Back to Journals » Neuropsychiatric Disease and Treatment » Volume 11
Psychiatric symptoms and leptin in obese patients who were bariatric surgery candidates
Authors Changchien T, Tai C, Huang C, Chien C, Yen Y
Received 7 May 2015
Accepted for publication 9 July 2015
Published 19 August 2015 Volume 2015:11 Pages 2153—2158
DOI https://doi.org/10.2147/NDT.S88075
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Wai Kwong Tang
Te-Chang Changchien,1 Chi-Ming Tai,2 Chih-Kun Huang,3 Chia-Chang Chien,1 Yung-Chieh Yen1,4
1Department of Psychiatry, E-Da Hospital, 2Department of Internal Medicine, E-Da Hospital, 3Bariatric and Metabolic International Surgery Center, E-Da Hospital, 4School of Medicine, I-Shou University, Kaohsiung, Taiwan
Objective: There is a significant relationship between obesity and common mental symptoms (depression and anxiety symptoms). But the association between depression (or anxiety symptoms) and serum leptin is still unclear and controversial, despite the growing body of evidence supporting the existence of “leptin resistance” in obese persons. So we investigated whether common mental symptoms, obesity, and the interactive effect of these two factors have a relationship with leptin in obese patients who were candidates for bariatric surgery.
Methods: In all, 139 participants (mean age: 31.4 years, standard deviation: 9.3 years, 73.4% female) were enrolled at an obesity treatment center in southern Taiwan. Serum leptin levels and body mass index (BMI) were measured. The Chinese Health Questionnaire and Taiwanese Depression Questionnaire were administered.
Results: The mean BMI of our participants was 39.4 kg/m2 (±6.8), and the mean leptin level was 24.5 ng/mL (±9.4). In the multivariate regression models, Chinese Health Questionnaire-by-BMI and Taiwanese Depression Questionnaire-by-BMI interaction terms remained significant predictors of leptin level (β=0.16, P<0.0001; β=0.04, P<0.0001, respectively), after adjustment for age, sex, and history of hypertension, diabetes, and hyperlipidemia, despite the inverse correlation between Chinese Health Questionnaire (or Taiwanese Depression Questionnaire) and leptin. In addition, female patients had significantly higher leptin levels than male patients.
Conclusion: The present findings confirmed that the relationship between common mental symptoms and leptin is modulated by obesity in severely obese patients. Future studies should focus on further measures of leptin receptors or signaling on the basis of these interactive effects in psychiatry.
Keywords: leptin, depression, anxiety, common mental disorder, obesity
Background
Depressive disorder is a prevalent mental disorder worldwide and is also a huge burden on public health globally.1 It is a complex and heterogeneous disorder and has diverse symptomatologies and psychopathologies. Common mental disorder is a diagnostic entity for nonpsychotic, depressive, and anxiety disorders. It leads to substantial morbidity worldwide, and its prevalence is increasing.2 Body mass index (BMI) is a simple index of weight-for-height (kilograms per meter squared) that is commonly used to classify underweight, overweight, and obesity. Obesity is defined by the WHO as BMI >30 kg/m2. Obesity leads to several chronic diseases such as hypertension, diabetes, hyperlipidemia, cardiovascular events, and stroke. Several epidemiological and clinical studies have suggested a link between obesity and psychiatric comorbidity.3 Obesity is positively associated with several mental disorders, especially mood and anxiety disorders.4 Adipose tissue secretes large amounts of proinflammatory cytokines. Hypercytokinemia, from a chronic inflammatory state of obesity, is frequently associated with depressive symptoms.5 At the same time, leptin may have its own role in the association between obesity and common mental disorders.
Leptin is an adipocyte hormone and is transported across the blood–brain barrier to exert its central effects. Leptin regulates human energy homeostasis. Its secretion is proportionate to the fat stores of the body; starvation or food deprivation reduces leptin levels.6 Other regulators of circulating leptin levels include sex, age, and food intake. In addition to the most common role of leptin in metabolism, its receptors are widely distributed in brain areas related to emotional responses, such as the hippocampus. However, the neurobiological mechanisms of leptin and their role in the regulation of moods are still unknown.7 In an animal study, leptin was dysregulated in chronic unpredictable stress models and chronic social defeat models, which were suggested to meet the criteria for the validity of animal models of symptoms of depression. Leptin treatment overcame behavioral deficits in the forced swim test.8 It could be speculated that leptin has a potential antidepressant efficacy. However, in obese humans, chronic overeating results in a tendency to develop leptin resistance, despite the high leptin levels observed. Clinical data on the association between leptin and depression are limited and controversial, even in obese patients.
It has been hypothesized that leptin resistance blunts its central action, despite the high levels of leptin in obese persons. In other words, it is not the absolute serum leptin concentration but its dysfunction in the central nervous system that may contribute to a causal relationship with depression.7 However, the high leptin levels in obese persons are still considered an indication of leptin resistance in clinical observation. Two previous studies reported an association between leptin and depressive symptoms that was modulated by abdominal adiposity in older community-dwelling persons.9,10 A key question to be addressed is whether the leptin hypothesis can serve as a common biological factor for comorbidity of obesity and common psychiatric symptoms. Therefore, we aimed to explore the association among leptin levels, adiposity, psychiatric symptoms, and their interaction in bariatric surgery candidates.
Methods
Subjects
Most of our subjects were patients with morbid obesity. Forty-six of the patients who were overweight or obese (BMI <35 kg/m2) had obesity-related health problems, such as diabetes, hypertension, gastroesophageal reflux disease, or metabolic syndrome. Our multidisciplinary team (including dietitians, endocrinologists, gastroenterologists, psychiatrists, and surgeons) surveyed and evaluated these patients who were unable to reduce body weight through diet control, behavioral modification, or pharmacological therapy, and determined they would be candidates for bariatric surgery at E-Da Hospital, Kaohsiung, Taiwan. From November 2006 to February 2009, a total of 139 severely obese bariatric surgery candidates were enrolled in our cross-sectional study. Patients with psychotic or eating disorders were excluded. All participants provided written informed consent. The study design was approved by the institutional review board of E-Da Hospital (Kaohsiung, Taiwan).
Serum leptin level
The leptin level is not stable throughout the day and has no clear diurnal pattern.6 In order to avoid the effect of food intake, fasting venous blood was withdrawn in the morning with no consumption of food overnight. Venous blood sampling was performed first, and then the serum was frozen at −80°C for later measurement of leptin. The serum leptin concentration was measured using a commercially available radioimmunoassay kit.
Screening tools for common psychiatric symptoms
Chinese Health Questionnaire
The Chinese Health Questionnaire (CHQ) is a 12-question, 2-reverse questions, 0–1-point questionnaire used to screen somatic and psychic anxiety symptoms, social dysfunction, self-confidence, and hope, all suggesting the nonpsychotic, depressive, and anxiety symptoms of common mental disorders. The questionnaire was derived from the General Health Questionnaire, with the addition of specially designed, culturally relevant items.11 For minor psychiatric morbidities, the questionnaire had sensitivity and specificity of 70% and 95%, respectively, in a community study.12 Cronbach’s α of 0.84 and internal consistency of 0.79 were demonstrated for the CHQ.13 The cutoff point in Taiwan community surveys is ≥5 points.
Taiwanese Depression Questionnaire
The Taiwanese Depression Questionnaire (TDQ) is an 18-question, 0–3-point questionnaire used for screening clinically depressive symptoms. The questionnaire is a culturally specific depression self-rating instrument for use in Taiwan.14 The TDQ has a Cronbach’s α of 0.90 and sensitivity and specificity of 0.89 and 0.92, respectively. The cutoff point in the Taiwan community population is ≥19 points.
Statistical analysis
Descriptive results regarding continuous variables were presented as the mean (standard deviation [SD]), and categorical variables were given as count and percentages. We also categorized the TDQ score by severity (no to minimal, mild, moderate to severe) to conduct an analysis to compare the leptin levels among each subgroup. All factors (age, sex, BMI, history of hypertension, diabetes, hyperlipidemia, CHQ score, and TDQ score) were included in five multiple linear regression models to identify abilities to independently predict leptin levels. In model 1, we included sex, age, BMI, history of hypertension, diabetes, and hyperlipidemia as independent variables. The CHQ score was added to the above six variables in model 2, and the TDQ score was added to the same six variables in model 4. In addition, we tested the interactive effect between the CHQ (or TDQ) score and BMI by standardizing the two and multiplying them with each other, and then used them as a new variable. So, in linear model 3 and model 5, the terms CHQ × BMI and TDQ × BMI were introduced, respectively, in addition to the preserved independent variables (sex, age, history of hypertension, diabetes, and hyperlipidemia, CHQ or TDQ score). All analyses were performed with SPSS 19.0 version for Windows (StataCorp LP, College Station, TX, USA). The study was approved by the Institutional Review Board of E-Da Hospital.
Results
Demographic and clinical characteristics
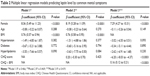
With regard to demographic and clinical characteristics, the female-to-male ratio of the obese patients was approximately 3:1, the average age was 31.4 years (±9.3), and BMI ranged from 26.3 kg/m2 to 67.5 kg/m2, with a mean value of 39.4 kg/m2 (±6.8). The mean CHQ and TDQ scores were 4.1 and 14.7, respectively. The leptin level ranged from 8 ng/mL to 56 ng/mL with a mean level of 24.5 ng/mL (±9.4). Approximately 25% of patients recruited had a medical history of hypertension and 10% had diabetes mellitus and hyperlipidemia. Women had more common mental symptoms and higher plasma leptin levels. Men had greater BMI and more history of hypertension (Table 1). There was no significant difference in leptin levels among the three subgroups by severity of depression (Figure 1).
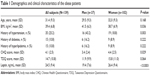  | Table 1 Demographics and clinical characteristics of the obese patients |
Multivariate linear regression models
In model 1, female sex (β coefficient =8.36, P<0.0001) and BMI (β coefficient =0.76, P<0.0001) were significantly associated with leptin level. In model 2, female sex and BMI were statistically significant (β coefficient =8.19, P<0.0001; β coefficient =0.76, P<0.0001, respectively) but not the CHQ score. In model 3, the CHQ-by-BMI interaction term (CHQ × BMI) was significantly associated with leptin (β coefficient =0.16, P<0.0001), indicating that CHQ-by-BMI interaction was detected. However, the CHQ score itself was inversely correlated to leptin (β coefficient =−6.20, P<0.0001; Table 2). The R2 value was 0.419. Based on the these findings, a positive interactive effect between CHQ score and BMI on leptin level was confirmed. In model 4, female sex and BMI were still significant predictors of leptin level (β coefficient =8.27, P<0.0001; β coefficient =0.76, P<0.0001, respectively). In model 5, the TDQ-by-BMI interaction term (TDQ × BMI) and female sex were significant predictors of leptin level (β coefficient =0.04, P<0.0001; β coefficient =7.44, P<0.0001, respectively), whereas the TDQ score was negatively correlated (β coefficient =−1.40, P<0.0001). The R2 value was 0.397 (Table 3). This model also confirmed that BMI significantly moderates the relationship between the TDQ score and the leptin level.
Discussion
In this study, our main findings included that obesity and the depression (or anxiety symptoms)-by-obesity interaction were both significantly associated with the leptin level, after adjustment for age, sex, and history of hypertension, diabetes, and hyperlipidemia, despite the inverse correlation between CHQ (or TDQ) and leptin. There are few studies that have focused on the prevalence of psychiatric disorders in Asian patients who seek obesity treatment. Lin et al explored the high prevalence of psychiatric disorders, especially mood disorders and anxiety disorders, in this population.15 The present study, to our knowledge, is the first to investigate the possible simultaneous influence and relationship among obesity, common psychiatric symptoms (depression and anxiety symptoms), and serum leptin level in ethnic Chinese morbidly obese patients. Some previous studies confirmed the significant association between leptin and depressive symptoms in an overlapping population (patients with metabolic syndrome and with type 2 diabetes),16,17 but the present study further focused on severely obese patients who were candidates for bariatric surgery. Milaneschi et al reported that the association between leptin and depressive symptoms was modulated by abdominal adiposity.10 But they used leptin-by-obesity as a predictive variable and depressive symptoms as a dependent variable instead. Leptin level, rather than depression, was used as a dependent variable in our study due to the presence of more confounders in predicting depression, such as other endocrine systems (estrogen, hypothalamus–pituitary–adrenal axis), and psychosocial factors that need to be controlled. Morris et al reported depression was a significant predictor of leptin levels, but further adjustment for BMI eliminated the association between them.18 This means the association between depression and leptin varies based on obesity status. Their study also used leptin levels as dependent variables and showed that overall severity of depression (by the Beck Depression Inventory II) was positively correlated with leptin levels.
The sex-specificity bias toward females, compared with males, showed higher levels of leptin in our study, consistent with the established literature.17,19,20 Even the finding that healthy women have higher leptin levels than men has been confirmed consistently.21 The reason for the sex difference needs to be elucidated. However, obese women usually have more self-distress from the stigma of obesity (especially in Asia, where the criteria for overweight/obesity is stricter and lower than in the Western world), more dissatisfaction with their body image, and more mood disorders and eating problems.15 So they should be afforded more attention and intervention in obesity treatment.
A few studies found that leptin levels were higher in patients with depression,22–24 with a bias toward females, even in premenopausal women.25 However, other studies reported that low leptin levels were associated with depression.26–28 Moreover, decreased leptin levels were observed in female patients with postpartum depression29 and in reproductive-age female populations.30 A lower cerebrospinal fluid leptin level was also found in suicide attempters with depression.31 In our study, the TDQ score was inversely associated with the leptin level, which meant that the negative relationship between severity of depressive symptoms and leptin was compatible with the role of leptin insufficiency in depression (or leptin might be a novel antidepressant). But in these subjects with greater BMI, depression was associated with leptin levels after adding an interactive effect. That is to say, the result of an interactive effect between depression and obesity in predicting leptin levels was consistent with the hypothesis of leptin resistance in depression. Leptin is ineffective in inhibiting food intake and increasing energy expenditure in obese persons but leads to a reduction in adipose tissue and weight loss in persons with normal weight.32 This suggests that leptin resistance in obese persons also blunts this hormone’s potential antidepressant efficacy. So we could expect that these obese patients with depression would have a better chance to respond to the leptin treatment after the obesity treatment. The underlying nature of leptin insufficiency versus leptin resistance in depression deserves further clinical research.
Data on the association between leptin and anxiety symptoms are relatively lacking. We reported a negative correlation between common mental symptoms (including anxiety symptoms) and leptin in our study. But another interactive effect between common mental symptoms and obesity also significantly predicted leptin levels. Two recent studies reported partially similar findings. Yoshida-Komiya et al reported that 29 reproductive-age women with mild depressive and anxious states (including five overweight subjects) had lower levels of leptin than healthy volunteers.30 Our study enrolled a higher total number of participants and number of obese participants than the aforementioned study. Lawson et al recruited 64 women and reported an inverse relationship between leptin and anxiety symptoms, but most Hamilton Rating Scale for Anxiety scores in the study were within the normal range.28 Our study used the CHQ as a localized and validated screening tool to present the distribution of scores.
There are some limitations in our study. First, this was a cross-sectional investigation and therefore, causality cannot be determined. Second, depressive and anxiety symptoms were obtained from self-reported questionnaires, with a lack of further clinical evaluation and diagnosis by mental health professionals. There perhaps was a possibility that the obese patients would conceal their true underlying psychopathologies and try not to let mental problems affect their optimal obesity treatment. Third, our study did not include the effect of the hypothalamus–pituitary–adrenal axis on obesity and depression or the mutual interaction between leptin and cortisol.33,34 Finally, as we mentioned earlier, leptin resistance may play an important role in the leptin hypothesis, and the interactive effect of leptin level and BMI may further indicate the severity of leptin resistance. We still need more structured and longitudinal study designs and analyses to clarify this interaction in association with psychiatric symptoms.
Conclusion
In conclusion, some of our findings have duplicated those of previous studies and have shown the importance of interactive effects among depression, anxiety, and obesity relative to leptin in psychoendocrinology, even in obese patients. Future studies should focus on further measures of leptin receptors or signaling on the basis of these interactive effects in psychiatry.
Acknowledgment
This study was supported by grants from E-Da Hospital, Taiwan (grant numbers EDAHI102004 and EDAHI103004). The funding sources had no involvement in the study design; the collection, analysis, or interpretation of the data; the writing of the report; or the submission of the paper for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. | ||
Fu TS, Lee CS, Gunnell D, Lee WC, Cheng AT. Changing trends in the prevalence of common mental disorders in Taiwan: a 20-year repeated cross-sectional survey. Lancet. 2013;381(9862):235–241. | ||
Simon GE, Von Korff M, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63(7):824–830. | ||
McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB. Are mood disorders and obesity related? A review for the mental health professional. J Clin Psychiatry. 2004;65(5):634–651. [quiz 730]. | ||
Elenkov IJ. Neurohormonal-cytokine interactions: implications for inflammation, common human diseases and well-being. Neurochem Int. 2008;52(1–2):40–51. | ||
Michalakis K, le Roux C. Gut hormones and leptin: impact on energy control and changes after bariatric surgery – what the future holds. Obes Surg. 2012;22(10):1648–1657. | ||
Lu XY. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Curr Opin Pharmacol. 2007;7(6):648–652. | ||
Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci U S A. 2006;103(5):1593–1598. | ||
Milaneschi Y, Simonsick EM, Vogelzangs N, et al; Health, Aging, and Body Composition Study. Leptin, abdominal obesity, and onset of depression in older men and women. J Clin Psychiatry. 2012;73(9):1205–1211. | ||
Milaneschi Y, Sutin AR, Terracciano A, et al. The association between leptin and depressive symptoms is modulated by abdominal adiposity. Psychoneuroendocrinology. 2014;42:1–10. | ||
Cheng TA, Williams P. The design and development of a screening questionnaire (CHQ) for use in community studies of mental disorders in Taiwan. Psychol Med. 1986;16(2):415–422. | ||
Cheng TA. A community study of minor psychiatric morbidity in Taiwan. Psychol Med. 1988;18(4):953–968. | ||
Cheng TA, Wu JT, Chong MY, Williams P. Internal consistency and factor structure of the Chinese Health Questionnaire. Acta Psychiatr Scand. 1990;82(4):304–308. | ||
Lee Y, Yang MJ, Lai TJ, Chiu NM, Chau TT. Development of the Taiwanese Depression Questionnaire. Chang Gung Med J. 2000;23(11):688–694. | ||
Lin HY, Huang CK, Tai CM, et al. Psychiatric disorders of patients seeking obesity treatment. BMC Psychiatry. 2013;13:1. | ||
Chirinos DA, Goldberg R, Gellman M, et al. Leptin and its association with somatic depressive symptoms in patients with the metabolic syndrome. Ann Behav Med. 2013;46(1):31–39. | ||
Labad J, Price JF, Strachan MW, et al; Edinburgh Type 2 Diabetes Study Investigators. Leptin levels and depressive symptoms in people with type 2 diabetes: the edinburgh type 2 diabetes study. Psychosom Med. 2012;74(1):39–45. | ||
Morris AA, Ahmed Y, Stoyanova N, et al. The association between depression and leptin is mediated by adiposity. Psychosom Med. 2012;74(5):483–488. | ||
Esel E, Ozsoy S, Tutus A, et al. Effects of antidepressant treatment and of gender on serum leptin levels in patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(4):565–570. | ||
Yang K, Xie G, Zhang Z, et al. Levels of serum interleukin (IL)-6, IL-1beta, tumour necrosis factor-alpha and leptin and their correlation in depression. Aust N Z J Psychiatry. 2007;41(3):266–273. | ||
Licinio J, Negrão AB, Mantzoros C, et al. Sex differences in circulating human leptin pulse amplitude: clinical implications. J Clin Endocrinol Metab. 1998;83(11):4140–4147. | ||
Antonijevic IA, Murck H, Frieboes RM, Horn R, Brabant G, Steiger A. Elevated nocturnal profiles of serum leptin in patients with depression. J Psychiatr Res. 1998;32(6):403–410. | ||
Pasco JA, Jacka FN, Williams LJ, et al. Leptin in depressed women: cross-sectional and longitudinal data from an epidemiologic study. J Affect Disord. 2008;107(1–3):221–225. | ||
Rubin RT, Rhodes ME, Czambel RK. Sexual diergism of baseline plasma leptin and leptin suppression by arginine vasopressin in major depressives and matched controls. Psychiatry Res. 2002;113(3):255–268. | ||
Cizza G, Nguyen VT, Eskandari F, et al; POWER Study Group. Low 24-hour adiponectin and high nocturnal leptin concentrations in a case-control study of community-dwelling premenopausal women with major depressive disorder: the Premenopausal, Osteopenia/Osteoporosis, Women, Alendronate, Depression (POWER) study. J Clin Psychiatry. 2010;71(8):1079–1087. | ||
Jow GM, Yang TT, Chen CL. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J Affect Disord. 2006;90(1):21–27. | ||
Kraus T, Haack M, Schuld A, Hinze-Selch D, Pollmacher T. Low leptin levels but normal body mass indices in patients with depression or schizophrenia. Neuroendocrinology. 2001;73(4):243–247. | ||
Lawson EA, Miller KK, Blum JI, et al. Leptin levels are associated with decreased depressive symptoms in women across the weight spectrum, independent of body fat. Clin Endocrinol. 2012;76(4):520–525. | ||
Skalkidou A, Sylven SM, Papadopoulos FC, Olovsson M, Larsson A, Sundstrom-Poromaa I. Risk of postpartum depression in association with serum leptin and interleukin-6 levels at delivery: a nested case-control study within the UPPSAT cohort. Psychoneuroendocrinology. 2009;34(9):1329–1337. | ||
Yoshida-Komiya H, Takano K, Fujimori K, Niwa SI. Plasma levels of leptin in reproductive-aged women with mild depressive and anxious states. Psychiatry Clin Neurosci. 2014;68(7):574–581. | ||
Westling S, Ahren B, Traskman-Bendz L, Westrin A. Low CSF leptin in female suicide attempters with major depression. J Affect Disord. 2004;81(1):41–48. | ||
Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282(16):1568–1575. | ||
Himmerich H, Zimmermann P, Ising M, et al. Changes in the hypothalamic-pituitary-adrenal axis and leptin levels during antidepressant treatment. Neuropsychobiology. 2007;55(1):28–35. | ||
Malendowicz LK, Rucinski M, Belloni AS, Ziolkowska A, Nussdorfer GG. Leptin and the regulation of the hypothalamic-pituitary-adrenal axis. Int Rev Cytol. 2007;263:63–102. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.