Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 11 » Issue 1
Pulmonary complications after abdominal surgery in patients with mild-to-moderate chronic obstructive pulmonary disease
Authors Kim TH, Lee JS, Lee SW , Oh YM
Received 9 August 2016
Accepted for publication 10 October 2016
Published 9 November 2016 Volume 2016:11(1) Pages 2785—2796
DOI https://doi.org/10.2147/COPD.S119372
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Tae Hoon Kim, Jae Seung Lee, Sei Won Lee, Yeon-Mok Oh
Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
Abstract: Postoperative pulmonary complications (PPCs) are one of the most important causes of postoperative morbidity and mortality after abdominal surgery. Although chronic obstructive pulmonary disease (COPD) has been considered a risk factor for PPCs, it remains unclear whether mild-to-moderate COPD is a risk factor. This retrospective cohort study included 387 subjects who underwent abdominal surgery with general anesthesia in a tertiary referral hospital. PPCs included pneumonia, pulmonary edema, pulmonary thromboembolism, atelectasis, and acute exacerbation of COPD. Among the 387 subjects, PPCs developed in 14 (12.0%) of 117 patients with mild-to-moderate COPD and in 13 (15.1%) of 86 control patients. Multiple logistic regression analysis revealed that mild-to-moderate COPD was not a significant risk factor for PPCs (odds ratio [OR] =0.79; 95% confidence interval [CI] =0.31–2.03; P=0.628). However, previous hospitalization for respiratory problems (OR =4.20; 95% CI =1.52–11.59), emergency surgery (OR =3.93; 95% CI =1.75–8.82), increased amount of red blood cell (RBC) transfusion (OR =1.09; 95% CI =1.05–1.14 for one pack increase of RBC transfusion), and laparoscopic surgery (OR =0.41; 95% CI =0.18–0.93) were independent predictors of PPCs. These findings suggested that mild-to-moderate COPD may not be a significant risk factor for PPCs after abdominal surgery.
Keywords: postoperative pulmonary complications, spirometry, risk factor, abdominal surgery, postoperative complications, postoperative care
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation and is associated with increased morbidity and mortality. The diagnosis of airflow limitation in COPD patients is based on the ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC); however, the severity of an airflow limitation is classified on the basis of FEV1, which was a predictor of mortality in COPD patients.1–3 Mild-to-moderate COPD is generally classified as an FEV1 ≥50% of the normal predicted value and an FEV1/FVC <0.7.4
Pulmonary complications, which are common after major abdominal surgery, increase mortality, length of hospital stay, intensive care unit (ICU) cares, hospital readmissions, and medical cost.5–7 Identification of the risk factors for predicting postoperative pulmonary complications (PPCs) is essential to provide proper perioperative management. Importantly, COPD has been previously shown to be associated with PPCs. Previous studies have shown that the prevalence of PPCs and the postoperative mortality rate increased in patients with COPD following general and thoracic surgery.8–11 However, in the majority of these studies, COPD was defined as clinically symptomatic COPD or severe-to-very severe COPD. Recent data have shown that patients with asymptomatic or mild airflow limitation could be diagnosed at earlier stages and that the overwhelming majority of COPD cases consist of patients with mild-to-moderate COPD.12 Until recently, the role of mild-to-moderate COPD in the development of PPCs was not studied well. Therefore, studies that assess the role of mild-to-moderate COPD are critical to provide accurate and appropriate perioperative management to patients. Furthermore, information on the relationship between mild-to-moderate COPD and PPCs is limited. In order to address this problem, patients who underwent abdominal surgery were retrospectively reviewed, and whether mild-to-moderate COPD was associated with the prevalence of PPC was evaluated. In addition, various candidate risk factors were investigated to determine whether they were good predictors of PPCs.
Materials and methods
Study design and patients
This retrospective cohort study was approved by the Institutional Review Board (2016-0428) of Asan Medical Center, which waived the need for informed consent. The medical records of patients who underwent abdominal surgery between January 2015 and December 2015 were reviewed, and the following data were extracted: demographic characteristics, comorbidities, spirometry results, preoperative respiratory symptoms, and operative conditions such as the lengths of anesthesia and surgery, surgical methods, surgical sites, surgical emergency, and the amount of perioperative red blood cell (RBC) transfusion. PPCs and postoperative care were also reviewed.
Patients who met the following criteria were included in the present study: preoperative pulmonology consultation because of comorbidity, old age, or abnormal lung function; abdominal surgery under general anesthesia; and age ≥40 years. Patients with the following characteristics were excluded from the final analysis: those without a lung function test within 1 month of surgery, those who underwent nonabdominal surgery or abdominal surgery in combination with thoracic surgery, those with a history of asthma or asthma–COPD overlap syndrome (ACOS), those who received preoperative mechanical ventilator care, and those with an unknown smoking history.
Definitions
COPD was defined as FEV1/FVC of <0.7 by preoperative spirometry evaluation. COPD patients were classified into two groups according to the severity defined by spirometry findings on the basis of Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines. Mild-to-moderate COPD was defined as FEV1 ≥50% of predicted value, and severe-to-very severe COPD was defined as FEV1 <50% of predicted value. Among patients with FEV1/FVC ≥0.7, those with FVC <80% of predicted value were found to have pulmonary disease with restrictive pattern, and those with FVC ≥80% of predicted value were defined as control subjects.
Abdominal surgery was classified into four categories based on the abdominal regions: upper abdominal, colorectal, genitourinary surgery, and others, by using the previously defined grouping method.13 Upper abdominal surgery included gastrectomy, pancreatectomy, hepatic resection, cholecystectomy, splenectomy, and excision of diaphragm, but esophagectomy was excluded. The amount of RBC transfusion during the perioperative period was estimated by the number of prepared packed RBCs between the day of surgery and the end of the first day after surgery. Surgeries were also categorized as emergency and scheduled, based on the surgical records.
The definition of PPCs was modified and applied from a previous study.14 PPCs included new-onset pneumonia, pulmonary edema, pulmonary thromboembolism, atelectasis, and acute exacerbation of COPD after surgery. Postoperative pneumonia was defined as new development of pulmonary infiltration on chest X-ray, acute respiratory symptoms, fever or elevated inflammatory markers, and requirement of antibiotic treatment. Pulmonary edema was defined as the presence of postoperative dyspnea with radiologically diagnosed pulmonary interstitial fluid accumulation or cardiomegaly or impaired cardiac function confirmed by echocardiography. Pulmonary thromboembolism was defined as postoperative dyspnea with decreased PaO2 on arterial blood gas analysis and the presence of thromboembolism on chest computed tomography. Atelectasis was defined as large-sized atelectasis of lobar involvement on chest X-ray. In COPD patients, episodes with postoperative wheezing requiring bronchodilator or glucocorticoid treatment were defined as acute exacerbation of COPD.
Statistical analysis
The characteristics of study subgroups were compared by using analysis of variance, Kruskal–Wallis test, unpaired t-test, or the Mann–Whitney U-test for continuous variables, and Fisher’s exact test was used for categorical variables. Variables with significance by univariate analysis and previously known risk factors, including age, male gender, body mass index (BMI), Charlson comorbidity index (CCI) score, American Society of Anesthesiologists (ASA) physical status score, previous hospitalization because of respiratory problems, blood urea nitrogen (BUN), emergency surgery, length of anesthesia, RBC transfusion, laparoscopic surgery, upper abdominal surgery, and spirometry findings, were evaluated by using multivariate analysis to determine the independent predictors of PPCs. Multivariate analysis using binary logistic regression was conducted by using all the variables mentioned above and also by using stepwise backward elimination method. First, all the variables related to an increased risk of PPCs were analyzed. Then, by using a stepwise backward elimination method, independent predictors of PPCs were determined. In order to confirm the role of mild-to-moderate COPD in the development of PPCs, the ever-smoker subgroup without a restrictive pattern was analyzed by using a sensitivity analysis. Statistical analyses were performed by using Statistical Package for the Social Sciences® Version 22.0 (IBM Corporation, Armonk, NY, USA). Data were expressed as mean ± standard deviation or medians and interquartile ranges (IQRs), and P-values <0.05 were considered significant.
Results
Baseline and operative characteristics
Among a total of 468 eligible patients, 81 patients were excluded from the final analysis (Figure 1). Table 1 shows the baseline characteristics of a total of 387 patients included in the present study. In this cohort, 258 (66.7%) patients were male with a median age of 69.0 years (range, 40–90 years). Overall median BMI was 23.0 kg/m2 (IQR =20.6–25.4 kg/m2). One hundred and ninety-seven (50.9%) patients were ever-smokers with a median smoking history of 30.0 pack-years (IQR =20.0–40.0 pack-years). Median ASA physical status score was 2.0 (IQR, 2.0–3.0), and 93.8% patients scored 1–3. Median CCI score (unadjusted for age) in this cohort was 3.0 (IQR =2.0–4.0). History of hospitalization for respiratory problems within 1 year of surgery was found in 24 (6.2%) patients, respectively.
  | Figure 1 Flow diagram for patient requirement. |
Lung function characteristics of the study subjects were as follows: median FVC, FEV1, and FEV1/FVC values were 2.8 L (IQR =2.1–3.4 L; 74.0% predicted [IQR =59.0%–86.0% predicted]), 1.9 L (IQR =1.4–2.4 L; 69.0% predicted [IQR =56.5%–86.0% predicted]), and 0.73 (IQR =0.64–0.80), respectively. Among a total of 387 patients included in the final analysis, 151 (39.0%) patients with obstructive spirometry pattern were divided into two categories: mild-to-moderate COPD was found in 117 (30.2%) patients and 34 (8.8%) patients had severe-to-very-severe COPD. Restrictive spirometry pattern was observed in 150 (38.8%) patients.
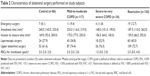
Table 2 shows abdominal surgery characteristics of the subjects. Forty-one (10.6%) patients underwent emergency abdominal surgery; the remaining patients underwent elective surgery. Laparoscopic surgery was performed in 145 (37.5%) patients. Upper abdominal area (71.1%) was the most commonly operated site in this study. Overall median length of anesthesia and surgery from incision to skin closure were determined as 205.0 (IQR =116–328) and 145.0 (IQR =75–264) min, respectively. In the perioperative period, a median of 2.0 (IQR =0.0–2.0) packs of RBCs were used for transfusion.
PPCs
Table 3 summarizes data on PPCs and postoperative management. Among a total of 387 patients who underwent abdominal surgery, PPCs occurred in 63 (16.3%) patients. The most common PPCs were pneumonia (8.3%) and pulmonary edema (6.5%). Other PPCs included atelectasis (4.9%), pulmonary thromboembolism (0.5%), and acute exacerbation of COPD (0.3%).
Among the entire cohort, 81 (20.9%) patients required intensive care during the postoperative period, and 57 (14.7%) patients received support by mechanical ventilation in ICU. The median duration of ICU stay was 4.0 (IQR =1.0–14.0) days, and the median number of days on a ventilator was 3.0 (IQR =2.0–14.0). Prolonged mechanical ventilation (>3 days) was needed for 34 patients (59.6% in patients with ventilator care). The most common cause of ICU care was complications associated with surgery (66.7% in patients with ICU care), and 27 patients (33.3% in patients with ICU care) required ICU care because of respiratory problems. After discharge, 31 (8.1%) patients required readmission; however, respiratory problems were not a common cause of readmission (only three patients). Mortality rate, including in-hospital and within 30 days after hospital discharge, was estimated to be 2.6%.
Comparison between patients with mild-to-moderate COPD and control subjects
There were no significant differences in PPCs (P=0.657) or requirement for intensive care, including ICU care (P=0.590) and mechanical ventilator support (P=0.506), between patients with mild-to-moderate COPD and control subjects, as shown in Table 3. Patients with mild-to-moderate COPD were older than control subjects (P<0.001) and were predominantly male (P<0.001). There were more ever-smokers among patients with COPD than control subjects (P<0.001); however, there was no significant difference in smoking duration between the groups (P=0.171). Patients with mild-to-moderate COPD had more comorbidities assessed by age-adjusted CCI score (P=0.043) and higher serum creatinine levels (P=0.003) than control subjects. There were more patients with poor physical status among those with mild-to-moderate COPD (P=0.045). Furthermore, deterioration in lung function was significantly worse (P<0.001), and dyspnea was significantly more frequent (P=0.039) in the mild-to-moderate COPD group than in the control group.
Risk factors for PPCs
Surprisingly, contrary to the findings of previous studies, mild-to-moderate COPD was not associated with an increased rate of PPCs by either univariate or multivariate analyses (Table 4). Accordingly, whether mild-to-moderate COPD increased the risk of PPCs was determined, and factors that could predict PPCs were identified. Applying the variables (see the “Statistical Analysis” section) to risk-adjusted analysis, the present study found that there was an increased risk of PPCs associated with a history of hospitalization for respiratory problems (P=0.012), emergency surgery (P=0.001), and amount of RBC transfusion (P=0.008).
Then, stepwise backward elimination was used to identify the best combination of risk factors for predicting PPCs. PPCs were independently predicted by six variables, including poor ASA physical status (OR =1.60; 95% CI =0.97–2.63; P=0.065), history of hospitalization for respiratory problems (OR =4.20; 95% CI =1.52–11.59; P=0.006), emergency surgery (OR =3.93; 95% CI =1.75–8.82; P=0.001), amount of RBC transfusion (OR =1.09; 95% CI =1.05–1.14; P<0.001 for one pack increase of RBC transfusion), laparoscopic surgery (OR =0.41; 95% CI =0.18–0.93; P=0.033), and severe-to-very severe COPD (OR =3.47; 95% CI =1.16–10.42; P=0.027). In this analysis, mild-to-moderate COPD was not a significant predictor of PPCs (P=0.628).
Risk factors for PPCs in ever-smokers
In order to confirm that mild-to-moderate COPD was not a predictor of PPCs, subgroup analysis was performed in ever-smoker patients excluding those with a restrictive pattern disease (Table 5), which revealed that mild-to-moderate COPD was not associated with an increased risk of PPCs. CCI scores were higher, hospitalization stays due to respiratory illness and emergency surgery were more frequent, and there were more RBC transfusions among smokers with PPCs than those without PPCs (data not shown).
Multivariate analysis using all the variables demonstrated that a history of hospitalization for respiratory problems (P=0.001) was significantly associated with PPCs. According to the multivariate analysis by using stepwise backward elimination, a history of hospitalization for respiratory problems (OR =18.75; 95% CI =3.47–101.25; P=0.001) and a higher amount of RBCs for transfusion (OR =1.17; 95% CI =1.02–1.34; P=0.023, for one pack increase of RBC transfusion) were independent predictors of PPCs. However, as consistent with results from analysis that included all the study subjects, mild-to-moderate COPD was not a risk factor for PPCs (P=0.152) by subgroup analysis.
Comparison between patients with and without PPCs
Tables S1 and S2 present the characteristics and surgical data of patients with and without PPCs. There were more PPCs in patients with lower BMI (P=0.049), poor ASA physical status (P<0.001), more medical comorbidities (ie, high CCI scores; P<0.001), history of hospitalization for respiratory causes (P=0.009), increased BUN (P=0.001) and creatinine (P=0.009), emergency surgery (P<0.001), nonlaparoscopic surgery (P<0.001), longer duration of anesthesia or surgery (P<0.001), and more RBC transfusions (P<0.001). However, abnormal preoperative spirometry findings, such as obstructive and restrictive patterns, and severity of obstruction were not associated with the prevalence of PPCs. There were no differences in age, gender, smoking history, respiratory symptoms, lung functions, or frequency of upper abdominal surgeries between patients with and without PPCs.
There were several differences in postoperative outcomes between patients with and without PPCs (Table S3). Patients with PPCs had a higher frequency and a longer duration of intensive care or mechanical ventilator support than those without PPCs. In addition, patients who received prolonged ventilator care had more PPCs. As part of the extended intensive care management, these patients had longer hospitalization times and delayed discharge. In-hospital mortality rate was also higher (11.1%) among patients with PPCs than those without PPCs (0.6%). Among these, only three patients had respiratory problems as a cause of death.
Discussion
Previous reports have shown that patients with COPD are at an increased risk of pulmonary complications after abdominal surgery.7,8,15 However, information on the relationship between PPCs and mild-to-moderate COPD remains limited. Therefore, in the present study, COPD patients who underwent abdominal surgery were investigated to determine whether mild-to-moderate COPD was associated with an increased risk of PPCs. Among a total of 387 patients who were included in the present study, 117 (30.2%) patients had mild-to-moderate COPD; however, there was no increased risk of PPCs, including pneumonia, pulmonary edema, pulmonary thromboembolism, atelectasis, and acute exacerbation of COPD.
The underlying mechanisms of PPCs are unclear,16 but previous reports have shown that COPD is an independent risk factor for PPCs in addition to other morbidities.8,15 Numerous studies have shown that severe COPD was associated with an increased prevalence of PPCs in various settings.7,9,10 Yet, most COPD patients suffer from the mild-to-moderate variant of the disease, and comorbidities were more common in mild-to-moderate COPD than in severe COPD.12,17 Thus, the medical team, including surgeons, anesthesiologists, and pulmonologists, has ample opportunity to evaluate patients with mild-to-moderate COPD for potential PPCs. In the present study, mild-to-moderate COPD was not associated with PPCs. There was no significant difference in the prevalence rate of PPCs between patients with mild-to-moderate COPD and control patients. In contrast, patients with severe-to-very severe COPD appeared to have more frequent PPCs than those without COPD or control subjects, although this finding did not reach a statistical significance. The risk-adjusted analysis also showed that mild-to-moderate COPD was not an independent risk factor for PPCs.
In the present study, PPCs occurred in 16.3% of the entire cohort and were found in 15.1% of the control subjects and 12.0% of the patients with mild-to-moderate COPD. PPCs were the second most common type of complication after surgery, and the incidence of PPC was increased in patients with respiratory diseases, ranging between 25% and 90%.7,18 However, the difference in incidence rates may result from selection bias because of differences in the characteristics of hospitals and study populations. The hospital in the present study was a relatively large-sized referral center and was one of the preferred hospitals in the region for major abdominal surgery. Thus, a large variety of difficult and complicated surgical cases such as liver transplantation and Whipple or pylorus-preserving pancreaticoduodenectomy were included. Furthermore, PPCs were diversely defined from transient hypoxia and bronchospasm to long-term respiratory failure, including weaning failure and reintubation.7,8,15,19 In the present study, the PPCs were first defined as postoperative pneumonia, pulmonary edema, pulmonary thromboembolism, atelectasis, and acute exacerbation of COPD. Then, the reason for postoperative respiratory distress could concretely be found out by these five categories of PPCs. A variable, prolonged ventilator care over 3 days was used to estimate postoperative respiratory failure, but still had a limitation to compare postoperative respiratory failure across papers.
A previous study indicated that the specific type and site of surgery were more significantly associated with an increased PPC risk than the preoperative pulmonary condition of the patient.7 Upper abdominal surgery plays an important role in postoperative diaphragmatic dysfunction, a well-known cause of PPCs.14,19–22 However, the study included esophagectomy, which has the highest rate of pulmonary complications and is commonly performed via the thoracic approach. Therefore, the present study strictly defined abdominal surgery as hepatobiliary, pancreatic, and gastric surgery and excluded esophagectomy. Accordingly, upper abdominal surgery was not a risk factor for PPCs in the present study.
Nonetheless, surgical or anesthesia-related factors remain important predictors of PPCs. There are contradictory reports about the effect of laparoscopic surgery on intraoperative or postoperative pulmonary mechanics.23–25 However, meta-analyses showed that patients who underwent laparoscopic surgery had better preservation of lung function and lower rates of pulmonary complications than those who underwent open surgery.26,27 Risk-adjusted analysis of patients in the present study supported the previous data showing that laparoscopic surgery could prevent PPCs. Under prolonged anesthesia, the combined effect of the supine position and exposure to general anesthetics can lead to an immediate decline in lung volume and alter immune responses and gas exchange capacity.16 Several previous studies have shown that both prolonged surgery and anesthesia were risk factors for PPCs.7,19,28 The present study showed that the duration of anesthesia and surgery were longer in patients with PPCs; however, neither was an independent predictor for PPCs in multivariate analysis.
Patients undergoing emergency surgery have shown higher rates of mortality as well as respiratory failure than nonemergent surgery patients.19,29 In agreement with these earlier findings, the present study observed that emergency surgery significantly increased the risk of PPCs and respiratory failure. Moreover, among surgery-related variables, emergency surgery was one of the strongest predictors of PPCs. Perioperative blood loss can also occur because of technical difficulty of surgery, in addition to the medical status of patients. A previous study of COPD patients showed that estimated blood loss was a risk factor in PPCs.14 In the present study, blood loss was estimated by the amount of prepared RBCs in the perioperative period. Patients with PPCs needed more RBC transfusions, which was an independent predictor of PPCs not only in the ever-smoker subgroup by a risk-adjusted analysis, but also among the entire cohort. Meanwhile, in a study about postoperative ARDS, the postoperative ARDS might be caused by transfusion-related acute lung injury, or massive transfusion itself might be an indicator of more aggressive resuscitation and predictive of future ARDS.30
Although several studies have shown that advanced age, smoking, obesity, and gender could impact postoperative complications,7,15,22,31 they have yet to be a consensus on these issues because of the limited study populations and scope of surgery type. However, one of the most important factors impacting postoperative outcome is the physical status of patients, including comorbidity. In the present study, patients with PPCs had higher ASA physical status and CCI scores. Among these patients, the predictive ability of ASA physical status score was not statistically significant. On the other hand, patients with PPCs had more episodes of hospitalization for respiratory illness. The present study found that a history of hospitalization for respiratory illness was the most powerful predictor of PPCs after surgery in ever-smoker subgroup as well as overall subjects in this study. The history of hospitalization for respiratory illness is a unique finding as a risk factor to predict PPCs. However, this result suggests that preoperative pulmonary status is also weighty according to other papers.19,28 Thus, even patients with no respiratory symptoms, a history of hospitalization for respiratory illness should be carefully assessed in preoperative stage.
The present study has several limitations. First, this was a retrospective study performed at a single center. Second, the low number of study subjects may have affected the statistical power of comparisons. A prospective multicenter study with a broad spectrum of patients, ranging from those with normal lung functions to patients with very severe COPD, is required to confirm the results of the present study. Third, the preoperative spirometry used to evaluate study subjects was not a routine practice for all surgical candidates; only those with respiratory symptoms, a history of respiratory illness, and advanced age were assessed by preoperative spirometry and received consultation from a pulmonologist. Therefore, the possibility of respiratory problems and complications was higher in the present study population than in the general population. In addition, preoperative conditions such as abdominal pain and abdominal inflammation could affect preoperative spirometry findings. Underestimation of spirometry results could lead to the inappropriate classification of patients. Thus, longitudinal studies using routine spirometry for all the subjects are required to evaluate the relationship between lung function and PPCs in ordinary times. Finally, prebronchodilator spirometry results were used without consideration of smoking history or exposure of other risk factors for COPD diagnosis; as per GOLD guidelines, this is not the recommended method of diagnosis. Several studies have shown that both the prevalence and incidence of COPD in the general population decreased substantially when COPD was defined on the basis of postbronchodilator spirometry values rather than prebronchodilator values.32,33 However, changes in FEV1 and FEV1/FVC values following bronchodilator use also decreased with age.34 As the study population in the present study consisted of relatively old patients, and given that subjects with a history of asthma or ACOS were excluded, the effect of bronchodilator use on the present findings was likely limited. In addition, the data showed that control subjects had fewer smokers and relatively shorter duration of smoking, compared to those with COPD. Furthermore, ever-smoker subgroup analysis was performed to increase the reliability of the present findings, which supported the initial finding that the role of mild-to-moderate COPD in PPCs was insignificant. Well-designed prospective studies with results of postbronchodilator spirometry and analysis of COPD risk factors are necessary to confirm that mild-to-moderate COPD was not associated with PPCs in patients who underwent abdominal surgery.
Conclusion
In conclusion, 12.0% of patients with mild-to-moderate (FEV1 ≥50% of predicted value) COPD suffered from PPCs after abdominal surgery; PPCs were observed in 15.1% of control subjects. Mild-to-moderate COPD was not associated with an increased risk of PPCs, even after adjusting for covariates. In the present study, a history of hospitalization for respiratory illness within the previous year, emergency surgery, and amount of RBCs for transfusion were the major significant risk factors for PPCs after abdominal surgery.
Disclosure
The authors report no conflicts of interest in this work.
References
Anthonisen NR, Wright EC, Hodgkin JE. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1986;133(1):14–20. | ||
Alfageme I, Reyes N, Merino M, et al. The effect of airflow limitation on the cause of death in patients with COPD. Chron Respir Dis. 2010;7(3):135–145. | ||
Mannino DM, Doherty DE, Sonia Buist A. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med. 2006;100(1):115–122. | ||
Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. | ||
Carey K, Stefos T, Shibei Z, Borzecki AM, Rosen AK. Excess costs attributable to postoperative complications. Med Care Res Rev. 2011;68(4):490–503. | ||
Sabate S, Mazo V, Canet J. Predicting postoperative pulmonary complications: implications for outcomes and costs. Curr Opin Anaesthesiol. 2014;27(2):201–209. | ||
Yang CK, Teng A, Lee DY, Rose K. Pulmonary complications after major abdominal surgery: National Surgical Quality Improvement Program analysis. J Surg Res. 2015;198(2):441–449. | ||
Gupta H, Ramanan B, Gupta PK, et al. Impact of COPD on postoperative outcomes: results from a national database. Chest. 2013;143(6):1599–1606. | ||
Kroenke K, Lawrence VA, Theroux JF, Tuley MR. Operative risk in patients with severe obstructive pulmonary disease. Arch Intern Med. 1992;152(5):967–971. | ||
Linden PA, Bueno R, Colson YL, et al. Lung resection in patients with preoperative FEV1 <35% predicted. Chest. 2005;127(6):1984–1990. | ||
Licker MJ, Widikker I, Robert J, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg. 2006;81(5):1830–1837. | ||
Choi SM, Lee J, Park YS, et al. Prevalence and global initiative for chronic obstructive lung disease group distribution of chronic obstructive pulmonary disease detected by preoperative pulmonary function test. PLoS One. 2015;10(1):e0115787. | ||
Manion SC, Brennan TJ. Thoracic epidural analgesia and acute pain management. Anesthesiology. 2011;115(1):181–188. | ||
Kim HJ, Lee J, Park YS, et al. Impact of GOLD groups of chronic pulmonary obstructive disease on surgical complications. Int J Chron Obstruct Pulmon Dis. 2016;11:281–287. | ||
Sakai RL, Abrao GM, Ayres JF, Vianna PT, Carvalho LR, Castiglia YM. Prognostic factors for perioperative pulmonary events among patients undergoing upper abdominal surgery. Sao Paulo Med J. 2007;125(6):315–321. | ||
Licker M, Schweizer A, Ellenberger C, Tschopp JM, Diaper J, Clergue F. Perioperative medical management of patients with COPD. Int J Chron Obstruct Pulmon Dis. 2007;2(4):493–515. | ||
Park HJ, Leem AY, Lee SH, et al. Comorbidities in obstructive lung disease in Korea: data from the fourth and fifth Korean National Health and Nutrition Examination Survey. Int J Chron Obstruct Pulmon Dis. 2015;10:1571–1582. | ||
Tzani P, Chetta A, Olivieri D. Patient assessment and prevention of pulmonary side-effects in surgery. Curr Opin Anaesthesiol. 2011;24(1):2–7. | ||
Canet J, Sabate S, Mazo V, et al. Development and validation of a score to predict postoperative respiratory failure in a multicentre European cohort: a prospective, observational study. Eur J Anaesthesiol. 2015;32(7):458–470. | ||
Berdah SV, Picaud R, Jammes Y. Surface diaphragmatic electromyogram changes after laparotomy. Clin Physiol Funct Imaging. 2002;22(2):157–160. | ||
Ferreyra G, Long Y, Ranieri VM. Respiratory complications after major surgery. Curr Opin Crit Care. 2009;15(4):342–348. | ||
Brooks-Brunn JA. Predictors of postoperative pulmonary complications following abdominal surgery. Chest. 1997;111(3):564–571. | ||
Kawamura H, Yokota R, Homma S, Kondo Y. Comparison of respiratory function recovery in the early phase after laparoscopy-assisted gastrectomy and open gastrectomy. Surg Endosc. 2010;24(11):2739–2742. | ||
Kawamura H, Homma S, Yokota R, Watarai H, Yokota K, Kondo Y. Assessment of pain by face scales after gastrectomy: comparison of laparoscopically assisted gastrectomy and open gastrectomy. Surg Endosc. 2009;23(5):991–995. | ||
Grabowski JE, Talamini MA. Physiological effects of pneumoperitoneum. J Gastrointest Surg. 2009;13(5):1009–1016. | ||
Jiang L, Yang KH, Guan QL, et al. Laparoscopy-assisted gastrectomy versus open gastrectomy for resectable gastric cancer: an update meta-analysis based on randomized controlled trials. Surg Endosc. 2013;27(7):2466–2480. | ||
Damiani G, Pinnarelli L, Sammarco A, Sommella L, Francucci M, Ricciardi W. Postoperative pulmonary function in open versus laparoscopic cholecystectomy: a meta-analysis of the Tiffeneau index. Dig Surg. 2008;25(1):1–7. | ||
Scholes RL, Browning L, Sztendur EM, Denehy L. Duration of anaesthesia, type of surgery, respiratory co-morbidity, predicted VO2max and smoking predict postoperative pulmonary complications after upper abdominal surgery: an observational study. Aust J Physiother. 2009;55(3):191–198. | ||
Visser A, Geboers B, Gouma DJ, Goslings JC, Ubbink DT. Predictors of surgical complications: a systematic review. Surgery. 2015;158(1):58–65. | ||
Blum JM, Stentz MJ, Dechert R, et al. Preoperative and intraoperative predictors of postoperative acute respiratory distress syndrome in a general surgical population. Anesthesiology. 2013;118(1):19–29. | ||
McAlister FA, Khan NA, Straus SE, et al. Accuracy of the preoperative assessment in predicting pulmonary risk after nonthoracic surgery. Am J Respir Crit Care Med. 2003;167(5):741–744. | ||
Johannessen A, Omenaas E, Bakke P, Gulsvik A. Incidence of GOLD-defined chronic obstructive pulmonary disease in a general adult population. Int J Tuberc Lung Dis. 2005;9(8):926–932. | ||
Johannessen A, Omenaas ER, Bakke PS, Gulsvik A. Implications of reversibility testing on prevalence and risk factors for chronic obstructive pulmonary disease: a community study. Thorax. 2005;60(10):842–847. | ||
Johannessen A, Lehmann S, Omenaas ER, Eide GE, Bakke PS, Gulsvik A. Post-bronchodilator spirometry reference values in adults and implications for disease management. Am J Respir Crit Care Med. 2006;173(12):1316–1325. |
Supplementary materials
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.