Back to Journals » Patient Preference and Adherence » Volume 16
Psychological Factors Affecting the Willingness to Accept a Possible Tyrosine Kinase Inhibitor (TKI) Discontinuation in Chronic Myeloid Leukaemia (CML) Patients
Authors Cutica I , Riva S, Orlandi EM, Iurlo A, Vener C , Elena C, Bucelli C, Cattaneo D , Tomezzoli E , Pravettoni G
Received 13 April 2022
Accepted for publication 29 July 2022
Published 31 October 2022 Volume 2022:16 Pages 2963—2975
DOI https://doi.org/10.2147/PPA.S369326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Ilaria Cutica,1,* Silvia Riva,2,* Ester Maria Orlandi,3 Alessandra Iurlo,4 Claudia Vener,1 Chiara Elena,3 Cristina Bucelli,4 Daniele Cattaneo,1,4 Elisa Tomezzoli,5 Gabriella Pravettoni1,5
1Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy; 2Department of Psychology and Pedagogic Science, St Mary’s University, London, UK; 3Hematology Unit, Foundation IRCCS Policlinico San Matteo, Pavia, Italy; 4Hematology Division, Foundation IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy; 5Applied Research Division for Cognitive and Psychological Science, European Institute of Oncology (IEO), IRCCS, Milan, Italy
*These authors contributed equally to this work
Correspondence: Ilaria Cutica, Department of Oncology and Hemato-Oncology, University of Milan, Via Santa Sofia 9/1, Milan, 20123, Italy, Tel +39 02 50321562, Fax +39 02 50318938, Email [email protected]
Purpose: Patients with chronic myeloid leukemia (CML) who present a sustained deep molecular response (DMR) for a stable period of time might benefit from discontinuing tyrosine kinase inhibitors (TKIs). A significant number of patients seem able to reach this stage due to the availability of TKIs. However, many patients remain reluctant about TKI discontinuation and may refuse treatment interruption. The purpose of this study was to explore the clinical and psycho-cognitive factors that may influence the decision to discontinue TKI therapy, thereby gaining a better understanding of patients’ viewpoints on TKI discontinuation.
Patients and Methods: One hundred and nineteen patients diagnosed with CML aged between 34 and 69 were enrolled (67 males and 52 females). Different clinical information and psycho-cognitive aspects such as attitude toward risk behaviours, risk preferences, need for cognitive closure, and tendency to resist to changes were assessed through the administration of a battery of questionnaires.
Results: A higher tendency toward risk behaviours and the tendency to focus on possible gain in the short term rather than on losses might represent important predictors for the willingness to accept TKI discontinuation. Possible relapses following interruption of the therapy are the most common reason for concern. Furthermore, lower levels of resistance to change and having previously experienced the desire to interrupt the therapy might lead patients to accept a higher probability of relapse risk when facing such a decision.
Conclusion: TKI discontinuation appears appealing and challenging at the same time for many CML patients, and different factors may influence this decision. Psychology plays a crucial role in assisting physician-patient communication and informed decision-making.
Keywords: TKI discontinuation, chronic myeloid leukaemia, patients’ perspective, risk preferences, decision-making
Introduction
Chronic myeloid leukaemia (CML) is a cancer of white blood cells characterised by an increased and unregulated growth of predominantly myeloid cells in the bone marrow and the accumulation of these cells in the blood.1 CML treatment is designed to attack cancer by focusing on the enzyme tyrosine kinase (TK), which allows cancer cells to grow and multiply.2 Currently, CML is mainly treated with drugs known as tyrosine kinase inhibitors (TKIs) that have dramatically improved long-term survival rates.3 TKIs such as imatinib (first-generation TKI), dasatinib, nilotinib, and bosutinib (second-generation TKIs) have been approved by the American Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as first-line treatments. Research in the field continues to advance to identify the best TKI for each patient, depending on efficacy, tolerability, early and late toxicity, drug costs, and patient’s quality of life: in comparison with imatinib, second- and third-generation TKIs improve clinical responses, even though the safer toxicity profile of imatinib might represent a better option for patients with comorbidities.4 Moreover, for most people, TKIs can help achieve a long-term disease-free remission.5
TKIs are effective in achieving long-term deep molecular responses (DMRs) – defined as BCR-ABL1 levels of molecular response MR4 and MR4.5 on the International Scale (IS) – in many patients,6 especially in Western countries. Patients who reach DMR after taking TKIs can be advised to discontinue the therapy, as recommended in the guidelines for CML treatment.6,7 For this reason, there has been an increasing focus on the identification of strategies to maximize the possibility of interrupting TKIs and gaining treatment-free remission (TFR) for patients who have a stable DMR.8–11 Several studies have examined the effectiveness of such a discontinuation: the French Stop Imatinib 1 Trial (STIM1)11 showed that 38% of the 100 CML patients enrolled maintained a molecular remission after a median follow-up of 77 months. The eligibility criterion for interrupting the treatment was to have reached a sustained undetectable molecular residual disease (UMRD) for at least 2 years. These results are consistent with a smaller study that applies the same eligibility criteria, namely the TWISTER study (Australian Leukaemia and Lymphoma Group CML8):12 18 (45%) of 40 patients maintained a molecular remission after a median follow-up of 42 months. Similarly, a systematic review and meta-analysis of 15 cohort studies,13 involving 509 patients, found a mean molecular relapse rate of 51%. More recently, several studies have investigated discontinuation from second-generation TKIs.14–16 For instance, the Pan-European Stop Tyrosine Kinase Inhibitor Study (EURO-SKI)14 showed that molecular relapse-free survival was 50% at 24 months for the 775 patients enrolled. Moreover, clinical data indicate that resuming treatment with a TKI even after a failed discontinuation attempt is feasible and can be successful.17
All these results showed that TKI discontinuation is a safe procedure; nonetheless, some studies also showed that several patients eligible for discontinuation prefer to continue with the usual treatment.18–20 The willingness of patients to discontinue TKI treatment ranges from 34% to 83%, according to different studies.21–29 Most frequent reasons for patients to discontinue TKI therapy are relief from side effects, as well as, to a lesser degree, relief from the inconvenience of taking daily medications, the opportunity to plan a pregnancy, and a sensible reduction of treatment costs. On the other hand, the most frequently patient-reported concerns involve the risk of CML recurrence and loss of disease control (from 80% to 93%), followed by the fear of not responding to the treatment in case of relapse (from 70% to 90%), and having more drug side effects after restarting the therapy (from 33% to 45%). Minor concerns included not having enough information about interrupting the treatment, fear of withdrawal syndrome, worry about inadequate medical assistance, and worry about dismaying family or friends. Adequate monitoring seems to be the most important prerequisite to accept TKI discontinuation.29
No research has been conducted to date on the cognitive and psychological characteristics, which may influence CML patients’ decision-making process regarding therapy discontinuation. In this context, evaluating the psychological profile of CML patients in terms of attitudes toward risk behaviours, risk perception, way of coping with ambiguous situations and resistance to change can shed light on the factors that might impact on the decision to accept or reject TKI discontinuation. It is thus the aim of this study to explore the cognitive and emotional factors that are specifically associated with patients’ willingness to discontinue TKI treatment, as well as the percentage of relapse risk they are prepared to accept. The second aim is to identify distinct clusters of patients based on their cognitive profiles and attitudes regarding risk and change.
Materials and Methods
Participants and Recruitment
Patients were recruited from two Italian medical centres included in the Lombardy Haematological network (http://www.rel-lombardia.net/) with large experience in CML treatment, namely the Foundation IRCCS Cà Granda Ospedale Maggiore Policlinico in Milan and the Foundation IRCCS Policlinico San Matteo in Pavia.
Inclusion criteria were (a) to be diagnosed with CML in chronic phase (CP) at data collection, (b) to be aged 18 or older, and (c) to be able to read and understand study materials (patient information and data protection form and patient-related questionnaires). Exclusion criteria were (a) to be affected by neurological and/or psychiatric disorders (ie, patients suffering from psychosis and/or personality disorders), and (b) to have mild or severe cognitive impairments. The CML stage was not included in the eligibility criteria since all patients might eventually face the possibility to discontinue TKIs.
Medical personnel contacted eligible patients a few days before their medical appointments, offering them the opportunity to participate in the study. Interested patients met with two research psychologists (IC and SR) who provided further details regarding the study procedure at their scheduled check-up. Patients who accepted to participate were asked to sign the informed consent prior to study commencement in accordance with the Declaration of Helsinki, and then to complete a battery of paper-pencil questionnaires. The completion required approximately 40 minutes and took place in a hospital room assigned for that purpose, in the presence of the researcher. Patients were not given any remuneration for their participation.
This study received approval by the Ethics Committee of both the Foundation IRCCS Cà Granda Ospedale Maggiore Policlinico in Milan and the Foundation IRCCS Policlinico San Matteo in Pavia. All the phases of the study were prepared, conducted, and described in accordance with the Italian Good Clinical Practice (D. M. 15 July 1997). The participants were informed that they could avoid answering any questions that might make them uncomfortable, and that they could withdraw from the study at any time.
Study Design and Psychological Methods
For this cross-sectional, non-controlled and non-randomized study, a three-part questionnaire was designed.
The first section included 4 socio-demographic questions (biological sex, age range, marital status, level of education), and 9 questions regarding disease history and current treatment (year of diagnosis, current disease stage, comorbidities, drug therapy, side effects, possible desire to interrupt the therapy, and related concerns).
In the second section, a hypothetical scenario22 was presented. The scenario illustrated the recent success of TKI-based therapies, including that in some cases the efficacy is high enough to result in a stable DMR, allowing the treatment to be interrupted. The scenario also suggested that approximately 40% of CML patients who discontinue TKIs do not relapse after 3 years of treatment, and that most of those who do relapse respond to TKI reinstatement. Following this, patients were asked whether they would agree to interrupt their therapy if suggested by their doctors and if properly followed up, the relapse rate they would consider acceptable to agree, and the factors that most concerned them about therapy discontinuation.
In the third part of the questionnaire, several psychological characteristics such as attitude toward risk behaviours, risk preferences, need for cognitive closure, and tendency to resist to changes were assessed. Specifically:
A) Attitude toward risk behaviours was assessed through the Stimulating-Instrumental Risk Inventory (SIRI),30 which consists of 17 items and response options are provided on a 4-point rating scale from 1 (does not describe me at all) to 4 (describes me very well). This scale also distinguishes two types of risk-taking through two subscales: (1) the Stimulating Risk Taking (SRT), a measure of impulsive risk behavior driven by emotional processes and positive arousal, focused on short-term consequences, unconcerned with the magnitude of potential losses; (2) the Instrumental Risk Taking (IRT), a measure of reflective risk behaviour characterised by cognitive processes and negative arousal, focused on long-term consequences and potential losses. In this study, both subscales showed an acceptable internal consistency, as Cronbach’s alpha was 0.70 and 0.80, respectively.
B) Risk preferences were assessed through the 7-item subscale of the Passive Risk-Taking Scale (PRT)31,32 that measures passive risk-taking in the medical and health domains. Passive risk-taking entails inaction rather than acting in order to reduce outcome variance, thus increasing the possibility of an undesired outcome. Answers were provided on an 8-point rating scale from 0 (strongly disagree) to 7 (strongly agree). Cronbach’s alpha in the present study was 0.75.
C) Need for cognitive closure, understood as the motivation to find an answer to an ambiguous situation as compared to confusion and uncertainty, was measured through the Need for Cognitive Closure Scale (NFCS).33,34 This scale includes 42 items that provide answers on a 6-point rating scale from 1 (strongly disagree) to 6 (strongly agree), and in this study, the overall Cronbach’s alpha was 0.72. The questionnaire also includes the following 5 subscales: Desire for predictability, Preference for order and structure, Discomfort with ambiguity, Decisiveness, Close-mindedness. In this study, the 5 subscales showed an acceptable internal consistency, as Cronbach’s alpha ranged from 0.71 to 0.83.
D) Resistance to change was assessed through the 17 items of the Resistance to Change (RTC) questionnaire,35 a personality measure of individuals’ tendency to resist to or avoid changes and to consider changes as aversive. This instrument provides answers on a 6-point Likert-type scale from 1 (strongly disagree) to 6 (strongly agree), and Cronbach’s alpha in the present study was 0.77.
Statistical Methods
Descriptive statistics have been computed for all clinical characteristics and outcome measures, as appropriate. In particular, mean (M), standard deviation (SD), median, and range were evaluated for continuous variables, whereas frequency (n) and percentage were evaluated for categorical variables. Missing data imputation has not been performed.
Chi-square test and independent sample t-test were, respectively, computed to evaluate whether the group of patients who would agree to interrupt the therapy and the group of those who would not showed significant differences with respect to gender and age range. Furthermore, chi-square tests were used to evaluate whether there was a statistically significant difference in terms of concerns related to a possible therapy discontinuation between both groups.
Univariable analyses were performed to identify possible associations between patients’ psycho-cognitive characteristics and variables regarding disease history and current treatment with two main outcomes: (1) patients’ willingness to interrupt the therapy, expressed as dichotomous variable (yes/no), and (2) probability of CML relapse risk they would accept to agree to interrupt TKIs, expressed as percentage (0–100%).
A multivariable analysis was then conducted by including covariates that resulted statistically significant with univariable analyses, in order to verify their association with the probability of CML relapse risk accepted by patients. The model selection analysis was adopted, fixing a 0.05 significance level both to entry and maintain a variable in the model. Pearson’s correlation coefficient was used to identify strongly correlated variables, in order to evaluate which ones to maintain in the model.
Cluster analyses were also conducted to group similar observations among patients, such that observations in the same cluster were similar to each other. Specifically, a two-step clustering strategy was applied to identify different clusters of patients based on risk and cognitive variables. First, a hierarchical cluster analysis was performed in order to identify the number of clusters. Since K-means clustering allows to group similar data points together, a supplementary K-means cluster analysis was performed to define cognitive profiles across patients. The final cluster centres were computed as the mean for each variable within each final cluster, and one-way ANOVA was performed to evaluate whether the clusters showed a homogeneous distribution with respect to Outcome 1 and Outcome 2.
Furthermore, in order to assess the reliability of the questionnaires, their internal consistency was determined by using Cronbach’s alpha, as reported in the method section (values ≥0.70 were considered acceptable).
All p-values were two-tailed and considered significant when p < 0.05. Descriptive statistics, chi-square tests, independent sample t-tests, univariable and multivariable analyses were carried out using SAS as statistical software (Version 9.4; SAS Institute Inc., Cary, NC, USA), and PROC LOGISTIC and PROC GLM were used for statistical model fitting. Cluster analyses and Cronbach’s alpha were conducted by IBM SPSS Statistics for Windows, Version 26.0.
Results
Descriptive Analyses
One hundred and nineteen patients diagnosed with CML from 1978 to 2016 were consecutively enrolled in this study. The sample consisted of 67 males and 52 females (M/F ratio: 1.29), aged from 34 to 69 (M = 52.9). Full records of the 119 patients have been collected for all the variables under study, except for disease phase and drug type (118 records). Median duration of the CML from diagnosis was 8 years (range: 1–39 years). Sixty-four patients out of 119 were receiving imatinib as first-line therapy (54%), 27 were taking dasatinib (23%), 24 nilotinib (20%), and 3 bosutinib (3%). All patients were in CP CML.
In Table 1 demographics and clinical characteristics of the sample are reported. Table 2 reports the answers (in terms of absolute and relative frequencies) to questions about experience with the current CML treatment, a possible already experienced desire to discontinue the therapy, the willingness to accept TKI discontinuation if suggested by doctors and if properly followed up, the percentage of relapse risk considered acceptable to discontinue the therapy, and the main factors that could prevent from TKI interruption.
 |
Table 1 Patients’ Demographic and Clinical Characteristics |
Most patients (64.71%) experienced side effects less than 6 days a month, and the vast majority (84.87%) never forgets to take prescriptions. Most patients (75.63%) would accept to interrupt the treatment, although many of them had not previously desired to cease it (77.31% of respondents). However, almost a quarter of our patients (24.37%) would refuse to interrupt the therapy. There were no significant differences with respect to age (t(118)=.798, p = 0.426) and gender (χ²(1)=2.052, p = 0.152) in patients who would consent to a possible therapy discontinuation and those who would not.
In general, patients reported to be more likely to stop TKIs if the relapse risk is no more than an average of 30%. On the other hand, patients who indicated that they would not interrupt the therapy would consent to discontinuation of TKIs if the maximum relapse risk was 8% on average (SD = 8.18), whereas patients who would interrupt the treatment would accept a maximum average risk of 38% (SD = 31.17).
Main concerns regarding TKI discontinuation involved possibility of disease recurrence (reported by 66% of patients) and fear of drug resistance in case of relapse (51%). Results related to differences in terms of frequencies of the main concerns with respect to TKI discontinuation are reported in Table 3. No significant differences emerged between patients who would accept to interrupt the treatment and those who would not with respect to concerns.
 |
Table 3 Patients’ Fears in Case of TKI Interruption. Absolute and Relative Frequencies of Main Concerns in the Case of TKIs Interrupting by Patients Who Would Accept or Not to Stop TKIs* |
Table 4 reports the mean values obtained in the psychological tests: SIRI, NCFS, PRT, and RTC.
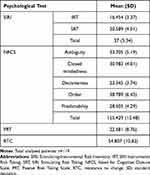 |
Table 4 Results to Psychological Tests |
Univariable Analyses
Table 5 reports univariable analysis results to identify the association between psycho-cognitive patients’ characteristics and the decision to accept TKI discontinuation (Outcome 1). Only SIRI Total [Odds Ratio (OR): 1.17 (1.07–1.29)] and SRT [OR: 1.47 (1.24–1.74)] covariates resulted singly associated with Outcome 1. Since they are strongly correlated (r = 0.77, p < 0.001), SIRI Total was not included in subsequent multivariable analysis.
 |
Table 5 Univariable Analysis Evaluating the Association Between Each Covariate, Singly Considered, and Outcome 1. Covariates are Analysed Continuously (A) or by Class (B) |
Table 6 reports covariates under study, singly considered, and their association with the percentage of relapse risk patients would accept to interrupt CML therapy (Outcome 2). Considering psychological covariates, only RTC (p = 0.007), SRT (p = 0.004), and IRT (p = 0.030) resulted singly associated with Outcome 2. IRT showed a negative coefficient value, so the result had no clinical meaning and it was not included as a covariate in the subsequent multivariable analysis.
 |
Table 6 Univariable Analysis Considering the Association Between Each Covariate, Singly Considered, and Outcome 2. Covariates are Analysed Continuously (A) or by Class (B) |
Moreover, covariates regarding both disease stage (Disease phase) and possibility of previously experiencing the desire to interrupt the therapy (Ever desired to stop) were significantly associated with Outcome 2, showing, respectively, p = 0.028 and p < 0.001. Table 7 reports the mean percentage of relapse risk that patients would accept in order to interrupt TKI therapy, by current disease phase and by the already experienced desire to stop.
 |
Table 7 Mean Percentage of Relapse Risk That Patients Would Accept in Order to Interrupt TKI Therapy by Current Disease Phase and by the Already Experienced Desire to Stop (Ever Desired to Stop) |
Multivariable Analysis
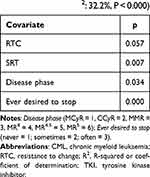
Statistically significant variables emerged from univariable analyses (ie, RTC, SRT, Ever desired to stop and Disease phase) were included in a multivariable analysis to verify their association with the probability of CML relapse risk accepted by patients in case of TKI discontinuation. No correlation between covariates included in the model was found. Interaction test was negative (final results are reported in Table 8). Therefore, the four covariates were included in the final model (R2: 32.2%, p < 0.001; only RTC showed a p-value of 0.057).
Furthermore, a multivariable analysis was conducted, starting from a complete model (which includes all variables reported in Table 6 except for covariates strongly associated with each other), eliminating one by one the less significant variables, and considering a significance level of 0.05 in order to keep the variable in the model. The final model included RTC, SRT, Ever desired to stop and Disease phase.
In Supplementary Materials, there are reported: the final model with least-squares means of Outcome 2 by Disease phase and by Ever desired to stop covariates (Table 1S); Outcome 2, RTC and SRT mean (SD) values by Disease phase classes (Table 2S) and by Ever desired to stop classes (Table 3S).
Cluster Analysis
The two-step cluster analysis (hierarchical and K-means) run with explorative purpose identified two distinct clusters. The final cluster centres reflect the characteristics of the typical case for each cluster (Figure 1 shows the final cluster centres mean). Cluster 1 (n = 51) was characterised by patients with higher tolerance to ambiguity and to life’s changes. Cluster 2 (n = 68) was characterised by patients that tend to show higher resistance to change on life events and higher cognitive closure.
One-way ANOVA showed a homogeneous distribution in the two clusters with respect to Outcome 1 and Outcome 2, but fears related to TKI discontinuation appeared to be tailored to each cluster where patients presenting higher tolerance to changes and ambiguity were also more exposed to fear and emotional response. Particularly, 23% of patients in Cluster 1 were more worried about the possibility to lose contact and miss routine assessments in their health centres, while in Cluster 2 only 6% of patients reported this fear (F = 7.051, p = 0.009). Similarly, 36% patients in Cluster 1 reported more concerns related to family worries than patients in Cluster 2 (17.6%) (F = 5.367, p = 0.022).
Discussion
This study examined the cognitive and psychological characteristics of CML patients that may influence their decision to interrupt CML therapy when suggested, in an effort to increase our understanding of patients’ views on TKI-based treatment discontinuation. We assessed patients’ attitude toward risk behaviours, risk preferences, way to cope with ambiguous situations, and tendency to resist to changes, along with several clinical data (eg, time from diagnosis, disease stage, current treatment, etc.).
Although many studies have confirmed the feasibility and safety of interrupting TKIs in selected patients, they might be reluctant.18–20 Our results are in line with a previous study29 showing that main concerns involve possible relapses and fear of not responding again to the treatment in case of recurrence. There was no difference as for concerns between those patients who would accept to discontinue their TKIs and those who would not, suggesting that these are both important aspects to discuss with all eligible patients when suggesting the discontinuation of the therapy. In our study, 24% of patients claim that they would not accept to interrupt the therapy, even if suggested by their doctors and properly followed up. In these situations, it is not cost-effective to continue the therapy, from both the patient’s and the welfare agency’s standpoints. TKI prescriptions weigh on public health expenses for treatments that are no longer necessary, and these therapies might impact on patients’ quality of life due to side effects or to lack of full compliance. In our sample, 64% of patients reported to experience side effects due to medications less than 6 days a month, and the vast majority (84%) never forgets to take prescriptions. Therefore, interrupting the therapy might represent a benefit for those who experience side effects daily or more than 15 days a month (23%) or from 7 to 15 days (39.5%), and those who forget to take medications from 1 to 5 times (14%) or more than 5 times a month (1.7%). However, our data indicate that neither experiencing more or less side effects nor the level of compliance in taking prescriptions are predictors of the decision to discontinue the therapy. In other words, even patients with frequent side effects or lower compliance may decide not to interrupt TKI assumption, even if it is safe to do so. Furthermore, our results showed that there is no difference in willingness to accept to interrupt the therapy between patients who actually were in deep molecular response (ie, patients who could be really asked to face such a decision) and those who were not.
This study also highlighted that attitude toward risk is a possible predictor for the willingness to accept TKI assumption. More specifically, patients who were more likely to accept exhibited a greater tendency toward risk behaviours. In particular, they showed higher levels of stimulating risk-taking, which reflects the tendency to accept risks because of the emotional excitement linked to risk behaviours, focusing mostly on possible gains in the short-term than on long-term consequences and the magnitude of possible losses. This result is consistent with findings according to which individuals who report higher levels of stimulating risk-taking tend to engage in activities connected with health risks.30
Not only did the stimulating risk-taking predict the willingness to interrupt TKIs, it also predicted the percentage of relapse risk regarded as acceptable by patients, along with resistance to change, having previously desired to therapy, and the stage of the disease. In particular, patients who reported a higher percentage of relapse risk as acceptable presented (a) higher levels of stimulating risk-taking, (b) lower levels of resistance to change (ie, the tendency to consider changes as aversive), (c) having previously desired to interrupt the therapy, and (d) MMR, MR4, MR4.5 or MR5 as current disease stage. However, it is to note that the latter result is not conclusive, since only a few patients (n = 7) reported MCyR, CCyR or MR5 as current disease stage. Multivariable analysis showed that these four variables (stimulating risk-taking, resistance to change, having previously desired to stop the therapy, and disease stage) account for 32.2% of the probability of CML relapse risk accepted by patients overall.
No association between psychological characteristics as passive risk-taking and need for cognitive closure had been found with our research outcomes. It would seem that neither inaction (even if it results in an undesirable outcome) nor the motivation to resolve ambiguous situations could predict whether or not an individual would be willing to interrupt the therapy or the probability of relapse risk they would accept. The results of cluster analysis might represent a pivotal aspect of communication that needs to be addressed to improve informed decisions in patients. Cluster 1 (n = 51) was characterised by patients with a higher tolerance to ambiguity, meaning they tend to have an individual’s disposition to better tolerate life’s changes. Cluster 2 (n = 68) was characterised by patients that tend to have higher resistance to change and higher cognitive closure (ie, the preference to find an answer to an ambiguous situation, as compared to confusion and uncertainty). This group of patients seems to be less tolerant to ambiguity. Particularly, patients in Cluster 1 were more worried about the possibility to lose contact and miss routine assessments in their health centres and reported more concerns related to family worries than patients in Cluster 2.
In regard to the main concerns expressed by all patients (ie, possible relapses and fear of not responding again to treatment in the event of relapses), these factors should be considered when communicating information regarding the discontinuation of TKIs to patients. In fact, the literature shows that a directive approach usually entails more reluctance on the part of patients,36 whereas empathy and active listening, even with respect to possible concerns, is more successful in terms of compliance with medical suggestions.37,38
A limitation of this study is the narrow sample size. Because of the small number of patients who reported MCyR, CCyR, or MR5 as current disease stage, definitive conclusions cannot be drawn regarding the effect of the disease stage on the percentage of relapse risk that can be considered as acceptable in order to interrupt the treatment. In future studies, other variables should be added to the scenario presented to patients, such as pros and cons for both therapy and therapy discontinuation, in order to assess the different impact each information has on the willingness to interrupt the therapy and on the percentage of relapse risk referred as acceptable to stop TKIs. Finally, it would be interesting to investigate other psychological variables that might be linked to both outcomes, so that a more patient-centred approach could be developed for treatment.
Conclusion
TKI discontinuation appears appealing and challenging at the same time for many CML patients, and different factors may play a role in taking such a decision. Many studies have already investigated several factors that impact on both the decision-making process and its outcome, but none of them took into account the psychological characteristics of patients. This study reveals that the main psychological factors that could contribute to affect the decision to accept to interrupt TKIs when suggested are higher levels of stimulating risk-taking, lower levels of resistance to change, and having already desired to interrupt the therapy. Globally, this study might assist in the identification of specific profiles and tailored treatment options for individual patients, in line with the current recommendation of personalised medicine.
Disclosure
Conflict of interest: Dr Chiara Elena reports advisory board from Gilead, advisory board from Blueprint, outside the submitted work. None of the authors has any other conflicts of interest related to this study.
References
1. Tefferi A. Classification, diagnosis and management of myeloproliferative disorders in the JAK2 V617F Era. ASH Educ Program Book. 2006;1:240–245.
2. Jabbour E, Cortes JE, Giles FJ, O’Brien S, Kantarjian HM. Current and emerging treatment options in chronic myeloid leukemia. Cancer. 2007;109(11):2171–2181. doi:10.1002/cncr.22661
3. Kimura S, Ashihara E, Maekawa T. New tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Curr Pharm Biotechnol. 2006;7(5):371–379. doi:10.2174/138920106778521532
4. Vener C, Banzi R, Ambrogi F, et al. First-line imatinib vs second-and third-generation TKIs for chronic-phase CML: a systematic review and meta-analysis. Blood Adv. 2020;4(12):2723–2735. doi:10.1182/bloodadvances.2019001329
5. Ross DM, Branford S, Seymour JF, et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia. 2010;24(10):1719–1724. doi:10.1038/leu.2010.185
6. Hochhaus A, Baccarani M, Silver RT, et al. European Leukemia Net 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966–984. doi:10.1038/s41375-020-0776-2
7. Radich JP, Deininger M, Abboud CN, et al. Chronic myeloid leukemia, version 1.2019, NCCN clinical practice guidelines in oncology. J National Comprehensive Cancer Network. 2018;16(9):1108–1135. doi:10.6004/jnccn.2018.0071
8. Guastafierro S, Falcone U, Celentano M, et al. Is it possible to discontinue imatinib mesylate therapy in chronic myeloid leukemia patients with undetectable BCR/ABL? A case report and a review of the literature. Leukemia Res. 2009;33:1079–1108. doi:10.1016/j.leukres.2008.11.027
9. Mahon FX, Fort MP, Etienne G, et al. Interferon alpha alone is able to cure chronic myeloid leukemia in a small subset of patients despite the persistence of leukemic cells: experience of long follow up after treatment discontinuation. Blood. 2010;116:2299. doi:10.1182/blood.V116.21.2299.2299
10. Mahon FX, Réa D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–1035. doi:10.1016/S1470-2045(10)70233-3
11. Etienne G, Guilhot J, Rea D, et al. Long-term follow-up of the French stop imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35:298–305. doi:10.1200/JCO.2016.68.2914
12. Ross DM, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122:515–522. doi:10.1182/blood-2013-02-483750
13. Campiotti L. Imatinib discontinuation in chronic myeloid leukaemia patients with undetectable BCR-ABL transcript level: a systematic review and a meta-analysis. Eur J Cancer. 2017;77:48–56. doi:10.1016/j.ejca.2017.02.028
14. Saussele S, Richter J, Guilhot J, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19:747–757. doi:10.1016/S1470-2045(18)30192-X
15. Takahashi N, Tauchi T, Kitamura K, Miyamura K, Saburi Y, Hatta Y; Japan Adult Leukemia Study Group. Deeper molecular response is a predictive factor for treatment-free remission after imatinib discontinuation in patients with chronic phase chronic myeloid leukemia: the JALSG-STIM213 study. Int J Hematol. 2018;107(2):185–193. doi:10.1007/s12185-017-2334-x
16. Okada M, Imagawa J, Tanaka H, et al. Final 3-year results of the dasatinib discontinuation trial in patients with chronic myeloid leukemia who received dasatinib as a second-line treatment. Clin Lymphoma Myeloma Leuk. 2018;18(5):353–360. doi:10.1016/j.clml.2018.03.004
17. Legros L, Nicolini FE, Etienne G, Rousselot P, Rea D, Giraudier S; French Intergroup for Chronic Myeloid Leukemias. Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer. 2017;123(22):4403–4410. doi:10.1002/cncr.30885
18. Cornelison M, Jabbour EJ, Welch MA. Managing side effects of tyrosine kinase inhibitor therapy to optimize adherence in patients with chronic myeloid leukemia: the role of the midlevel practitioner. J Supportive Oncol. 2012;10(1):14–24. doi:10.1016/j.suponc.2011.08.001
19. Cortes J, Goldman MG, Hughes T. Current issues in chronic myeloid leukemia: monitoring, resistance, and functional cure. J National Comprehensive Cancer Network. 2012;10:S13. doi:10.6004/jnccn.2012.0184
20. Sweet K, Oehler V. Discontinuation of tyrosine kinase inhibitors in chronic myeloid leukemia: when is this a safe option to consider? Hematology 2013. Am Soc Hematol Educ Program Book. 2013;1:184–188. doi:10.1182/asheducation-2013.1.184
21. Boquimpani CM, Szczudlo T, Mendelson E, et al. Attitudes and perceptions of patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) toward treatment-free remission (TFR). Blood. 2014;124(21):4547. doi:10.1182/blood.V124.21.4547.4547
22. Sanford D, Kyle R, Lazo–Langner A, et al. Patient preferences for stopping tyrosine kinase inhibitors in chronic myeloid leukemia. Curr Oncol. 2014;21(2):241–249. doi:10.3747/co.21.1736
23. Goldberg S, Hamarman S. Patients with chronic myelogenous leukemia may not want to discontinue tyrosine kinase inhibitor therapy. Blood. 2015;126(23):1584. doi:10.1182/blood.V126.23.1584.1584
24. Jiang Q, Liu ZC, Zhang SX, et al. Young age and high cost are associated with future preference for stopping tyrosine kinase inhibitor therapy in Chinese with chronic myeloid leukemia. J Cancer Res Clin Oncol. 2016;142(7):1539–1547. doi:10.1007/s00432-016-2159-7
25. Lou J, Huang J, Wang Z, et al. Chronic myeloid leukemia patients and treatment-free remission attitudes: a multicenter survey. Patient Prefer Adherence. 2018;12:1025–1032. doi:10.2147/PPA.S163393
26. Villemagne Sanchez LA, O’Callaghan C, Gough K, et al. Patient perceptions of treatment-free remission in chronic myeloid leukemia. Leuk Lymphoma. 2018;59(2):406–415. doi:10.1080/10428194.2017.1337114
27. Flynn KE, Myers JM, D’Souza A, Schiffer CA, Thompson JE, Atallah E. Exploring Patient Decision Making Regarding Discontinuation of Tyrosine Kinase Inhibitors for Chronic Myeloid Leukemia. Oncologist. 2019;24(9):1253–1258. doi:10.1634/theoncologist.2018-0831
28. Sharf G, Marin C, Bradley JA, et al. Treatment-free remission in chronic myeloid leukemia: the patient perspective and areas of unmet needs. Leukemia. 2020;34(8):2102–2112. doi:10.1038/s41375-020-0867-0
29. Vashti NMF, Jeroen J, Janssen WM, Westerweel PE, Inge GP. Tyrosine kinase inhibitor treatment discontinuation in chronic myeloid leukemia: patient views. Leuk Lymphoma. 2021;62:649–658. doi:10.1080/10428194.2020.1839655
30. Zaleskiewicz T. Beyond risk seeking and risk aversion: personality and the dual nature of economic risk taking. Eur J Pers. 2001;15(S1):S105–S122. doi:10.1002/per.426
31. Keinan R, Bereby-Meyer Y. “Leaving it to chance”—Passive risk taking in everyday life. Judgm Decis Mak. 2012;7(6):705–715. doi:10.1002/per.426
32. Riva S, Gorini A, Cutica I, Mazzocco K, Pravettoni G. Translation, cross-cultural adaptation, and reliability, of the Italian version of the Passive Risk Taking (PRT) Scale. Judgm Decis Mak. 2015;10(6):597–604.
33. Webster DM, Kruglanski AW. Individual differences in need for cognitive closure. J Pers Soc Psychol. 1994;67(6):1049–1062. doi:10.1037/0022-3514.67.6.1049
34. Pierro A. Caratteristiche strutturali della versione italiana di Bisogno di Chiusura Cognitiva (di Webster & Kruglanski) [Structural characteristics of the Italian version of the Need for Cognitive Closure Scale (by Webster & Kruglanski)]. TPM. 1995;2:125–142.
35. Oreg S. Resistance to change: developing an individual differences measure. J Applied Psychol. 2003;88(4):680–693. doi:10.1037/0021-9010.88.4.680
36. Miller WR, Rollnick S. Motivation Interviewing. Guilford: New York; 1991.
37. Rollnick S, Miller WR, Butler C. Motivational Interviewing in Health Care: Helping Patients Change Behavior. Guilford Press; 2008.
38. Copes A. Aderenza e comportamenti autoprotettivi [Adherence and self-protective behaviour]. In: Ricci Bitti PE, Gremigni P, editors. Psicologia Della Salute [Health Psychology]. Roma: Carocci; 2013.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.