Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Psychometrics of the Concise Health Risk Tracking Self-Report (CHRT-SR16) Assessment of Suicidality in a Sample of Adults with Moderate to Severe Methamphetamine Use Disorder: Findings from the ADAPT-2 Randomized Trial
Authors Trombello JM, Kulikova A, Mayes TL , Nandy K, Carmody T, Bart G, Nunes EV , Schmitz J, Kalmin M, Shoptaw S, Trivedi MH
Received 2 February 2023
Accepted for publication 28 April 2023
Published 22 June 2023 Volume 2023:19 Pages 1443—1454
DOI https://doi.org/10.2147/NDT.S406909
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Joseph M Trombello,1 Alexandra Kulikova,2 Taryn L Mayes,1 Karabi Nandy,1,3 Thomas Carmody,1,3 Gavin Bart,4 Edward V Nunes,5 Joy Schmitz,6 Mariah Kalmin,7 Steven Shoptaw,7 Madhukar H Trivedi1
1Center for Depression Research and Clinical Care, Peter O’Donnell Jr. Brain Institute and Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas, TX, USA; 2Department of Educational Psychology, University of North Texas, Denton, TX, USA; 3Peter O’Donnell Jr. School of Public Health, University of Texas Southwestern Medical Center, Dallas, TX, USA; 4Department of Medicine, University of Minnesota, Hennepin County Medical Center, Minneapolis, MN, USA; 5Department of Psychiatry, Columbia University Irving Medical Center and New York State Psychiatric Institute, New York, NY, USA; 6Faillace Department of Psychiatry and Behavioral Sciences, University of Texas (UT Health) at Houston, Houston, TX, USA; 7Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA, USA
Correspondence: Madhukar H Trivedi, Center for Depression Research and Clinical Care, Peter O’Donnell Jr. Brain Institute and Department of Psychiatry, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX, 75390-9119, USA, Tel +214-648-0188, Fax +214-648-0167, Email [email protected]
Background: The co-occurrence of suicidality and substance use disorders has been well established, but rating scales to examine suicidal behavior and risk are sparse among participants with substance use disorders. We examined the psychometric properties of the 16-item Concise Health Risk Tracking Scale – Self Report (CHRT-SR16) to measure suicidality among adults with moderate-to-severe methamphetamine use disorder.
Methods: Participants (n = 403) with moderate-to-severe methamphetamine use disorder completed the CHRT-SR16 as part of a randomized, double-blind, placebo-controlled pharmacotherapy trial. The CHRT-SR16 factor structure was assessed using confirmatory factor analysis (CFA). Internal consistency was estimated with coefficients alpha (α) and omega (ω), test-retest reliability with intraclass correlation coefficient (ICC) and standard error of measurement, and convergent validity using Spearman’s ρ rank order correlation coefficient test between CHRT-SR16 factors and the Patient Health Questionnaire (PHQ-9). The analyses utilized baseline and week 1 data (for test-retest reliability only).
Results: CFA revealed a seven-factor model of Pessimism, Helplessness, Social Support, Despair, Impulsivity, Irritability, and Suicidal Thoughts as the best-fitting model. The CHRT-SR16 also exhibited strong internal consistency (α = 0.89; ω = 0.89), test-retest reliability (ICC = 0.78) and convergent validity with the PHQ-9 total score (ρ = 0.62).
Conclusion: The CHRT-SR16 showed strong psychometric properties in a sample of participants with primary methamphetamine use disorder.
Clinicaltrials.gov Identifier: NCT03078075.
Keywords: stimulant use disorder, confirmatory factor analysis, irritability, impulsivity, propensity, PHQ-9
Introduction
Substance use disorders and suicidal ideation are highly prevalent and concerning public health problems; nearly 20 million Americans aged 12 or older had a substance use disorder in 2017,1 and in 2019, 47,511 Americans died by suicide.2 Suicide rates have also been steadily rising from 1999 to 2018,3 and individuals with substance use disorders are at increased risk for suicide.4,5 Beyond outcomes like suicide attempts and completion, specific components of substance misuse are also associated with suicide risk factors. Despair, hopelessness/helplessness, and lack of social support may be risk factors for suicidality,6,7 and may be more common in those with substance abuse disorders.8 Impulsivity and substance use disorders are strongly associated,9 and impulsivity more than doubles the odds of a suicide attempt.10 Irritability is also associated with substance use and withdrawal,11,12 and there is some evidence that irritability may be a risk factor for suicidality, though this relationship may vary by patient population.13,14 Recent work found irritability to be predictive of suicidal ideation in adults in residential treatment for stimulant use disorder, and this relationship persisted when controlling for depression.15 Thus, it is clear that studying the relationship between suicidal ideation and risk factors among those with primary substance use disorders is vital.
Given the clear public health problem represented by suicidal ideation and behaviors, several self-report and clinician-rated measures have been developed to measure suicidal ideation, behaviors, and risk factors. Perhaps the most widely used suicide assessment is the Columbia Suicide Severity Rating Scale (CSSRS),16 a clinician rated measure that classifies possibly suicidal events into the Columbia Classification Algorithm for Suicide Assessment (C-CASA) categories. Another popular and validated measure has been the self-report Concise Health Risk Tracking Index (CHRT-SR).17 Unlike the CSSRS, the CHRT-SR is a self-report measure and includes not only suicidal ideation and behaviors, but also other factors associated with increased risk of suicide. Prior research has validated multiple versions of the CHRT-SR for both unipolar and bipolar depression (the 7-item, 12-item, and 14-item CHRT-SR7, CHRT-SR12, and CHRT-SR14 respectively),18–20 for use in adolescents (CHRT-SR14),21 and for use in treatment studies (CHRT-SR12).22–24 Specifically, one paper25 investigated the psychometrics of the CHRT-SR12 in a sample of participants who were primarily receiving an education or exercise-based intervention to treat stimulant use disorder, and found that the psychometric properties of the CHRT-SR12 were consistent with prior work (ie, Propensity and Suicidal Thoughts factors, and four domains within the Propensity subscale), and that the scale demonstrated strong evidence of reliability and convergent validity.
This research contributes to the literature by examining a 16-item version of the CHRT-SR (CHRT-SR16), which adds four additional items that assess impulsivity and irritability to the original CHRT-SR12. It is worth noting that prior work found that the inclusion of the two impulsivity items to the CHRT-SR12 (creating the CHRT-SR14) slightly worsened the psychometric properties of the scale in a sample of individuals with substance use disorder.25 However, assessment of non-depressive risk factors, such as impulsivity and irritability, is a unique feature of the CHRT-SR16, and there may be value in including these items to assess these factors in people with methamphetamine use disorder.
We conducted psychometric analyses of the CHRT-SR16 in a sample of adult participants receiving an intervention for moderate-to-severe methamphetamine use disorder, with a goal to assess this measure’s factor structure, reliability, and convergent validity among individuals with substance use disorders. Specifically, this study investigated the following questions:
- What is the overall factor structure of the CHRT-SR16 among a sample of participants receiving an intervention for a primary methamphetamine use disorder and how does this compare to the factor structure observed in other samples?
- What is the test-retest reliability (from baseline to week 1) and internal consistency/coefficient alpha values for the domains of the CHRT-SR16?
- What is the convergent validity as measured by correlations between the CHRT-SR16 total score (and domains) and the nine-item Patient Health Questionnaire (PHQ-9)26 total score and the score on the PHQ-9 suicidal ideation item?
Materials and Methods
Design
This study utilized data from a previously published Accelerated Development of Additive Treatment for Methamphetamine Disorder (ADAPT-2) clinical trial, a 12-week randomized, double-blind, sequential parallel comparison design trial evaluating the efficacy of combination injectable naltrexone and oral bupropion versus placebo in outpatient adults with moderate-to-severe methamphetamine use disorder.27 The analyses utilized baseline ADAPT-2 data to evaluate the psychometric properties of the CHRT-SR16, including internal consistency and convergent validity. Baseline and week 1 data (average 6.5 days between visits) were used to assess test-retest reliability of the scale.
Participants
The detailed inclusion and exclusion criteria for the ADAPT-2 study are described elsewhere.27 The baseline sample consisted of 403 adults between 18 and 65 years of age who used methamphetamine, while complete baseline and week 1 data (for test-retest reliability) were available for 277 participants. Randomized participants met criteria for moderate-to-severe methamphetamine use disorder based on the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5),12 and reported methamphetamine use on at least 18 out of the last 30 days before the consent. Participants undergoing substance use treatment, needing opioid medications during the study, or having other contraindicated medical conditions were excluded from the study. In addition, those with suicidal or homicidal ideation requiring immediate attention at baseline were excluded and referred to appropriate psychiatric care. The ADAPT-2 trial was carried out in accordance with the Declaration of Helsinki. All participants provided written informed consent approved by a central institutional review board and each site’s local institutional review board prior to initiating any study procedures.
Measures
The CHRT-SR16 is a Likert-type ordinal scale with item-level responses ranging from 0 (“Strongly Disagree”) to 4 (“Strongly Agree”) to each item/statement when considering the past week. Total score ranges from 0 to 64, with higher CHRT scores indicative of greater suicidality risk and higher frequency of suicidal thoughts. Baseline and Week 1 CHRT-SR16 data were utilized in the analysis.
The self-report Patient Health Questionnaire (PHQ-9) consists of nine items corresponding to the degree of frequency of being bothered by the nine symptoms of major depressive disorder over the last two weeks, where item-level responses are measured on a scale from 0 (“not at all”) to 3 (“nearly every day”) and the total score ranges from 0 to 27. Higher scores on the PHQ-9 are indicative of greater depressive symptom severity. Item 9 of the scale assesses suicidal ideation.
Statistical Analyses
A series of confirmatory factor analyses (CFA) were performed to evaluate the factor structure of the CHRT-SR16. The following four models were tested with factors identified a priori based on prior literature:16 (1) a single-factor model with 16 items loading on one factor of Suicidal Risk (CHRT-SR16 total); (2) a two-correlated-factor model with items 1–13 loading on the Propensity factor and items 14–16 loading on the Suicidal Thoughts factor; (3) a seven-correlated-factor model with items 1–13 loading on the Pessimism, Helplessness, Social Support, Despair, Impulsivity, and Irritability factors and items 14–16 loading on the Suicidal Thoughts factor; and (4) we tested the hypothesis that the 16-items on the CHRT-SR would load on seven first-order factors (Pessimism, Helplessness, Social Support, Despair, Impulsivity, Irritability, and Suicidal Thoughts), with six factors (Pessimism through Irritability) loading on a second-order latent factor of Propensity.
All CFA models were fitted using diagonally weighted least squares mean and variance adjusted (WLSMV) estimators with robust standard errors. WLSMV does not assume multivariate normality among observed indicators, resulting in less biased estimates of factor loadings compared to maximum and robust maximum likelihood estimators, particularly among large sample sizes and ordinal-level responses.28,29 The following fit indices and cutoffs were used as indicators of satisfactory model fit: root mean square error of approximation (RMSEA) ≤ 0.06 (90% CI ≤ 0.08), standardized root mean square residual (SRMR) ≤ 0.08, comparative fit index (CFI) ≥ 0.95, Tucker-Lewis Index (TLI) ≥ 0.95,30,31 and the weighted root mean square residual (WRMR) ≤ 1.00.32
The internal consistency reliability of the CHRT-SR16 was examined for the entire scale, two main factors (Propensity and Suicidal Thoughts), and six Propensity subscales (Pessimism, Helplessness, Social Support, Despair, Impulsivity, and Irritability) using coefficients alpha (α) and omega (ω). Acceptable internal consistency was indicated by coefficient values ≥ 0.70.33 Coefficient ω does not assume equal factor loadings (essential tau equivalence), making it a more general estimator of reliability.34
The test-retest reliability was assessed between baseline and week 1 (mean 6.5 days apart) using the intraclass correlation coefficient (ICC) as a relative measure of reliability and the standard error of measurement (SEM) as an absolute reliability index. Specifically, we used a two-way mixed effects ICC measurement, with subjects as random effects and visits as fixed effects.35,36 The SEM formula was computed as follows: 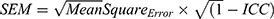 .36 ICC and SEM scores were obtained for CHRT-SR16 overall scale, the second-order Propensity factor and each of the first-order factors. Convergent validity was evaluated by assessing the Spearman ρ correlation coefficient between PHQ-9 total score and the CHRT-SR16 total score, including the first- and second-order factors.
.36 ICC and SEM scores were obtained for CHRT-SR16 overall scale, the second-order Propensity factor and each of the first-order factors. Convergent validity was evaluated by assessing the Spearman ρ correlation coefficient between PHQ-9 total score and the CHRT-SR16 total score, including the first- and second-order factors.
All statistical analyses were conducted using R statistical software (version 4.1.1), with CFA models fitted using package lavaan,37 and reliability and validity estimates obtained using package psych.38
Results
The baseline sample consisted of 403 participants randomized into the study. Full descriptive and demographic information, including substance use history, is presented in Table 1, and has also been previously published.27 The participants were primarily male (68.7%), middle-aged (M = 41.0 years; SD = 10.1), and White (71.2%), with 55 (13.6%) and 48 (11.9%) participants of Hispanic or Latino ethnicity and identifying as Black, respectively. All participants met DSM-5 criteria for moderate-to-severe methamphetamine use disorder, with an average of 26.7 days (SD = 4.1) of methamphetamine use in the 30 days prior to consent.
 |
Table 1 Baseline Characteristics of the Sample (N = 403) |
CHRT-SR16 descriptive statistics, including means and SDs at baseline, are included in Table 1. Most participants scored 0 (“strongly disagree”) or 1 (“disagree”) to items related to Suicidal Thoughts and the subfactors of pessimism, helplessness, and despair. Particularly for item 16, an item regarding having a suicide plan, 382 individuals (94.8%) reported that they “strongly disagree[d]” (79.2%) or “disagree[d]” (15.6%) with the statement. The median Suicidal Thoughts factor total score was 0, with 59.8% of participants having a total score of 0 (out of a possible 12) across three suicidality items. Items of impulsivity (M = 4.4, SD = 2.2) and irritability (M = 4.0, SD = 2.4) were more frequently endorsed.
CHRT-SR16 Fit Indices
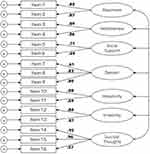
The one-factor (Model 1; CHRT-SR16) model, the two-factor (Model 2; Propensity latent factor and Suicidal Thoughts factor) model, and model 4 (Propensity latent factor as a second-order factor determined from the correlation among the factors) did not adequately fit the observed data. Only model 3 (7-factor structure) exhibited acceptable model fit. Table 2 shows fit indices across all models, and Figure 1 shows Model 3 factor structure.
 |
Table 2 Goodness-of-Fit Indicators (N = 403) |
CHRT-SR16 Standardized Factor Loadings
In Model 3, the majority of items loaded strongly on their underlying factors and exhibited strong inter-item correlation. Items 7 and 16 had the weakest loading on their respective factors of Despair (0.61) and Suicidal Thoughts (0.57). Item 7 also showed a moderate correlation with Items 8 (ρ = 0.44) and 9 (ρ = 0.46) – the other items in the Despair factor. In comparison, Items 8 and 9 correlated strongly with each other at ρ = 0.77. For the Suicidal Thoughts factor, Items 14 and 15 had excellent factor loadings of 0.92 and 0.96, respectively, while Item 16 had a weak factor loading of 0.57. Item 16 also showed lower correlation with other items on the Suicidal Thoughts factor (ρ = 0.63 and ρ = 0.67 with Items 14 and 15) compared to the correlation between Items 14 and 15 (ρ = 0.84). In general, Item 16 correlated weekly with most other items on CHRT-SR16, with inter-item correlations ranging between 0.10 (Item 5) to 0.46 (Item 8). The standardized factor loadings for Model 3 are presented in Table 3.
 |
Table 3 Standardized Factor Loadings for Model 3 (N = 403) |
CHRT-SR16 Internal Consistency
The internal consistency for CHRT-SR16 was 0.89 for both coefficients α and ω, suggesting acceptable internal consistency. The internal consistency for individual factors was also acceptable, with the exception of the Social Support factor (both α and ω = 0.66), which was slightly lower than the rest of the factors. Both Propensity and Suicidal Thoughts demonstrated excellent internal consistency at Baseline, with α = 0.89 and ω = 0.89 for Propensity, and α = 0.86 and ω = 0.90 for Suicidal Thoughts. The correlations between six first-order factors (Pessimism, Helplessness, Social Support, Despair, Impulsivity, and Irritability) loading on a second-order latent Propensity factor ranged from 0.63 (Impulsivity) to 0.80 (Helplessness) and were all statistically significant at p < 0.001. The lowest first-order inter-correlations were observed between the Impulsivity factor and the other first-order factors (correlation coefficients ranging between 0.29 and 0.42). Additionally, the individual items showed strong and statistically significant correlations with underlying factors, with all item-factor correlations greater than 0.70. Table 4 and Table 5 provide a summary of inter-factor and inter-item correlations for the CHRT-SR16.
 |
Table 4 Inter-Subscale Correlations for CHRT-SR16 Total and Subscale Scores (ρ, Spearman’s Rho Rank Order Coefficient Test) |
 |
Table 5 Item-Subscale Correlations for CHRT-SR16 (ρ, Spearman’s Rho Rank Order Coefficient Test) |
CHRT-SR16 Test-Retest Reliability
The CHRT-SR16 total score and the Propensity factor had acceptable test-retest reliability between Baseline and week 1 (ICC = 0.78 for both). Among the first-order factors, the ICC ranged from 0.64 (Helplessness) to 0.74 (Despair). The lowest ICC was observed for the Suicidal Thoughts factor (ICC = 0.49). Table 6 presents the ICC and SEM scores for the scale’s total score and individual factors.
 |
Table 6 CHRT-SR16 Internal Consistency and Test-Retest Reliability Between Baseline and Visit 1 for CHRT-SR16 |
CHRT-SR16 Convergent Validity
The association between the CHRT-SR16 total score and PHQ-9 total score was moderate-to-high (ρ = 0.62, p < 0.001), as was the association between the Propensity factor and the PHQ-9 total score (ρ = 0.63, p < 0.001). The Suicidal Thoughts factor had the weakest correlation with the PHQ-9 total score, but the relationship was still statistically significant (ρ = 0.31, p < 0.001). Among the first-order factors that load on Propensity, the correlation coefficients (ρ) with PHQ-9 total score ranged from 0.32 (Social Support) to 0.53 (Despair) and all were statistically significant at p < 0.001. Similar to the CHRT-SR16 Suicidal Thoughts factor (items 14–16), the distribution of the suicidality item (#9) on the PHQ-9 was positively skewed, with 80.9% of participants endorsing “not at all” (no thoughts that they would be better off dead or hurting themselves) over the past week. The suicidality item on the PHQ-9 exhibited the strongest correlation with the Suicidal Thoughts factor on the CHRT-SR16 (ρ = 0.51, p < 0.001). Table 7 shows the CHRT-SR16 factor correlations with the PHQ-9 total score and the suicidality item (item 9).
 |
Table 7 Convergent Validity (Spearman ρ Coefficients) |
Discussion
With a goal of examining a more comprehensive, 16-item version of the self-report Concise Health Risk Tracking Scale, we validated this measure’s psychometric properties and suitability for use in people seeking treatment for moderate-to-severe methamphetamine use disorder. Specifically, confirmatory factor analysis determined that all 16 items loaded onto a series of factors and subfactors encapsulating the propensity for suicide risk as well as a second factor of Suicidal Thoughts. Test-retest reliability and internal consistency for the factors and subfactors were generally highly acceptable. Convergent validity between the CHRT-SR16 and the suicidal ideation item from the PHQ-9 was also established.
Testing four potential models of factor structure, one model was deemed acceptable which established six first-order factors loading on a second-order latent factor of Propensity, along with a separate Suicidal Thoughts factor. This model aligns with prior CHRT-SR psychometric research and theory on how the factors of pessimism, helplessness, social support, despair, impulsivity, and irritability are described as suicide propensity.17 Although the current study tested four models based upon theory and prior research, other models may exist that fit the data just as well. Thus, the good model fit demonstrated by one of our models does not indicate that it is “correct or true”, only plausible and well supported.
Across several metrics in our sample, the three-item Suicidal Thoughts factor and its individual items did not perform as well as other CHRT items in this sample. Low base-rate prevalence may mostly explain these findings, as nearly 60% of participants responded “strongly disagree” to all three items on the Suicidal Thoughts factor, and only 19.6% responded in the affirmative on all three items, with the majority endorsing 0 or 1 responses. In addition, the final item of this factor regarding a current suicidal plan was especially infrequently endorsed, as 95% of participants reported “strongly disagree” or “disagree” to this item at baseline, and only 0.2% and 0.7% reported “agree” or “strongly agree” respectively. These low base rates are to be expected given study eligibility criteria that excluded severe current suicidality at screening. It is therefore not surprising that this item performed relatively poorly in intercorrelation with all other items. In addition, variability across time on all three suicide thoughts items and overall factor was relatively low, with very infrequent endorsement. Low variability among items and a small number of time points can contribute to low ICC,39 and both issues are present in our current study.
Study strengths include a large sample size and the inclusion of individuals with a primary stimulant use disorder – a relatively understudied population (compared to participants with a primary mood disorder) with respect to suicidality, but a critical one from a public health perspective, given the association between substance use disorders and suicide mortality.4
Study limitations include low endorsement of several items and factors, particularly those regarding Suicidal Thoughts and planning. The sample also had a relatively low proportion of women, who are more likely to experience suicidal thoughts and plans as compared to men.40–42 Inclusion of a greater proportion of women may have permitted a more robust and accurate test of the performance of the Suicidal Thoughts factor in individuals with substance use disorders. This work also did not assess for this scale’s predictive validity for future suicide attempts or suicidal behaviors among those with a primary stimulant use disorder, although this predictive validity has been established with another version of the CHRT-SR among adolescents treated for suicidality in an intensive outpatient program.43
Nonetheless, our research adds to prior literature by confirming the psychometric factor structure, reliability, and convergent validity of the CHRT-SR16 among a sample of participants receiving treatment for a primary stimulant use disorder. To improve our understanding of the Suicidal Thoughts factor, future research should examine the reliability, validity, and psychometrics of the Suicidal Thoughts factor and its constituent items among a sample of participants where suicidal thoughts are more frequent, and where data are obtained at more than two timepoints. In addition, determining the CHRT’s predictive validity of suicidal events (ie, suicide attempts, hospitalizations, and deaths by suicide) among a sample of participants with a substance use disorder is an important future direction which will require larger sample sizes. Finally, investigating the extent to which suicidal ideation as assessed by the CHRT-SR16 predicts substance use overdoses is especially important given substance users’ frequent reports of suicidal intent before overdosing.44
Conclusion
The results of this study offer evidence for strong reliability and convergent validity of the CHRT-SR16, among the first validated instruments to measure Suicidal Thoughts and behavior in a population that is disproportionately experiencing overdose and death due to methamphetamine use.45 The CHRT-SR16 builds upon prior work with the CHRT-SR,17,25 and allows for the novel assessment of impulsivity and irritability as possible risk factors for suicidal behavior.
Abbreviations
CHRT-SR, Concise Health Risk Tracking Self Report; CSSRS, Columbia Suicide Severity Rating Scale; PHQ-9, Patient Health Questionnaire; ADAPT-2, Accelerated Development of Additive Treatment for Methamphetamine Disorder; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, 5th Edition; CFA, Confirmatory Factor Analyses; WLSMV, Weighted Least Squares Mean and Variance Adjusted; RMSEA, Root Mean Square Error of Approximation; SRMR, Standardized Root Mean Square Residual; CFI, Comparative Fit Index; TLI, Tucker-Lewis Index; WRMR, Weighted Root Mean Square Residual; ICC, Intraclass Correlation Coefficient; SEM, Standard Error of Measurement.
Data Sharing Statement
The complete de-identified patient data set, study protocols, and case report forms are available at https://datashare.nida.nih.gov/study/nidactn0068, with no end date set for availability. Data are available for anyone requesting access, and website users will have to register a name and valid e-mail address in order to download data and to accept their responsibility for using data in accordance with the NIDA Data Share Agreement.
Ethics Statement
The ADAPT-2 trial was carried out in accordance with the Declaration of Helsinki. All participants provided written informed consent approved by a central institutional review board (The University of Texas Southwestern Medical Center institutional review board) and each site’s local institutional review board prior to initiating any study procedures.
Acknowledgments
We thank the study participants, without whom this research would not have been possible. We also thank the staff at the following participating study sites for their collaboration and diligent work conducting CTN-0068 ADAPT-2: Behavioral Health Services of Pickens County, SC; CODA, Inc., Portland, OR; Hennepin County Medical Center – Berman Center for Research, Minneapolis, MN; New York State Psychiatric Institute – Substance Use Research Center, New York, NY; Substance Use Research Unit at the San Francisco Department of Public Health, San Francisco, CA; University of California Los Angeles Center for Behavioral Addiction Medicine, Los Angeles, CA; University of Texas Health Center for Neurobehavioral Research on Addiction, Houston, TX; University of Texas Southwestern Medical Center, Dallas, TX. Most importantly, we thank the individuals who volunteered to participate in the study.
In addition, we thank our collaborators during the study: the Clinical Coordinating Center at The Emmes Company, particularly Matthew Wright and Eve Jelstrom; the Data and Statistics Center at The Emmes Company, particularly Catherine Mudrick, Ashley Case, and Jacquie King; AiCure, particularly Brien Hawley, Laura Schafner, and Gordon Kessler; Angela Casey-Willingham for her assistance monitoring study implementation. We thank Kathryn Forbes for her administrative assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Institute on Drug Abuse (NIDA) of the National Institutes of Health (UG1DA020024, 2017), and the Department of Health and Human Services (HHSN271201500065C and HHSN271201400028C, 2016). Alkermes provided Vivitrol (naltrexone for extended-release injectable suspension) and matched placebo free of charge for use in this trial under a written agreement with NIDA. Licensing and distribution of the CHRT is managed by Mapi Research Trust on behalf of the copyright holder, University of Texas Southwestern Medical Center. At the time of publishing, the CHRT is available without charge to non-commercial users. Fees may apply to commercial users, IT companies, funded academic users or health-care organizations. Requests for information and licensing of the CHRT should be submitted through Mapi Research Trust’s ePROVIDE platform (https://eprovide.mapi-trust.org/).
Disclosure
Dr. Trombello reports owning stock in Merck and has served as a paid consultant for Alto Neuroscience LLC. He is currently a Clinical Assistant Professor of Psychiatry at UT Southwestern Medicine and was employed as an Assistant Professor of Psychiatry when the study was conducted. He is now employed by Janssen Research and Development.
Dr. Carmody has served as a consultant for Alkermes, Inc.
Dr. Bart reported receiving grants from the National Institute on Drug Abuse and National Institute of Diabetes and Digestive and Kidney Diseases during the conduct of the study, and personal fees from the American Academy of Addiction Psychiatry in honoraria for content expertise for ongoing participation in PCSS (Providers Clinical Support System) Exchange, an educational webinar series for opioid use disorder education.
Dr. Nunes has served as a consultant without compensation for Alkermes, Pear Therapeutics, and Camurus. He has been/currently serves as an investigator on NIH funded studies that received in-kind donation of medication from Alkermes, Indivior, and Braeburn, and in-kind donations of digital therapeutics from Pear Therapeutics and CHESS Health.
Dr. Shoptaw has received clinical research supplies from Gilead Sciences, Inc., Alkermes, Inc., and Indivior, Inc., and has consulted for Aelis Farma, Inc., and Sparian Biosciences, Inc.
Dr. Trivedi has served as a consultant or advisor for Acadia Pharmaceuticals, Inc., Akili Interactive, Alkermes, Inc. (Pub Steering Comm-ALKS5461), Allergan Sales LLC, Alto Neuroscience, Inc., Applied Clinical Intelligence, LLC (ACI), Axome Therapeutics, Boehringer Ingelheim, Engage Health Media, Gh Research, GreenLight VitalSign6, Inc., Heading Health, Inc., Health Care Global Village, Janssen – Cilag.SA, Janssen Research and Development, LLC (Adv Committee Esketamine), Janssen Research and Development, LLC (panel for study design for MDD relapse), Janssen - ORBIT, Legion Health, Jazz Pharmaceuticals, Lundbeck Research U.S.A, Medscape, LLC, Merck Sharp & Dohme Corp., Mind Medicine (MindMed) Inc., Myriad Neuroscience, Neurocrine Biosciences Inc, Navitor, Pharmaceuticals, Inc., Noema Pharma AG, Orexo US Inc., Otsuka Pharmaceutical Development & Commercialization, Inc. (PsychU, MDD Section Advisor), Otsuka America Pharmaceutical, Inc. (MDD expert), Pax Neuroscience, Perception Neuroscience Holdings, Inc., Pharmerit International, LP, Policy Analysis Inc., Sage, Therapeutics, Rexahn Pharmaceuticals, Inc., Sage Therapeutics, Signant Health, SK Life Science, Inc., Takeda Development Center Americas, Inc., The Baldwin Group, Inc., and Titan Pharmaceuticals, Inc. Dr. Trivedi also received editorial compensation from Oxford University Press. The other authors report no conflicts of interest in this work.
References
1. Substance Abuse and Mental Health Services Administration. Data From: Key Substance Use and Mental Health Indicators in the United States: Results from the 2016 National Survey on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2017.
2. Centers for Disease Control and Prevention. Data From: Web-Based Injury Statistics Query and Reporting System (WISQARS) Fatal Injury Reports. Centers for Disease Control and Prevention; 2020.
3. Hedegaard H, Curtin SC, Warner M. Increase in suicide mortality in the United States, 1999–2018. NCHS Data Brief. 2020;362:1–8.
4. Bohnert KM, Ilgen MA, Louzon S, McCarthy JF, Katz IR. Substance use disorders and the risk of suicide mortality among men and women in the US veterans health administration. Addiction. 2017;112(7):1193–1201. doi:10.1111/add.13774
5. Lynch FL, Peterson EL, Lu CY, et al. Substance use disorders and risk of suicide in a general US population: a case control study. Addict Sci Clin Pract. 2020;15(1):14. doi:10.1186/s13722-020-0181-1
6. Brown GK, Beck AT, Steer RA, Grisham JR. Risk factors for suicide in psychiatric outpatients: a 20-year prospective study. J Consult Clin Psychol. 2000;68(3):371–377. doi:10.1037/0022-006X.68.3.371
7. Bridge JA, Goldstein TR, Brent DA. Adolescent suicide and suicidal behavior. J Child Psychol Psychiatry. 2006;47(3–4):372–394. doi:10.1111/j.1469-7610.2006.01615.x
8. Copeland WE, Gaydosh L, Hill SN, et al. Associations of despair with suicidality and substance misuse among young adults. JAMA Netw Open. 2020;3(6):e208627. doi:10.1001/jamanetworkopen.2020.8627
9. Kozak K, Lucatch AM, Lowe DJE, Balodis IM, MacKillop J, George TP. The neurobiology of impulsivity and substance use disorders: implications for treatment. Ann N Y Acad Sci. 2019;1451(1):71–91. doi:10.1111/nyas.13977
10. Liu RT, Trout ZM, Hernandez EM, Cheek SM, Gerlus N. A behavioral and cognitive neuroscience perspective on impulsivity, suicide, and non-suicidal self-injury: meta-analysis and recommendations for future research. Neurosci Biobehav Rev. 2017;83:440–450. doi:10.1016/j.neubiorev.2017.09.019
11. Chen XJ, Wang CG, Li YH, Sui N. Psychophysiological and self-reported responses in individuals with methamphetamine use disorder exposed to emotional video stimuli. Int J Psychophysiol. 2018;133:50–54. doi:10.1016/j.ijpsycho.2018.08.011
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™.
13. Jha MK, Minhajuddin A, Chin Fatt C, et al. Association between irritability and suicidal ideation in three clinical trials of adults with major depressive disorder. Neuropsychopharmacology. 2020;45(13):2147–2154. doi:10.1038/s41386-020-0769-x
14. Orri M, Perret LC, Turecki G, Geoffroy MC. Association between irritability and suicide-related outcomes across the life-course. Systematic review of both community and clinical studies. J Affect Disord. 2018;239:220–233. doi:10.1016/j.jad.2018.07.010
15. Jha MK, Minhajuddin A, Chin Fatt C, et al. Irritability as an independent predictor of concurrent and future suicidal ideation in adults with stimulant use disorder: findings from the STRIDE study. J Affect Disord. 2021;292:108–113. doi:10.1016/j.jad.2021.04.019
16. Posner K, Brown GK, Stanley B, et al. The Columbia-suicide severity rating scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. doi:10.1176/appi.ajp.2011.10111704
17. Trivedi MH, Wisniewski SR, Morris DW, et al. Concise Health Risk Tracking scale: a brief self-report and clinician rating of suicidal risk. J Clin Psychiatry. 2011;72(6):757–764. doi:10.4088/JCP.11m06837
18. Ostacher MJ, Nierenberg AA, Rabideau D, et al. A clinical measure of suicidal ideation, suicidal behavior, and associated symptoms in bipolar disorder: psychometric properties of the concise health risk tracking self-report (CHRT-SR). J Psychiatr Res. 2015;71:126–133. doi:10.1016/j.jpsychires.2015.10.004
19. Reilly-Harrington NA, Shelton RC, Kamali M, et al. A tool to predict suicidal ideation and behavior in bipolar disorder: the concise health risk tracking self-report. J Affect Disord. 2016;192:212–218. doi:10.1016/j.jad.2015.12.036
20. Villegas AC, DuBois CM, Celano CM, et al. A longitudinal investigation of the concise health risk tracking self-report (CHRT-SR) in suicidal patients during and after hospitalization. Psyc Res. 2018;262:558–565. doi:10.1016/j.psychres.2017.09.044
21. Mayes TL, Kennard BD, Killian M, et al. Psychometric properties of the concise health risk tracking (CHRT) in adolescents with suicidality. J Affect Disord. 2018;235:45–51. doi:10.1016/j.jad.2018.03.007
22. Trombello JM, Killian MO, Grannemann BD, et al. The concise health risk tracking-self report: psychometrics within a placebo-controlled antidepressant trial among depressed outpatients. J Psychopharmacol. 2019;33(2):185–193. doi:10.1177/0269881118817156
23. De La Garza N, Rush AJ, Killian MO, Grannemann BD, Carmody TJ, Trivedi MH. The Concise Health Risk Tracking Self-Report (CHRT-SR) assessment of suicidality in depressed outpatients: a psychometric evaluation. Depress Anxiety. 2019;36(4):313–320. doi:10.1002/da.22855
24. Zisook S, Lesser IM, Lebowitz B, et al. Effect of antidepressant medication treatment on suicidal ideation and behavior in a randomized trial: an exploratory report from the combining medications to enhance depression outcomes study. J Clin Psychiatry. 2011;72(10):1322–1332. doi:10.4088/JCP.10m06724
25. Sanchez K, Killian MO, Mayes TL, et al. A psychometric evaluation of the Concise Health Risk Tracking Self-Report (CHRT-SR)- A measure of suicidality-in patients with stimulant use disorder. J Psychiatr Res. 2018;102:65–71. doi:10.1016/j.jpsychires.2018.03.012
26. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi:10.1046/j.1525-1497.2001.016009606.x
27. Trivedi MH, Walker R, Ling W, et al. Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med. 2021;384(2):140–153. doi:10.1056/NEJMoa2020214
28. Li CH. Confirmatory factor analysis with ordinal data: comparing robust maximum likelihood and diagonally weighted least squares. Behav Res Methods. 2016;48(3):936–949. doi:10.3758/s13428-015-0619-7
29. Suh Y. The performance of maximum likelihood and weighted least square mean and variance adjusted estimators in testing differential item functioning with nonnormal trait distributions. Struct Equ Mod. 2015;22(4):568–580. doi:10.1080/10705511.2014.937669
30. Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Mod. 1999;6:1–55. doi:10.1080/10705519909540118
31. Brown TA. Confirmatory Factor Analysis for Applied Research. Guilford publications; 2015.
32. DiStefano C, Liu J, Jiang N, Shi D. Examination of the weighted root mean square residual: evidence for trustworthiness? Struct Equ Mod. 2018;25(3):453–466. doi:10.1080/10705511.2017.1390394
33. Hair JF, Black B, Babin B, Anderson R, Tatham RL. Multivariate Data Analysis. Person Education; 2006.
34. Hayes AF, Coutts JJ. Use omega rather than cronbach’s alpha for estimating reliability. Commun Methods Meas. 2020;14(1):1–24. doi:10.1080/19312458.2020.1718629
35. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. doi:10.1037//0033-2909.86.2.420
36. Hopkins WG. Measures of reliability in sports medicine and science. Sports Med. 2000;30(1):1–15. doi:10.2165/00007256-200030010-00001
37. Rosseel Y. lavaan: an R package for structural equation modeling. J Stat Softw. 2012;48(2):1–36. doi:10.18637/jss.v048.i02
38. Revelle WR. Psych: Procedures for Personality and Psychological Research. Northwestern University; 2017.
39. Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. Pearson/Prentice Hall; 2009.
40. Crosby AE, Han B, Ortega LA, Parks SE, Gfroerer J. Suicidal thoughts and behaviors among adults aged ≥18 years--United States, 2008–2009. MMWR Surveill Summ. 2011;60(13):1–22.
41. Milton AC, Davenport TA, Iorfino F, Flego A, Burns JM, Hickie IB. Suicidal thoughts and behaviors and their associations with transitional life events in men and women: findings from an international web-based sample. JMIR Ment Health. 2020;7(9):e18383. doi:10.2196/18383
42. Nock MK, Green JG, Hwang I, et al. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the national comorbidity survey replication adolescent supplement. JAMA Psychiatry. 2013;70(3):300–310. doi:10.1001/2013.jamapsychiatry.55
43. Mayes TL, Killian M, Rush AJ, et al. Predicting future suicidal events in adolescents using the Concise Health Risk Tracking Self-Report (CHRT-SR). J Psychiatr Res. 2020;126:19–25. doi:10.1016/j.jpsychires.2020.04.008
44. Connery HS, Taghian N, Kim J, et al. Suicidal motivations reported by opioid overdose survivors: a cross-sectional study of adults with opioid use disorder. Drug Alcohol Depend. 2019;205:107612. doi:10.1016/j.drugalcdep.2019.107612
45. Han B, Compton WM, Jones CM, Einstein EB, Volkow ND. Methamphetamine use, methamphetamine use disorder, and associated overdose deaths among US adults. JAMA Psychiatry. 2021;78(12):1329–1342. doi:10.1001/jamapsychiatry.2021.2588
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.