Back to Journals » Psoriasis: Targets and Therapy » Volume 11
Psoriasis and Cardiometabolic Diseases: The Impact of Inflammation on Vascular Health
Authors Teklu M, Parel PM , Mehta NN
Received 13 May 2021
Accepted for publication 6 July 2021
Published 21 July 2021 Volume 2021:11 Pages 99—108
DOI https://doi.org/10.2147/PTT.S320016
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Uwe Wollina
Meron Teklu, Philip M Parel, Nehal N Mehta
National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA
Correspondence: Nehal N Mehta
Lasker Senior Investigator, Chief, Section of Inflammation and Cardiometabolic Diseases, National Heart, Lung and Blood Institute, National Institutes of Health, 10 Center Drive, Clinical Research Center, Room 5-5140, Bethesda, MD, 20892, USA
Tel +1-301-827-0483
Fax +1-301-827-0915
Email [email protected]
Abstract: Psoriasis is a common chronic inflammatory condition associated with a higher risk of cardiovascular disease. Psoriasis confers a dose-dependent increase in risk for the metabolic syndrome and its components. The metabolic syndrome and its components have been associated with higher coronary atherosclerosis in psoriasis and cardiovascular events in the general population. In this review, we discuss the role of inflammation and psoriasis in cardiometabolic diseases with a focus on the metabolic syndrome and its components. We highlight the relationship between psoriasis and important cardiovascular risk factors encompassed by obesity, dyslipidemia, insulin resistance and hypertension. Furthermore, we briefly highlight literature on anti-inflammatory therapies and their impact on the components of the metabolic syndrome as well as directly quantified coronary atherosclerosis burden.
Keywords: psoriasis, inflammation, metabolic syndrome, atherosclerosis
Inflammation and Atherosclerosis
Inflammation plays a critical role from initiation to progression to rupture of atherosclerotic plaques.1 The healthy endothelium is integral to the normal function of the vessel. It serves a homeostatic role by acting as a barrier, producing pro- or anti-thrombotic molecules and modulating between vasoconstrictive and vasodilatory states.2 The initiating event of atherosclerosis is believed to be injury to this vital structure by physical disruptions like sheer stress or metabolic abnormalities like hyperlipidemia.3 This disruption triggers a localized inflammatory response that alters the protective role of the endothelium and leads to well-known events of atherosclerosis including trapping and oxidation of lipoproteins within the vessel wall, further localized inflammation and formation of an atherosclerotic plaque.4 The progression and stability of these plaques depends on the balance between pro- and anti-inflammatory forces.5,6 Beyond its localized role, inflammation is intricately linked to risk factors for atherosclerosis.7–12 While states such as hyperlipidemia, hypertension and obesity themselves promote inflammation, they are also increased in states of chronic inflammation.13
Psoriasis as a Human Model for Inflammation
Psoriasis is a chronic inflammatory dermatologic condition affecting 1–9% of the adult population depending on geographical location.14 In addition to the localized skin inflammation caused by the scaling plaques for which this disease is well known,15 psoriasis also leads to systemic,16,17 and especially vascular,18 inflammation. Psoriasis is associated with a higher risk of cardiometabolic diseases, including a higher risk of myocardial infarctions compared to the general population.19 Interestingly, the biggest difference in risk is seen in younger patients with severe disease defined as use of systemic therapy.19 This adds to other bodies of literature in supporting the hypothesis that not only is psoriasis associated with a higher risk of cardiovascular disease but that it also accelerates this risk. In psoriasis, skin disease gives a view into vascular health.20 While patients with psoriasis are known to have higher levels of vascular inflammation, the severity of the dermatologic manifestation of psoriasis may also be important to vascular health (Figure 1).18 The psoriasis area and severity index score, a measure of psoriatic skin burden, associates with aortic vascular inflammation,18 which itself has been associated with high-risk coronary atherosclerosis features.21 Psoriasis, especially severe psoriasis, also confers a higher risk of major adverse cardiovascular events.22,23
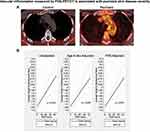 |
Figure 1 Vascular inflammation measured by FDG-PET/CT is associated with psoriasis skin disease severity. (A), Tomographic-fused positron emission tomography (PET) image of the aortic arch from a patient with severe skin disease and a control patient. (B), Regression plots for multivariable regression analysis of vascular inflammation as measured by target-to-background ratio (TBR) with psoriasis area and severity index (PASI) score.Notes: Reproduced with permission from Naik HB, Natarajan B, Stansky E, et al. Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study. Arterioscler Thromb Vasc Biol. 2015;35(12):2667-2676. Copyright © 2015, Wolters Kluwer Health.18 Abbreviations: CI, confidence interval; and FRS, Framingham risk score. |
Psoriasis and the Metabolic Syndrome
The metabolic syndrome is a group of risk factors that is strongly associated with future risk of cardiovascular events.24,25 While there are various criteria for the metabolic syndrome, all include some component of obesity, dyslipidemia, insulin resistance and hypertension (Figure 2).26 Systemic inflammation, as assessed by c-reactive protein levels, has been associated with the metabolic syndrome and its components.9,27–29 In addition, the stratification of participants with metabolic syndrome based on c-reactive protein levels has been shown to have an added prognostic role in predicting future cardiovascular events.30 Patients with psoriasis have a complex interplay with the metabolic syndrome and its individual components. Psoriasis confers an independent dose-dependent risk for the metabolic syndrome and its individual components.31 Furthermore, those with psoriasis and the metabolic syndrome have been shown to have higher coronary subclinical atherosclerosis burden that increases in a stepwise manner with each additional criterion met (Figure 3).32 Below, we will discuss each component of the metabolic syndrome and its role in psoriasis.
 |
Figure 3 Non-calcified coronary burden increases with the number of metabolic syndrome criteria met. Average non-calcified burden by number of metabolic syndrome criteria met. 7 patients met 5 criteria and are combined with those who met 4 criteria.Notes: Reproduced with permission from Teklu M, Zhou W, Kapoor P, et al. Metabolic syndrome and its factors are associated with noncalcified coronary burden in psoriasis: An observational cohort study. J Am Acad Dermatol. 2021;84(5):1329-1338. Copyright 2021, with permission from Elsevier.32 |
Psoriasis and Obesity
Patients with psoriasis are known to have higher measures of both total adiposity and specific fat depots such as visceral, hepatic and epicardial adipose tissue.13,33–35 The directionality of the relationship between psoriasis and obesity is complicated with some studies suggesting obesity as the risk factor for psoriasis36–38 and others suggesting psoriasis as the culprit.39–41 Chronic inflammation may be a risk factor for obesity, but obesity itself is a state of low-grade chronic inflammation.42,43 Psoriasis and obesity share common inflammatory pathways.44–46 Furthermore, the psychosocial impact of living with psoriasis, including but not limited to higher rates of depression,47 anxiety, and stress48 can contribute to body fat distributions with an unfavorable cardiometabolic profile.49 Those with joint involvement may also have decreased access to regular physical activity.50–52 Hence, psoriasis may confer a higher risk of obesity but obesity itself may be a risk factor for psoriasis. For the metabolic syndrome, this is especially important as the component relating to central adiposity may be the initiating factor. Specifically, abdominal visceral adipose tissue, a metabolically active fat depot that has been associated with systemic inflammation in psoriasis,53 may play a critical role in insulin resistance,54 hyperlipidemia55 and hypertension.56 In psoriasis, the waist circumference criterion of the metabolic syndrome has a uniquely strong association with subclinical atherosclerosis independent of traditional cardiovascular risk factors including the other components of the metabolic syndrome.32 Furthermore, there is a strong association between the metabolic syndrome and abdominal visceral adipose tissue volume in psoriasis.32
Psoriasis and Dyslipidemia
Inflammation alters lipid structure and function.57,58 Inflammation can lead to increased production and decreased clearance of triglycerides59–62 as well as alteration of the content and function of high-density lipoprotein cholesterol (HDL).57,58 Psoriasis is associated with dyslipidemia in a possibly dose-dependent manner.63 Patients with psoriasis are known to have higher triglycerides64,65 and lower serum HDL levels63,66 than the general population. Beyond traditional lipid measures, more detailed nuclear magnetic resonance lipid profiling has shown a more atherogenic profile in psoriasis including higher very-low-density lipoprotein concentrations and lower LDL particle size,66 which has been shown to be lower in those with metabolic syndrome.67 In psoriasis, HDL has been shown to contain less apoA-1, phospholipids and cholesterol while containing higher levels of apoA-II and acute phase reactants such as serum amyloid A, prothrombin, and α-1-acid glycoprotein 1.68 Furthermore, HDL cholesterol efflux capacity is impaired in psoriasis and negatively correlates with the psoriasis area and severity index score.68
Psoriasis and Insulin Resistance
Chronic inflammation hinders insulin signaling and promotes insulin resistance. For example, c-Jun NH2-terminal kinase is in part activated by TNF- α and may alter insulin signaling through phosphorylation of insulin receptor substrate 1.69,70 In states of insulin resistance, there are higher levels of inflammatory mediators and markers of systemic inflammation.71–73 Furthermore, systemic inflammation as assessed by c-reactive protein may predict future risk of type 2 diabetes.27,74 Patients with psoriasis have a higher prevalence of type 2 diabetes and elevated blood glucose and insulin compared to controls.75,76 In one study, the psoriasis area and severity index score correlated with hemoglobin A1c and those treated with anti-IL-17A had a significant reduction in hemoglobin A1c alongside an improvement in disease severity, though change in psoriasis severity did not correlate with change in hemoglobin A1c. In the same study, imiquimod was used to induce systemic and cutaneous inflammation with human psoriasis features in mice. These mice initially showed features of insulin resistance and subsequently decreased fasting glucose levels after administration of anti-IL-17A treatment.77 Adiponectin, which has anti-inflammatory, atheroprotective and insulin sensitizing effects,78 is lower in psoriasis79 and may increase with psoriasis-targeted therapy.80 In a study assessing links between adiponectin and psoriasis, adiponectin deficient mice had more severe epidermal hyperplasia and inflammatory cell infiltration and the skin lesions showed higher levels of inflammatory mediators, especially IL-17A. Treatment with exogenous adiponectin improved psoriasis-like skin lesions in these mice.81 These complex pathways once again show an intricate bidirectional relationship between psoriasis and components of the metabolic syndrome.
Psoriasis and Hypertension
From pathogenesis to end organ damage, inflammation plays a key role in hypertension, with preclinical studies showing that T cells may even be essential for the development of hypertension.82,83 Psoriasis and hypertension share important common inflammatory pathways. For example, murine models have shown that IL-17 knockout mice do not sustain an elevation in blood pressure, preserve vascular function and have reduced T cell infiltration of the aorta after infusion with angiotensin II.84 IL-17 may play an essential role in psoriasis, in which increased levels of IL-17 are seen both in the skin85 and the bloodstream.86 IL-17A upregulates keratin 17 expression, which is strongly expressed within psoriatic lesions.87 Similar to obesity, the directionality of the association between hypertension and psoriasis may be complex. While many studies have reported a higher prevalence and risk of hypertension in patients with psoriasis,13,88–90 there is also evidence that hypertension may be a risk factor for psoriasis.91,92 In a large prospective analysis of the Nurses’ Health Study, women with hypertension for six or more years had an increased risk of developing psoriasis compared to normotensive women.92 In psoriasis, the hypertension component of the metabolic syndrome independently associates with noncalcified burden after adjustment for traditional cardiovascular risk factors and after adjustment for other components of the metabolic syndrome,32 demonstrating its unique importance as a cardiovascular risk factor in psoriasis.
Psoriasis Treatment and Cardiometabolic Health
One fascinating aspect of psoriasis as a human model for inflammation and atherosclerosis is that treatment of the skin disease has often been associated with improvement in cardiometabolic profiles.93 Anti-inflammatory psoriasis therapy has been shown to be associated with a reduction in vascular inflammation,94,95 high-risk coronary atherosclerosis burden96,97 and some components of the metabolic syndrome. HDL content and cholesterol efflux capacity improves after anti-psoriatic treatment independent of serum HDL levels.98 Patients with psoriasis on 6 months of anti-TNF-α therapy showed an improvement in insulin sensitivity,99 and there was lower incidence of diabetes in a cohort of patients with psoriasis or rheumatoid arthritis on anti-TNF-α therapy.100 As noted above, IL-17A inhibition may have a role in reducing fasting glucose or hemoglobin A1c in mouse and human models.77 In psoriasis, anti-TNF-α therapy may associate with weight gain101–103 and anti-interleukin 12/23 and anti-interleukin-17 therapy may be neutral.102,104 However, a reduction of visceral adipose tissue concurrent with a reduction in c-reactive protein levels has been demonstrated.53 Further work is needed on fat depot specific effects of psoriasis therapy, especially on visceral adipose tissue. While there is some evidence that biologic therapy may reduce systolic and diastolic blood pressure in patients with inflammatory conditions,105 anti-TNF-α therapy may also be associated with a higher risk of hypertension in rheumatoid arthritis.106 In pre-clinical studies, immune suppression, especially knockout of interleukin-6, has been shown to have a protective effect against the systemic consequences of hypertension such as endothelial and renal damage.107–109 The effect of biologic therapy on blood pressure parameters in psoriasis requires further investigation. Finally, anti-inflammatory psoriasis therapy may also reduce rates of cardiovascular events.110,111
Patients with psoriasis and higher body weight or body mass index may experience lower efficacy of certain biologic therapies.112–115 Given the importance of the adiposity component to the metabolic syndrome, recent work has focused on assessing the efficacy and safety of biologic therapies based on metabolic syndrome status. In a post hoc analysis of two Phase 3 randomized controlled trials (reSURFACE 1 and reSURFACE 2), the percentage of patients with chronic plaque psoriasis treated with tildrakizumab who had a ≥75% improvement in the psoriasis area and severity index score at 12 and 52 weeks was similar by metabolic syndrome status.116 The reduction in psoriasis severity from baseline was also similar in those with and without metabolic syndrome. Furthermore, percentage of patients with ≥1 serious adverse event was similar based on metabolic syndrome status.116 Given work showing the importance of the metabolic syndrome to early coronary atherosclerosis in psoriasis,32 these findings and the potential for anti-psoriatic therapy to address cardiometabolic risk factors portend a positive outlook for the care of patients with metabolic syndrome and psoriasis.
Conclusions
Collectively, these findings highlight the importance of psoriasis as a model for the cardiovascular consequences of chronic inflammation, the need for heightened awareness of these consequences amongst patients and providers, and the value of further study of this disease state to understand the role of inflammation and anti-inflammatory treatment on cardiometabolic health.
Abbreviations
ApoA-II, apolipoprotein A-II; HDL, high-density lipoprotein; IL-17, interleukin 17; LDL, low-density lipoprotein; TNF, tumor necrosis factor.
Disclosure
Dr. Mehta is a full-time US government employee and has received research grants from AbbVie, Janssen, Novartis Corp, and Celgene, outside the submitted work. The other authors have no conflicts of interest in this work.
References
1. Libby P, Ridker PM, Maseri A. Inflammation and Atherosclerosis. Circulation. 2002;105(9):1135–1143. doi:10.1161/hc0902.104353
2. Cahill PA, Redmond EM. Vascular endothelium - Gatekeeper of vessel health. Atherosclerosis. 2016;248:97–109. doi:10.1016/j.atherosclerosis.2016.03.007
3. Davignon J, Ganz P. Role of Endothelial Dysfunction in Atherosclerosis. Circulation. 2004;109(23_suppl_1):
4. Lilly LS. Pathophysiology of Heart Disease: A Collaborative Project of Medical Students and Faculty. Philadelphia: Wolters Kluwer; 2016.
5. Shah PK, Falk E, Badimon JJ, et al. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92(6):1565–1569.
6. Naruko T, Ueda M, Haze K, et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002;106(23):2894–2900. doi:10.1161/01.CIR.0000042674.89762.20
7. McLaughlin T, Liu LF, Lamendola C, et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 2014;34(12):2637–2643. doi:10.1161/ATVBAHA.114.304636
8. Marques-Vidal P, Mazoyer E, Bongard V, et al. Prevalence of insulin resistance syndrome in southwestern France and its relationship with inflammatory and hemostatic markers. Diabetes Care. 2002;25(8):1371–1377. doi:10.2337/diacare.25.8.1371
9. Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19(4):972–978. doi:10.1161/01.ATV.19.4.972
10. Joshi AA, Lerman JB, Aberra TM, et al. GlycA Is a Novel Biomarker of Inflammation and Subclinical Cardiovascular Disease in Psoriasis. Circ Res. 2016;119(11):1242–1253. doi:10.1161/CIRCRESAHA.116.309637
11. Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107(3):391–397. doi:10.1161/01.CIR.0000055014.62083.05
12. Wang Y, Li W, Zhao T, et al. Interleukin-17-Producing CD4(+) T Cells Promote Inflammatory Response and Foster Disease Progression in Hyperlipidemic Patients and Atherosclerotic Mice. Front Cardiovasc Med. 2021;8:667768. doi:10.3389/fcvm.2021.667768
13. Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55(5):829–835. doi:10.1016/j.jaad.2006.08.040
14. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385. doi:10.1038/jid.2012.339
15. Lin WJ, Norris DA, Achziger M, Kotzin BL, Tomkinson B. Oligoclonal expansion of intraepidermal T cells in psoriasis skin lesions. J Invest Dermatol. 2001;117(6):1546–1553. doi:10.1046/j.0022-202x.2001.01548.x
16. Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F. The inflammatory response in mild and in severe psoriasis. Br J Dermatol. 2004;150(5):917–928. doi:10.1111/j.1365-2133.2004.05984.x
17. Sajja AP, Joshi AA, Teague HL, Dey AK, Mehta NN. Potential Immunological Links Between Psoriasis and Cardiovascular Disease. Front Immunol. 2018;9:1234. doi:10.3389/fimmu.2018.01234
18. Naik HB, Natarajan B, Stansky E, et al. Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study. Arterioscler Thromb Vasc Biol. 2015;35(12):2667–2676. doi:10.1161/ATVBAHA.115.306460
19. Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741. doi:10.1001/jama.296.14.1735
20. Harrington CL, Dey AK, Yunus R, Joshi AA, Mehta NN. Psoriasis as a human model of disease to study inflammatory atherogenesis. Am J Physiol Heart Circ Physiol. 2017;312(5):H867–h873. doi:10.1152/ajpheart.00774.2016
21. Joshi AA, Lerman JB, Dey AK, et al. Association Between Aortic Vascular Inflammation and Coronary Artery Plaque Characteristics in Psoriasis. JAMA Cardiol. 2018;3(10):949–956. doi:10.1001/jamacardio.2018.2769
22. Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145(6):700–703. doi:10.1001/archdermatol.2009.94
23. Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31(8):1000–1006. doi:10.1093/eurheartj/ehp567
24. Ninomiya JK, L’Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109(1):42–46. doi:10.1161/01.CIR.0000108926.04022.0C
25. Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–2716. doi:10.1001/jama.288.21.2709
26. Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of Metabolic Syndrome. Circulation. 2004;109(3):433–438. doi:10.1161/01.CIR.0000111245.75752.C6
27. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi:10.1001/jama.286.3.327
28. Freeman DJ, Norrie J, Caslake MJ, et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. 2002;51(5):1596–1600. doi:10.2337/diabetes.51.5.1596
29. Fröhlich M, Imhof A, Berg G, et al. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 2000;23(12):1835–1839. doi:10.2337/diacare.23.12.1835
30. Ridker PM, Buring JE, Cook NR, Rifai N. C-Reactive Protein, the Metabolic Syndrome, and Risk of Incident Cardiovascular Events. Circulation. 2003;107(3):391–397.
31. Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 Pt 1):556–562. doi:10.1038/jid.2011.365
32. Teklu M, Zhou W, Kapoor P, et al. Metabolic syndrome and its factors are associated with noncalcified coronary burden in psoriasis: an observational cohort study. J Am Acad Dermatol. 2021;84(5):1329–1338. doi:10.1016/j.jaad.2020.12.044
33. Balci A, Balci DD, Yonden Z, et al. Increased amount of visceral fat in patients with psoriasis contributes to metabolic syndrome. Dermatology. 2010;220(1):32–37. doi:10.1159/000254482
34. Torres T, Bettencourt N, Mendonça D, et al. Epicardial adipose tissue and coronary artery calcification in psoriasis patients. J Eur Acad Dermatol Venereol. 2015;29(2):270–277. doi:10.1111/jdv.12516
35. van der Voort EA, Koehler EM, Dowlatshahi EA, et al. Psoriasis is independently associated with nonalcoholic fatty liver disease in patients 55 years old or older: results from a population-based study. J Am Acad Dermatol. 2014;70(3):517–524. doi:10.1016/j.jaad.2013.10.044
36. Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125(1):61–67. doi:10.1111/j.0022-202X.2005.23681.x
37. Snekvik I, Smith CH, Nilsen TIL, et al. Obesity, Waist Circumference, Weight Change, and Risk of Incident Psoriasis: prospective Data from the HUNT Study. J Invest Dermatol. 2017;137(12):2484–2490. doi:10.1016/j.jid.2017.07.822
38. Soltani-Arabshahi R, Wong B, Feng BJ, Goldgar DE, Duffin KC, Krueger GG. Obesity in early adulthood as a risk factor for psoriatic arthritis. Arch Dermatol. 2010;146(7):721–726. doi:10.1001/archdermatol.2010.141
39. Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2(12):e54. doi:10.1038/nutd.2012.26
40. Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141(12):1527–1534. doi:10.1001/archderm.141.12.1527
41. Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159(4):895–902. doi:10.1111/j.1365-2133.2008.08707.x
42. Bermudez EA, Rifai N, Buring J, Manson JE, Ridker PM. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler Thromb Vasc Biol. 2002;22(10):1668–1673. doi:10.1161/01.ATV.0000029781.31325.66
43. Aronson D, Bartha P, Zinder O, et al. Obesity is the major determinant of elevated C-reactive protein in subjects with the metabolic syndrome. Int J Obes Relat Metab Disord. 2004;28(5):674–679. doi:10.1038/sj.ijo.0802609
44. Cai Y, Fleming C, Yan J. New insights of T cells in the pathogenesis of psoriasis. Cell Mol Immunol. 2012;9(4):302–309. doi:10.1038/cmi.2012.15
45. Zhou H, Liu F. Regulation, Communication, and Functional Roles of Adipose Tissue-Resident CD4(+) T Cells in the Control of Metabolic Homeostasis. Front Immunol. 2018;9:1961. doi:10.3389/fimmu.2018.01961
46. Jensen P, Skov L. Psoriasis and Obesity. Dermatology. 2016;232(6):633–639. doi:10.1159/000455840
47. Jensen P, Ahlehoff O, Egeberg A, Gislason G, Hansen PR, Skov L. Psoriasis and New-onset Depression: a Danish Nationwide Cohort Study. Acta Derm Venereol. 2016;96(1):39–42. doi:10.2340/00015555-2183
48. Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146(8):891–895. doi:10.1001/archdermatol.2010.186
49. Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220–229. doi:10.1001/archgenpsychiatry.2010.2
50. Hernández-Hernández V, Ferraz-Amaro I, Díaz-González F. Influence of disease activity on the physical activity of rheumatoid arthritis patients. Rheumatology. 2014;53(4):722–731. doi:10.1093/rheumatology/ket422
51. Shih M, Hootman JM, Kruger J, Helmick CG. Physical activity in men and women with arthritis National Health Interview Survey, 2002. Am J Prev Med. 2006;30(5):385–393. doi:10.1016/j.amepre.2005.12.005
52. Hootman JM, Macera CA, Ham SA, Helmick CG, Sniezek JE. Physical activity levels among the general US adult population and in adults with and without arthritis. Arthritis Rheum. 2003;49(1):129–135. doi:10.1002/art.10911
53. Sajja A, Abdelrahman KM, Reddy AS, et al. Chronic inflammation in psoriasis promotes visceral adiposity associated with noncalcified coronary burden over time. JCI Insight. 2020;5(22). doi:10.1172/jci.insight.142534
54. Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48(4):839–847. doi:10.2337/diabetes.48.4.839
55. Hwang YC, Fujimoto WY, Hayashi T, Kahn SE, Leonetti DL, Boyko EJ. Increased Visceral Adipose Tissue Is an Independent Predictor for Future Development of Atherogenic Dyslipidemia. J Clin Endocrinol Metab. 2016;101(2):678–685. doi:10.1210/jc.2015-3246
56. Sironi AM, Gastaldelli A, Mari A, et al. Visceral fat in hypertension: influence on insulin resistance and beta-cell function. Hypertension. 2004;44(2):127–133. doi:10.1161/01.HYP.0000137982.10191.0a
57. McGillicuddy FC, de la Llera Moya M, Hinkle CC, et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119(8):1135–1145. doi:10.1161/CIRCULATIONAHA.108.810721
58. de la Llera Moya M, McGillicuddy FC, Hinkle CC, et al. Inflammation modulates human HDL composition and function in vivo. Atherosclerosis. 2012;222(2):390–394. doi:10.1016/j.atherosclerosis.2012.02.032
59. Feingold KR, Staprans I, Memon RA, et al. Endotoxin rapidly induces changes in lipid metabolism that produce hypertriglyceridemia: low doses stimulate hepatic triglyceride production while high doses inhibit clearance. J Lipid Res. 1992;33(12):1765–1776. doi:10.1016/S0022-2275(20)41334-3
60. Nonogaki K, Moser AH, Pan XM, Staprans I, Grunfeld C, Feingold KR. Lipoteichoic acid stimulates lipolysis and hepatic triglyceride secretion in rats in vivo. J Lipid Res. 1995;36(9):1987–1995. doi:10.1016/S0022-2275(20)41116-2
61. Feingold KR, Soued M, Adi S, et al. Effect of interleukin-1 on lipid metabolism in the rat. Similarities to and differences from tumor necrosis factor. Arterioscler Thromb. 1991;11(3):495–500. doi:10.1161/01.ATV.11.3.495
62. Nonogaki K, Fuller GM, Fuentes NL, et al. Interleukin-6 stimulates hepatic triglyceride secretion in rats. Endocrinology. 1995;136(5):2143–2149. doi:10.1210/endo.136.5.7720663
63. Ma C, Harskamp CT, Armstrong EJ, Armstrong AW. The association between psoriasis and dyslipidaemia: a systematic review. Br J Dermatol. 2013;168(3):486–495. doi:10.1111/bjd.12101
64. Ma C, Schupp CW, Armstrong EJ, Armstrong AW. Psoriasis and dyslipidemia: a population-based study analyzing the National Health and Nutrition Examination Survey (NHANES). J Eur Acad Dermatol Venereol. 2014;28(8):1109–1112. doi:10.1111/jdv.12232
65. Akkara Veetil BM, Matteson EL, Maradit-Kremers H, McEvoy MT, Crowson CS. Trends in lipid profiles in patients with psoriasis: a population-based analysis. BMC Dermatol. 2012;12:20. doi:10.1186/1471-5945-12-20
66. Mehta NN, Li R, Krishnamoorthy P, et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis. 2012;224(1):218–221. doi:10.1016/j.atherosclerosis.2012.06.068
67. Hulthe J, Bokemark L, Wikstrand J, Fagerberg B. The Metabolic Syndrome, LDL Particle Size, and Atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20(9):2140–2147. doi:10.1161/01.ATV.20.9.2140
68. Holzer M, Wolf P, Curcic S, et al. Psoriasis alters HDL composition and cholesterol efflux capacity. J Lipid Res. 2012;53(8):1618–1624. doi:10.1194/jlr.M027367
69. Paz K, Hemi R, LeRoith D, et al. A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation. J Biol Chem. 1997;272(47):29911–29918. doi:10.1074/jbc.272.47.29911
70. Kanety H, Feinstein R, Papa MZ, Hemi R, Karasik A. Tumor necrosis factor alpha-induced phosphorylation of insulin receptor substrate-1 (IRS-1). Possible mechanism for suppression of insulin-stimulated tyrosine phosphorylation of IRS-1. J Biol Chem. 1995;270(40):23780–23784. doi:10.1074/jbc.270.40.23780
71. Katsuki A, Sumida Y, Murashima S, et al. Serum levels of tumor necrosis factor-alpha are increased in obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83(3):859–862. doi:10.1210/jcem.83.3.4618
72. Zinman B, Hanley AJ, Harris SB, Kwan J, Fantus IG. Circulating tumor necrosis factor-alpha concentrations in a native Canadian population with high rates of type 2 diabetes mellitus. J Clin Endocrinol Metab. 1999;84(1):272–278. doi:10.1210/jcem.84.1.5405
73. Pradhan AD, Cook NR, Buring JE, Manson JE, Ridker PM. C-reactive protein is independently associated with fasting insulin in nondiabetic women. Arterioscler Thromb Vasc Biol. 2003;23(4):650–655. doi:10.1161/01.ATV.0000065636.15310.9C
74. Barzilay JI, Abraham L, Heckbert SR, et al. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001;50(10):2384–2389. doi:10.2337/diabetes.50.10.2384
75. Ucak S, Ekmekci TR, Basat O, Koslu A, Altuntas Y. Comparison of various insulin sensitivity indices in psoriatic patients and their relationship with type of psoriasis. J Eur Acad Dermatol Venereol. 2006;20(5):517–522. doi:10.1111/j.1468-3083.2006.01499.x
76. Wan MT, Shin DB, Hubbard RA, Noe MH, Mehta NN, Gelfand JM. Psoriasis and the risk of diabetes: a prospective population-based cohort study. J Am Acad Dermatol. 2018;78(2):315–322.e311. doi:10.1016/j.jaad.2017.10.050
77. Ikumi K, Odanaka M, Shime H, et al. Hyperglycemia Is Associated with Psoriatic Inflammation in Both Humans and Mice. J Invest Dermatol. 2019;139(6):1329–1338.e1327. doi:10.1016/j.jid.2019.01.029
78. Goldstein BJ, Scalia R. Adiponectin: a novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004;89(6):2563–2568. doi:10.1210/jc.2004-0518
79. Shibata S, Saeki H, Tada Y, Karakawa M, Komine M, Tamaki K. Serum high molecular weight adiponectin levels are decreased in psoriasis patients. J Dermatol Sci. 2009;55(1):62–63. doi:10.1016/j.jdermsci.2009.02.009
80. Shibata S, Tada Y, Hau C, et al. Adiponectin as an anti-inflammatory factor in the pathogenesis of psoriasis: induction of elevated serum adiponectin levels following therapy. Br J Dermatol. 2011;164(3):667–670. doi:10.1111/j.1365-2133.2010.10123.x
81. Shibata S, Tada Y, Hau CS, et al. Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from γδ-T cells. Nat Commun. 2015;6:7687. doi:10.1038/ncomms8687
82. Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449–2460. doi:10.1084/jem.20070657
83. Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;298(4):R1089–1097. doi:10.1152/ajpregu.00373.2009
84. Madhur MS, Lob HE, McCann LA, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55(2):500–507. doi:10.1161/HYPERTENSIONAHA.109.145094
85. Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128(5):1207–1211. doi:10.1038/sj.jid.5701213
86. El-Moaty Zaher HA, El-Komy MHM, Hegazy RA, Mohamed El Khashab HA, Ahmed HH. Assessment of interleukin-17 and vitamin D serum levels in psoriatic patients. J Am Acad Dermatol. 2013;69(5):840–842. doi:10.1016/j.jaad.2013.07.026
87. Shi X, Jin L, Dang E, et al. IL-17A upregulates keratin 17 expression in keratinocytes through STAT1- and STAT3-dependent mechanisms. J Invest Dermatol. 2011;131(12):2401–2408. doi:10.1038/jid.2011.222
88. Armesto S, Coto-Segura P, Osuna CG, Camblor PM, Santos-Juanes J. Psoriasis and hypertension: a case-control study. J Eur Acad Dermatol Venereol. 2012;26(6):785–788. doi:10.1111/j.1468-3083.2011.04108.x
89. Cohen AD, Weitzman D, Dreiher J. Psoriasis and hypertension: a case-control study. Acta Derm Venereol. 2010;90(1):23–26. doi:10.2340/00015555-0741
90. Qureshi AA, Choi HK, Setty AR, Curhan GC. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145(4):379–382. doi:10.1001/archdermatol.2009.48
91. Kim HN, Han K, Song SW, Lee JH. Hypertension and risk of psoriasis incidence: an 11-year nationwide population-based cohort study. PLoS One. 2018;13(8):e0202854. doi:10.1371/journal.pone.0202854
92. Wu S, Han J, Li WQ, Qureshi AA. Hypertension, antihypertensive medication use, and risk of psoriasis. JAMA Dermatol. 2014;150(9):957–963. doi:10.1001/jamadermatol.2013.9957
93. Aksentijevich M, Lateef SS, Anzenberg P, Dey AK, Mehta NN. Chronic inflammation, cardiometabolic diseases and effects of treatment: psoriasis as a human model. Trends Cardiovasc Med. 2020;30(8):472–478. doi:10.1016/j.tcm.2019.11.001
94. Kim BS, Lee WK, Pak K, et al. Ustekinumab treatment is associated with decreased systemic and vascular inflammation in patients with moderate-to-severe psoriasis: feasibility study using (18) F-fluorodeoxyglucosePET/CT. J Am Acad Dermatol. 2019;80(5):1322–1331. doi:10.1016/j.jaad.2018.03.011
95. Gelfand JM, Shin DB, Alavi A, et al. A Phase IV, Randomized, Double-Blind, Placebo-Controlled Crossover Study of the Effects of Ustekinumab on Vascular Inflammation in Psoriasis (the VIP-U Trial). J Invest Dermatol. 2020;140(1):85–93.e82. doi:10.1016/j.jid.2019.07.679
96. Choi H, Uceda DE, Dey AK, et al. Treatment of Psoriasis With Biologic Therapy Is Associated With Improvement of Coronary Artery Plaque Lipid-Rich Necrotic Core: results From a Prospective, Observational Study. Circ Cardiovasc Imaging. 2020;13(9):e011199. doi:10.1161/CIRCIMAGING.120.011199
97. Elnabawi YA, Dey AK, Goyal A, et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res. 2019;115(4):721–728. doi:10.1093/cvr/cvz009
98. Holzer M, Wolf P, Inzinger M, et al. Anti-psoriatic therapy recovers high-density lipoprotein composition and function. J Invest Dermatol. 2014;134(3):635–642. doi:10.1038/jid.2013.359
99. Pina T, Armesto S, Lopez-Mejias R, et al. Anti-TNF-α therapy improves insulin sensitivity in non-diabetic patients with psoriasis: a 6-month prospective study. J Eur Acad Dermatol Venereol. 2015;29(7):1325–1330. doi:10.1111/jdv.12814
100. Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305(24):2525–2531. doi:10.1001/jama.2011.878
101. Tan E, Baker C, Foley P. Weight gain and tumour necrosis factor-alpha inhibitors in patients with psoriasis. Australas J Dermatol. 2013;54(4):259–263. doi:10.1111/ajd.12044
102. Takamura S, Takahashi A, Inoue Y, Teraki Y. Effects of tumor necrosis factor-α, interleukin-23 and interleukin-17A inhibitors on bodyweight and body mass index in patients with psoriasis. J Dermatol. 2018;45(9):1130–1134. doi:10.1111/1346-8138.14526
103. Florin V, Cottencin AC, Delaporte E, Staumont-Sallé D. Body weight increment in patients treated with infliximab for plaque psoriasis. J Eur Acad Dermatol Venereol. 2013;27(2):e186–190. doi:10.1111/j.1468-3083.2012.04571.x
104. Gisondi P, Conti A, Galdo G, Piaserico S, De Simone C, Girolomoni G. Ustekinumab does not increase body mass index in patients with chronic plaque psoriasis: a prospective cohort study. Br J Dermatol. 2013;168(5):1124–1127. doi:10.1111/bjd.12235
105. Sandoo A, Panoulas VF, Toms TE, et al. Anti-TNFα therapy may lead to blood pressure reductions through improved endothelium-dependent microvascular function in patients with rheumatoid arthritis. J Hum Hypertens. 2011;25(11):699–702. doi:10.1038/jhh.2011.36
106. Zhao Q, Hong D, Zhang Y, Sang Y, Yang Z, Zhang X. Association between anti-TNF therapy for rheumatoid arthritis and hypertension: a meta-analysis of randomized controlled trials. Medicine. 2015;94(14):e731. doi:10.1097/MD.0000000000000731
107. Muller DN, Shagdarsuren E, Park JK, et al. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol. 2002;161(5):1679–1693. doi:10.1016/S0002-9440(10)64445-8
108. Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol. 2007;27(12):2576–2581. doi:10.1161/ATVBAHA.107.153080
109. Brands MW, Banes-Berceli AK, Inscho EW, Al-Azawi H, Allen AJ, Labazi H. Interleukin 6 knockout prevents angiotensin II hypertension: role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension. 2010;56(5):879–884. doi:10.1161/HYPERTENSIONAHA.110.158071
110. Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273(2):197–204. doi:10.1111/j.1365-2796.2012.02593.x
111. Tzellos T, Kyrgidis A, Zouboulis CC. Re-evaluation of the risk for major adverse cardiovascular events in patients treated with anti-IL-12/23 biological agents for chronic plaque psoriasis: a meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol. 2013;27(5):622–627. doi:10.1111/j.1468-3083.2012.04500.x
112. Strober B, Menter A, Leonardi C, et al. Efficacy of risankizumab in patients with moderate-to-severe plaque psoriasis by baseline demographics, disease characteristics and prior biologic therapy: an integrated analysis of the Phase III UltIMMa-1 and UltIMMa-2 studies. J Eur Acad Dermatol Venereol. 2020;34(12):2830–2838. doi:10.1111/jdv.16521
113. Papp KA, Reich K, Blauvelt A, et al. Efficacy of tildrakizumab for moderate-to-severe plaque psoriasis: pooled analysis of three randomized controlled trials at weeks 12 and 28. J Eur Acad Dermatol Venereol. 2019;33(6):1098–1106. doi:10.1111/jdv.15400
114. Menter MA, Papp KA, Cather J, et al. Efficacy of Tofacitinib for the Treatment of Moderate-to-Severe Chronic Plaque Psoriasis in Patient Subgroups from Two Randomised Phase 3 Trials. J Drugs Dermatol. 2016;15(5):568–580.
115. Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371(9625):1675–1684. doi:10.1016/S0140-6736(08)60726-6
116. Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy and safety are not altered by metabolic syndrome status in patients with psoriasis: post hoc analysis of 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2020;82(2):519–522. doi:10.1016/j.jaad.2019.09.042
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.