothyroidism and thyroid autoimmunity are closely associated with premature birth and miscarriage in pregnancy, untreated or in some cases undiagnosed thyroid diseases may lead to infertility, which may be induced by high levels of prolactin (PRL), anovulatory cycles and female hormone defects.20,21 Accordingly, we found that subclinical hypothyroidism can lead to coagulation disorders and DVT: in a large-scale study, Danescu et al found that patients with hypothyroidism had an increased risk of VTE.22,23 Case 2 developed GDM. It has been suggested that hyperglycemia promotes vascular inflammation in patients with GDM and diabetes, thereby increasing the risk of cardiovascular events.24 Many studies have studied many risk factors and confirmed the association between diabetes and PE in pregnancy. A Norwegian registry-based case–control study showed that diabetes increases the risk of VTE by 4-fold, but they do not find that GDM predicts postpartum VTE, suggesting that prenatal and postpartum VTE may be involved in different mechanisms.25,26
In addition, the patients both had a history of C-section (caesarean section), just as mentioned above, postpartum is at high risk of VTE, especially after C-section. It has been recognised that C-section is an independent risk factor for PE. In a Swedish retrospective study, C-section increased the risk by 5-fold and 3-fold in the Norway study mentioned above.27 The etiology of PE in pregnancy is multifactorial. Crucial mechanisms include gradual activation of the hemostatic system, progressive development of hormone-induced regional stasis of blood flow and pelvic venous compression from the enlarging uterus. The impacts of these mechanisms reach a peak in the late pregnancy. C-section surgery can further enhance hypercoagulable state, because tissue damage, catecholamine release and systemic inflammatory responses resulting from surgery synergistically activate coagulation factors and platelets. Besides, it can also decrease deep vein blood flow in legs and aggravate regional stasis. These mechanisms reasonably explain the high incidence of PE in women with caesarean section.28
Clinical manifestations of PE in pregnancy are widely variable, typical signs and symptoms can be present, but PE remains more often a non-specific event with atypical or mild clinical presentation, or signs and symptoms often overlap with physiological changes that occur during normal pregnancy, which can delay timely diagnosis.10 When several clinical features were present in pregnant and postpartum women with suspected PE, no significant correlation was found between any individual or group characteristics and PE. Predictive value could be improved by improving the specificity of the experiment, such as chest radiography. It was estimated that pregnant or lactating women are at a higher risk exposure due to breast tissue proliferation, even increasing the risk of breast cancer (Note: case 2 had a history of breast hyperplasia). Albeit to an unknown extent, this is an important consideration.27
The modified Wells score (WMS)29 was initially described by Wells in 1995 and subsequently modified,independent of imaging or biomarkers has been recognized in the non-pregnant population, which can also predict the pretest probability of PE in pregnant women to some extent. A recent small study in 100 pregnant women validated the WMS and showed adequate sensitivity and specificity, as well as high negative predictive value for cut-off points, compared with CTPA (100%), although the positive predictive value remained poor at 36%.10 In our case, the WMS score of case 1 was 1.5 (history of caesarean section within 4 weeks) and the score of case 2 was 1.5 (history of caesarean section within 4 weeks), which indicated that some defects existed in the early detection and diagnosis.
High level of D-dimer is a reliable predictor, which has high sensitivity and moderate specificity in non-pregnant women compared with that of ECG, imaging examination and arterial blood gas.27 D-dimer is a minor protein product with negligible levels in the circulation due to endogenous fibrinolysis. Many studies have examined the level of D-dimer during pregnancy and showed that D-dimer values were higher during pregnancy compared to nonpregnancy. In addition, the level of D-dimer was also increased in the third trimester of normal pregnancy. However, the reason for the increase of D-dimer included proclotting factors produced during normal placental growth, hormone changes, blood flow slowed down, activity reduced, and vascular damage increased due to anatomical changes.10 This results in a physiological increase in the concentration of D-dimer in the blood of the mother during pregnancy until delivery, which is not necessarily associated with thromboembolism.14 Therefore, D-dimer may be less effective as a PE screening indicator in these populations. As noted before, D-dimer increased gradually during pregnancy and peaked on the first day after delivery.27 Considering the physiological status of pregnant women, the recommended reference range of plasma normal D-dimer levels (<500ng/mL) is not suitable for pregnant and perinatal women to screen VTE, which increases the false positive rate and may lead to unnecessary imaging examination and anticoagulation therapy. Several studies have attempted to increase the critical value of pregnancy-associated VTE or finding a higher reference range of D-dimer to improve the predictive value of the D-dimer test that maintain a high sensitivity while improving the specificity.30 For example, a prospective study shows raising the D-dimer standard to 1000ng/mL with none of the following three criteria: clinical signs of DVT, hemoptysis, and PE as the most likely diagnosis throughout pregnancy.31 Another retrospective analysis pointed out that the women suspected of PE after cesarean section, the level of D-dimer within 24 hours had an important diagnostic value, and the cut-off value was suggested to be 800ng/mL. It is also recommended that patients with suspected PE after cesarean section with D-dimer levels above 1000ng/mL should start anticoagulation and perform diagnostic imaging examination, PE can basically be ruled out when the D-dimer level is below 800 ng/mL.27 There is no international consensus on the diagnostic guidelines for PE during pregnancy due to the lack of strong evidence to verify the diagnostic algorithm. In our two study, the two cases delivered by C-section measured D-dimer at 7470ng/mL and 5940ng/mL, respectively, strongly suggesting the possibility of PE, imaging and anticoagulation therapy were necessary. When PE was diagnosed, D-dimer of case 2 was significantly lower than that after delivery, which may be related to the longer time interval of thrombosis. Case 2 was diagnosed on the 18th day compared to 14th day. In addition, it may be related to the extent of thrombotic load, and studies have shown that more extensive PE-related degradation of larger embolism that assessed by CT angiography does induce greater D-dimer release.32,33 As shown in the above pictures, there were three vascular embolisms with larger area in case 1. While in case 2, only the right lower pulmonary artery was not completely embolized. In addition, case 2 took some measures to prevent thrombosis, such as elastic socks and early exercise, which may promote blood circulation to some extent.
We also noticed that case 2 suffered from postpartum depression, insomnia and other adverse emotions, and received short-term drug treatment. These unhealthy emotions were considered to be associated with VTE development. It was presumed that these factors promote the hypercoagulable state through autonomic and neuroendocrine pathways. Several studies have found a significant relationship between antidepressant use and VTE.34 Existing available epidemiological observations suggest that both depression and antidepressant use may be associated with an increased risk of VTE. This association is more consistent among women.35
Acute treatment consists of anticoagulants as the first-line treatment. Low molecular weight heparin (LMWH) and unfractionated heparin (UFH) can both be used safely in pregnancy, since they are unable to cross placental barrier. However, LMWH is the first anticoagulant of choice for VTE in pregnancy due to its better bioavailability, low risk of bleeding, and low rates of heparin-induced thrombocytopenia and osteopenia. The standard dose of enoxaparin is 1mg/kg subcutaneous injection every 12 hours.36 When LMWH is not available, UFH can also be considered, because it has a short half-life and is readily reversible and is also preferred in cases of renal dysfunction.37 There are no adequately powered randomised controlled trials comparing UFH with LMWH for the treatment of VTE during pregnancy. However, in nonpregnant patients, it was demonstrated that patients treated with LMWH had lower thrombotic complications, bleeding, and mortality than UFH.4 Warfarin, an oral vitamin K antagonist (VKA), is not used in pregnancy, as it crosses the placenta and can be teratogenic. In the postpartum period, coumadin is breastfeeding-compatible. Postpartum patients receiving therapeutic anticoagulation may need to be bridged from LMWH or UFH to warfarin to avoid the risk of recurrent VTE.38,39 In our cases, both of them were patients with postpartum PTE, but elevation of D-dimer in postpartum is not necessarily associated with VTE. Anyway, the levels of 7470ng/mL and 5940ng/mL are highly suggestive of the possibility of VTE. According to WMS score, the patients both are low-risk, so they were discharged and asked to follow up after symptomatic treatment. This matters because case 2 did have suspicious symptoms such as chest tightness, which was similar to the symptoms in the third trimester of pregnancy, and was very likely to be overlooked, but case 1 was asymptomatic, so the follow-up seems important. In the treatment after the diagnosis of PTE, all of our patients received regular anticoagulation therapy and gradually transitioned to oral warfarin, and they all achieved relatively good prognosis.
Conclusion
From these two case reports, we can infer that IVF, cesarean section, GDM, hypothyroidism and even depression may be related to the occurrence of postpartum PTE. The increase of D-dimer has certain reference significance, but because of the special physiological changes in pregnancy, the reference range that can improve the predictive value of D-dimer is inconclusive; for patients with postpartum PTE, the recommended treatment is generally intensive anticoagulation with LWMH, followed by transition to oral warfarin.
Statement
The manuscript is plead to be peer reviewed. The patients provided informed consent to publish their case details and any accompanying images.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the CARE guidelines (https://www.care-statement.org/).13 The study was approved by Ethics Committee of the Taicang Hospital Affiliated of Soochow University (2022-KY-155), and informed consent was taken from all the patients.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Practice Bulletin No. 183: Postpartum Hemorrhage, WHO. Maternal mortality. Fact sheet. Geneva; 2019.
2. Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(2):e323–33. doi:10.1016/S2214-109X(14)70227-X
3. Sayinzoga F, Bijlmakers L, van Dillen J, et al. Maternal death audit in Rwanda 2009–2013: a nationwide facility-based retrospective cohort study. BMJ Open. 2016;6(1):e009734. doi:10.1136/bmjopen-2015-009734
4. McLintock C, Brighton T, Chunilal S, et al. Recommendations for the diagnosis and treatment of deep venous thrombosis and pulmonary embolism in pregnancy and the postpartum period. Aust N Z J Obstet Gynaecol. 2012;52(52):14–22. doi:10.1111/j.1479-828X.2011.01361.x
5. Abe K, Kuklina EV, Hooper WC, et al. Venous thromboembolism as a cause of severe maternal morbidity and mortality in the United States. Semin Perinatol. 2019;43(43):200–204. doi:10.1053/j.semperi.2019.03.004
6. Sultan AA, West J, Tata LJ, et al. Risk of first venous thromboembolism in and around pregnancy: a population-based cohort study. Br J Haematol. 2012;156(3):366–373. doi:10.1111/j.1365-2141.2011.08956.x
7. Zhao Z, Zhou Q, Li X, et al. Missed opportunities for venous thromboembolism prophylaxis during pregnancy and the postpartum period: evidence from mainland China in 2019. BMC Pregnancy Childbirth. 2021;21(1):400. doi:10.1186/s12884-021-03863-w
8. Henriksson P. Cardiovascular problems associated with IVF therapy. J Intern Med. 2021;289(1):2–11. doi:10.1111/joim.13136
9. Tan TC, Goh CMY, Tan SSX, et al. Epidemiology of pregnancy-associated pulmonary embolism in South Asian multi-ethnic country: mortality trends over the last four decades. J Obstet Gynaecol Res. 2021;47(47):174–183. doi:10.1111/jog.14450
10. Conti E, Zezza L, Ralli E, et al. Pulmonary embolism in pregnancy. J Thromb Thrombolysis. 2014;37(37):251–270. doi:10.1007/s11239-013-0941-9
11. Szecsi PB, Jørgensen M, Klajnbard A, et al. Haemostatic reference intervals in pregnancy. Thromb Haemost. 2010;103(4):718–727. doi:10.1160/TH09-10-0704
12. Kim SJ, Ahn HJ, Park JY, et al. The clinical significance of D-dimer concentrations in patients with gestational hypertensive disorders according to the severity. Obstet Gynecol Sci. 2017;60(6):542–548. doi:10.5468/ogs.2017.60.6.542
13. Riley DS, Barber MS, Kienle GS, et al. CARE explanation and elaborations: reporting guidelines for case reports. J Clin Epidemiol. 2017;89:218–235. doi:10.1016/j.jclinepi.2017.04.026
14. Borsi SH, Shoushtari MH. Comparison of the D-dimer concentration in pregnant women with or without pulmonary thromboembolism. J Family Med Prim Care. 2020;9(8):4343–4347. doi:10.4103/jfmpc.jfmpc_1070_19
15. Heit JA, Kobbervig CE, James AH, et al. Trends in the incidence of venous thrombo embolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. 2005;143(10):697–706. doi:10.7326/0003-4819-143-10-200511150-00006
16. Hansen AT, Kesmodel US, Juul S, et al. Increased venous thrombosis incidence in pregnancies after in vitro fertilization. Hum Reprod. 2014;29(3):611–617. doi:10.1093/humrep/det458
17. Henriksson P, Westerlund E, Wallen H, et al. Incidence of pulmonary and venous thromboembolism in pregnancies after in vitro fertilisation: cross sectional study. BMJ. 2013;346:e8632. doi:10.1136/bmj.e8632
18. Rova K, Passmark H, Lindqvist PG, et al. Venous thromboembolism in relation to in vitro fertilization: an approach to determining the incidence and increase in risk in successful cycles. Fertil Steril. 2012;97(1):95–100. doi:10.1016/j.fertnstert.2011.10.038
19. Blumenfeld Z. The Ovarian Hyperstimulation Syndrome. Vitam Horm. 2018;107:423–451.
20. Olausson N, Discacciati A, Nyman AI, et al. Incidence of pulmonary and venous thromboembolism in pregnancies after in vitro fertilization with fresh respectively frozen-thawed embryo transfer: nationwide cohort study. J Thromb Haemost. 2020;18(8):1965–1973. doi:10.1111/jth.14840
21. Koyyada A, Orsu P. Role of hypothyroidism and associated pathways in pregnancy and infertility: clinical insights. Tzu Chi Med J. 2020;32(4):312–317. doi:10.4103/tcmj.tcmj_255_19
22. Danescu LG, Badshah A, Danescu SC, et al. Venous thromboembolism in patients hospitalized with thyroid dysfunction. Clin Appl Thromb Hemost. 2009;15(6):676–680. doi:10.1177/1076029609336856
23. Hostiuc M, Curca GC, Dermengiu D, et al. Can subclinical hypothyroidism explain some sudden deaths due to pulmonary embolism without evident risk factors? Med Hypotheses. 2011;76(6):855–857. doi:10.1016/j.mehy.2011.02.035
24. Heit JA, Leibson CL, Ashrani AA, et al. Is diabetes mellitus an independent risk factor for venous thromboembolism? Arterioscler Thromb VascBiol. 2009;29(9):1399–1405. doi:10.1161/ATVBAHA.109.189290
25. Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism, a meta-analysis. Circulation. 2008;117(1):93–102. doi:10.1161/CIRCULATIONAHA.107.709204
26. Won HS, Kim DY, Yang MS, et al. Pregnancy-induced hypertension, but not gestational diabetes mellitus, is a risk factor for venous thromboembolism in pregnancy. Korean Circ J. 2011;41(1):23–27. doi:10.4070/kcj.2011.41.1.23
27. Zhang L, Chen Y, Liu W, et al. Predictive value of D-dimer and analysis of risk factors in pregnant women with suspected pulmonary embolism after cesarean section. BMC Pulm Med. 2021;21(1):391. doi:10.1186/s12890-021-01757-3
28. Wang HC, Tsai PS, Li K-Y, et al. Perioperative risk factors for postpartum pulmonary embolism in Taiwanese Cesarean section women. Asian J Anesthesiol. 2017;55(2):35–40. doi:10.1016/j.aja.2017.05.002
29. Wells PS, Hirsh J, et &. Accuracy of clinical assessment of deep-vein thrombosis. Lancet (London, England). 1995;345(8961):1326–1330. doi:10.1016/s0140-6736(95)92535-x
30. Kovac M, Mikovic Z, Rakicevic L, et al. The use of D-dimer with new cutoff can be useful in diagnosis of venous thromboembolism in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2010;148(1):27–30. doi:10.1016/j.ejogrb.2009.09.005
31. van der Pol LM, Tromeur C, Bistervels IM, et al. Pregnancy-adapted YEARS algorithm for diagnosis of suspected pulmonary embolism. N Engl J Med. 2019;380(12):1139–1149. doi:10.1056/NEJMoa1813865
32. Becattini C, Lignani A, Masotti L, et al. D-dimer for risk stratification in patients with acute pulmonary embolism. J Thromb Thrombolysis. 2012;33(1):48–57. doi:10.1007/s11239-011-0648-8
33. Galle C, Papazyan J-P, Miron M-J, et al. Prediction of pulmonary embolism extent by clinical findings, D-dimer level and deep vein thrombosis shown by ultrasound. Thromb Haemost. 2001;86(11):1156–1160. doi:10.1055/s-0037-1616044
34. Parkin L, Balkwill A, Sweetland S, et al. Antidepressants, depression, and venous thromboembolism risk: large prospective study of UK women. J Am Heart Assoc. 2017;6(5):e005316. doi:10.1161/JAHA.116.005316
35. Kunutsor SK, Seidu S, Khunti K, et al. Depression, antidepressant use, and risk of venous thromboembolism: systematic review and meta-analysis of published observational evidence. Ann Med. 2018;50(6):529–537. doi:10.1080/07853890.2018.1500703
36. Dado CD, Levinson AT, Bourjeily G, et al. Pregnancy and Pulmonary Embolism. Clin Chest Med. 2018;39(3):525–537. doi:10.1016/j.ccm.2018.04.007
37. Bates SM. Pregnancy-associated venous thromboembolism: prevention and treatment. Semin Hematol. 2011;48(4):271–284. doi:10.1053/j.seminhematol.2011.08.003
38. De Swiet M, Lewis PJ. Excretion of anticoagulants in human milk. N Engl J Med. 1977;297(26):1471.
39. Brandjes DP, Heijboer H, Büller HR, et al. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N Engl J Med. 1992;327(21):1485–1489. doi:10.1056/NEJM199211193272103
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.