Back to Journals » Clinical and Experimental Gastroenterology » Volume 16
PSMA-Targeted PET Radiotracer [18F]DCFPyL as an Imaging Biomarker in Inflammatory Bowel Disease
Authors Ismail MS, Peters DE, Rowe SP, Salavati A, Sharma S , Anders RA, Pomper M , Slusher BS, Selaru FM
Received 1 March 2023
Accepted for publication 3 August 2023
Published 8 December 2023 Volume 2023:16 Pages 237—247
DOI https://doi.org/10.2147/CEG.S404009
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Santosh Shenoy
Mohamed Saleh Ismail,1,* Diane E Peters,2,3,* Steven P Rowe,4,5,* Ali Salavati,6 Sowmya Sharma,1 Robert A Anders,7 Martin Pomper,2,4,5,* Barbara S Slusher,2,3,8,* Florin M Selaru1,5,*
1Division of Gastroenterology, Johns Hopkins Medical Institutions, Baltimore, MD, USA; 2Department of Pharmacology and Molecular Sciences, Johns Hopkins Medical Institutions, Baltimore, MD, USA; 3Johns Hopkins Drug Discovery, Baltimore, MD, USA; 4Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins Medical Institutions, Baltimore, MD, USA; 5The Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins Medical Institutions, Baltimore, MD, USA; 6Ahmanson Translational Theranostics Division, Department of Molecular and Medical Pharmacology, University of California Los Angeles (UCLA), Los Angeles, CA, USA; 7The Bloomberg-Kimmel Institute for Cancer Immunotherapy, Johns Hopkins Medical Institutions, Baltimore, MD, USA; 8Department of Neurology, Johns Hopkins Medical Institutions, Baltimore, MD, USA
*These authors contributed equally to this work
Correspondence: Florin M Selaru; Barbara S Slusher, Email [email protected]; [email protected]
Background: Prostate-specific membrane antigen (PSMA) is highly and specifically upregulated in active-inflamed mucosa of patients with inflammatory bowel disease (IBD). We hypothesized that this upregulation would be detectable using a PSMA-targeted positron emission tomography/computed tomography (PET/CT) imaging agent, [18F]DCFPyL, enabling non-invasive visualization of inflammation. A noninvasive means of detecting active inflammation would have high clinical value in localization and management of IBD.
Study: We performed [18F]DCFPyL imaging in three IBD patients with active disease. Abnormally increased gastrointestinal [18F]DCFPyL uptake was observed in areas with endoscopic, histologic, and immunohistochemical inflammation, demonstrating partial overlap of segments of bowel with abnormal [18F]DCFPyL uptake and active inflammation.
Conclusion: This study demonstrates that PSMA-targeted [18F]DCFPyL PET can effectively detect regions of inflamed mucosa in patients with IBD, suggesting its utility as a non-invasive imaging agent to assess location, extent, and disease activity in IBD.
Keywords: IBD, disease activity, PSMA, GCPII, [18F]DCFPyL
Background
Inflammatory bowel disease (IBD) is a chronic inflammatory condition of the gastrointestinal tract that is characterized by remission and relapse.1,2 Diagnosis of remission can be made based upon clinical findings, biochemical markers, endoscopic, and/or histologic criteria. Achievement of endoscopic remission has emerged as a goal for therapeutic management, as visibly normal mucosa has been associated with improvement in the natural course of IBD and prevention of long-term disease sequelae.2–4 Although endoscopic remission is a goal of treat-to-target disease management, it is recognized that submucosal inflammation can persist in the absence of mucosal injury, and there is no single test that can accurately detect asymptomatic inflammation in patients with known IBD.5 Histologic remission is an emerging concept. However, its reliability is challenging, as existing histologic scoring systems are subjective and difficult to reproduce.6 The ability to accurately and noninvasively detect and monitor gastrointestinal inflammation in IBD patients would have broad clinical impact by contributing to therapeutic management decisions, avoiding unnecessary treatments, and enhancing monitoring during periods of both treatment and remission.
Prostate-specific membrane antigen (PSMA), also referred to as glutamate carboxypeptidase II (GCPII), has emerged as a promising biomarker and therapeutic target in IBD.7–10 PSMA is a metallopeptidase responsible for hydrolysis of glutamate peptides including the neurotransmitter N-acetyl-aspartyl-glutamate and the essential dietary nutrient folate polyglutamate.11 Recent studies have also shown that PSMA is expressed in the endothelium of tumor-associated blood vessels and in activated macrophages, indicating its role in inflammation.12 PSMA is minimally expressed in the normal gastrointestinal tract,13–17 but is highly and specifically upregulated in inflamed endoscopic biopsies of both Crohn’s disease (CD) and ulcerative colitis (UC) patients.8,9,18
PSMA is also highly upregulated in prostate cancer.17,19 Recently, the PSMA-targeted positron emission tomography (PET) imaging agent [18F]DCFPyL (piflufolastat F 18, tradename PylarifyTM) has received US FDA approval for diagnostic imaging in prostate cancer.20,21 This has led to widespread availability of the agent, facilitating investigations into off-label use. We hypothesized that the PSMA upregulation occurring in active IBD would be detectable using [18F]DCFPyL imaging, and further hypothesized that a positive signal would indicate tissue inflammation. The use of PSMA-targeted [18F]DCFPyL PET imaging in inflammatory bowel disease has only been reported in a single-case report.22 This was performed for a patient with rising PSA serology levels in the absence of any genitourinary symptoms. [68 Ga]Ga-PSMA-11 PET/CT was performed, showing uptake in the distal ileum.22 The patient retrospectively reported a previous diagnosis of Crohn’s disease, with recent increase in diarrheal episodes, bringing the authors to the conclusion that these findings were likely secondary to the patients known history of inflammatory bowel disease.22 We performed [18F]DCFPyL PET in patients hospitalized due to active IBD to present data that PSMA imaging can be used to noninvasively identify active sites of inflammation in IBD patients during a flare.
Methods
Patients with inflammatory bowel disease (IBD), either labeled as UC, CD, or IBD-Unclassified, presenting with symptoms of a flare who required admission to the hospital, were identified for the study. As per the usual work-up of IBD flares, they underwent routine laboratory testing, as well as endoscopic evaluation. Additionally, [18F]DCFPyL PET/CT imaging was performed under a United States Food and Drug Administration (FDA) Investigational New Drug application (IND 121064). Institutional approval for this study was granted by the Johns Hopkins Medicine Institutional Review Board. Written informed consent was obtained from each patient before the study. All consent forms included participant approval to publish results. The authors declare that the procedures were followed according to the regulations established by the Clinical Research and Ethics Committee and the Helsinki Declaration of the World Medical Association. [18F]DCFPyL PET/CT images were acquired on a Siemens Biograph mCT 128-slice scanner (Siemens Healthineers, Erlangen, Germany), approximately 60 minutes after intravenous administration of approximately 333 MBq (9 mCi) [18F]DCFPyL. The results of [18F]DCFPyL PET/CT images of each patient were used to corroborate endoscopic findings.
Results
Crohn’s Disease
To demonstrate the use of PSMA-targeted [18F]DCFPyL PET/CT in patients with CD, two patients with varying phenotypes were chosen.
The first phenotype was long-standing ileocolonic CD in a 42-year-old male presenting with symptoms of flare, including abdominal pain and diarrhea of more than 20 bowel movements per day. Inflammatory markers were elevated with an erythrocyte sedimentation rate (ESR) of 36 mm/h (normal <15 mm/h), C-reactive protein (CRP) of 7 mg/dL (normal <0.5 mg/dL), and fecal calprotectin of 241 ug/g (normal <50 ug/g). He had been maintained on ustekinumab every 6 weeks and subcutaneous methotrexate 25 mg weekly. Previous medications included infliximab, adalimumab, vedolizumab, and 6-mercaptopurine. All of these medications failed to control the disease, and he had ongoing inflammation with resulting ileal strictures.
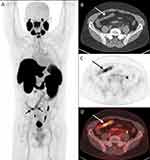
The PET/CT scan demonstrated a long segment of abnormally increased [18F]DCFPyL uptake in the terminal ileum (Figure 1). Ileocolonoscopy was performed and demonstrated inflammation and pseudopolyps from the anal verge up to 20 cm from the point of entry, with otherwise normal colonic mucosa (Figure 2). Of note, terminal ileal inflammation was noted with erythema, edema, and ulceration. A non-traversable short stricture was noted in the terminal ileum, 5 cm from the ileocecal valve, which was dilated with a through-the-scope (TTS) balloon up to 12 mm. A further non-traversable stricture was noted 3 cm proximal to the first stricture.
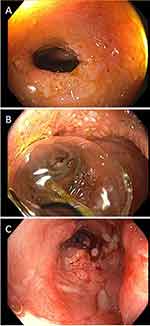 |
Figure 2 Ileocolonoscopy with inflammation and pseudopolyposis from the anal verge up to 20cm and terminal ileum stricture (A) requiring balloon dilatation (B) with successful dilatation (C). |
Biopsies from the terminal ileum and rectum demonstrated chronic inflammatory disease. To assess PSMA expression in the region of distal colon located 20 cm from the anal verge, where inflammation was visualized on endoscopic exam but was not detected by [18F]DCFPyL PET/CT, PSMA immunohistochemistry (IHC) was performed on full-thickness distal colon sections, acquired during his abdominoperineal resection using the validated antibody 1A11 (Figure 3) according to previously described methods.7,23 Interestingly, heterogeneously increased PSMA expression was detected in colon epithelial cells adjacent to sites of severe inflammation, where it displayed an intracellular, cytoplasmic expression pattern (Figure 3C), and thus may have been inaccessible to the radiotracer, explaining the apparent discrepancy between endoscopic and histologic disease, and [18F]DCFPyL imaging at this site. In agreement with this hypothesis, IHC performed on terminal ileum biopsies from this patient exhibited a strikingly different pattern of PSMA expression, with concentrated signal at the apical brush border membrane in addition to increased intracellular expression (Figure 3D). Notably, in the ileal specimen, PSMA was observed to be upregulated in 100% of epithelia examined, while in the colonic sections, PSMA upregulation was patchy and detected in ~20% of epithelia in inflamed regions. It is therefore likely that decreased magnitude of PSMA upregulation in the colon of this patient, relative to ileum, may also have contributed to the observed differences in [18F]DCFPyL uptake.
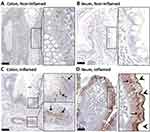 |
Figure 3 PSMA immunohistochemistry (IHC) on full-thickness distal colon sections. Arrows and arrowheads identifying area of PSMA staining on various cross-sections of the colon and ileum (A–D). |
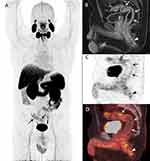
The second CD phenotype involved colonic involvement and perianal fistula in a 36-year-old male with two setons in place who was admitted to the hospital with worsening rectal pain and bloody diarrhea. He had recently been switched to ustekinumab after secondary loss of response to adalimumab. Inflammatory markers were elevated with ESR of 33 mm/h, CRP of 7.6 mg/dL and fecal calprotectin of >8,000 ug/g. MRI demonstrated wall thickening and inflammation of the sigmoid colon and rectum with no new fistula. His colonoscopy showed marked erythema, friability, and deep ulcerations in the rectum and sigmoid colon (Figure 4). Biopsies demonstrated chronic active inflammation and PSMA IHC showed increased numbers of PSMA positive cells in inflamed sections (Supplementary Figure 1). He received intravenous steroids and infliximab with no response. He underwent abdominoperineal resection. [18F]DCFPyL PET/CT imaging was performed prior to his surgery. A written, informed consent was obtained prior to the scan. PET/CT scan demonstrated abnormally increased [18F]DCFPyL uptake in the sigmoid colon and rectum (Figure 5), in areas of mucosal inflammation.
 |
Figure 4 Erythema, friability, and deep ulcerations in the rectum and sigmoid colon on colonoscopy (A and B). |
Ulcerative Colitis
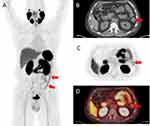
[18F]DCFPyL PET/CT imaging utility was investigated in ulcerative colitis with a 66-year-old man with left-sided disease presenting to the emergency department with symptoms of flare including bloody diarrhea of more than 30 bowel movements per day, urgency, and abdominal pain. He had recently been switched to vedolizumab after secondary loss of response to infliximab. His last colonoscopy, performed while on infliximab every 4 weeks, demonstrated marked erythema, friability, spontaneous bleeding, and ulcerations in the descending colon, sigmoid colon, and rectum (Mayo endoscopic subscore 2–3), with no active disease beyond the splenic flexure. He was admitted to the hospital. Inflammatory markers were elevated with ESR of 47 mm/h, CRP of 6.6 mg/dL and fecal calprotectin of 867 ug/g. Stool studies, including Clostridioides difficile test, were negative. He underwent a flexible sigmoidoscopy to 20 cm from the anal verge which demonstrated spontaneous bleeding and ulcerations throughout the examined mucosa (Mayo endoscopic subscore 3) (Figure 6). Biopsies confirmed severe active chronic inflammatory disease, with negative immunostain for cytomegalovirus. IHC of inflamed biopsies, identified occasionally increased PSMA expression in the apical epithelial membrane, and also numerous PSMA positive cells throughout the mucosa (Supplementary Figure 2). [18F]DCFPyL PET/CT imaging was performed. A written, informed consent was obtained preceding the scan. PET/CT demonstrated abnormally increased [18F]DCFPyL uptake in the descending colon, sigmoid colon, and rectum (Figure 7), in a distribution corresponding to the findings on endoscopy.
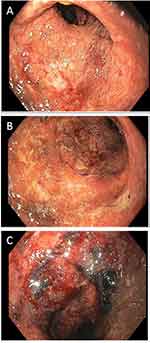 |
Figure 6 Flexible sigmoidoscopy demonstrating Mayo endoscopic subscore 3 (A–C). |
Discussion
The accurate and non-invasive diagnosis of active mucosal inflammation in a patient with known IBD can be a clinical challenge. There is no single non-invasive gold standard test, biomarker, or imaging agent to reliably diagnose inflamed mucosa during a flare or to monitor disease activity after treatment is initiated.5 Existing laboratory tests, including CRP, ESR, and fecal calprotectin, each present their own limitations. CRP, a widely utilized indicator of the acute phase inflammatory response, is highly specific for inflammation but lacks sensitivity and can be normal during an acute flare.24,25 Therefore, while CRP is a valuable adjunct to clinical and endoscopic evaluation, it cannot be reliably used to rule out flares.25
ESR, an indirect measure of systemic inflammation, is another commonly used biomarker of inflammation in IBD. However, ESR correlates even less with endoscopic disease activity than CRP.5 Calprotectin is a neutrophil marker that correlates with neutrophil migration to the gastrointestinal tract and, by extension, should correlate well with inflammation.26 Fecal calprotectin is also a useful adjunct in determining if a flare is occurring, although false positives have been reported.5 Lastly, calprotectin tends to be more useful in colonic inflammation in UC and CD but is less accurate in small bowel inflammation in CD.27 In addition, none of these non-invasive tests can localize inflammation.
Given the imperfect nature of the blood- and stool-based markers of inflammation, ileocolonoscopy is the gold standard for the diagnosis of active mucosal inflammation and flares in patients with IBD.28 However, endoscopy is an invasive procedure requiring adequate bowel preparation. In addition, there is a significant rate of complications, including perforation, in patients undergoing endoscopy while having bowel inflammation.29 Therefore, there is a strong clinical need for alternative, non-invasive diagnostic tools to aid in the accurate diagnosis and monitoring of mucosal inflammation in patients with IBD.
Numerous imaging modalities have been studied in patients with inflammatory bowel disease, each with its advantages and disadvantages. CT imaging, although widely available, has been shown to have low sensitivity for assessing the extent of inflammation in Crohn’s disease,30 and it produces concerns about radiation as recurrent imaging is often required.31 MRI has a similar diagnostic accuracy for imaging IBD; however, it is more time-consuming and expensive. Intestinal ultrasound is a promising imaging modality as it is easily accessible, non-invasive, radiation-free, and cost-efficient.32 However, although this imaging has become widely available in Europe, it remains limited in North America.32
The use of molecular imaging may be a promising non-invasive approach to assess the location and disease activity in patients with IBD. As PSMA is specifically increased in active, inflamed CD and UC patient biopsies relative to uninvolved areas,8 we investigated whether PSMA-targeted imaging could be used to detect active inflammation. We evaluated the PET imaging agent [18F]DCFPyL (PylarifyTM) for this purpose. [18F]DCFPyL is FDA-approved as a diagnostic agent in prostate cancer and has been found to be sensitive and specific in identifying PSMA-positive prostate cancer.21 [18F]DCFPyL has a favorable safety profile.20 While there is uptake of [18F]DCFPyL in the normal GI tract, the biodistribution is predominantly within the proximal small bowel, such that positive sites in the more distal small bowel and colon are readily identifiable. The recent development of PET/computed tomography (CT) combines the physiological sensitivity of PET with the anatomical accuracy of CT, increasing the specificity of PET.32
Utility in Crohn’s disease (CD) was established in our patient with active ileocolonic CD, where [18F]DCFPyL uptake was abnormally increased in the terminal ileum, corresponding to the inflammation observed in the terminal ileum on ileocolonoscopy and supported by histology on mucosal biopsies. Immunohistochemistry (IHC) demonstrated markedly upregulated prostate-specific membrane antigen (PSMA) expression at this site, with increases in both membrane-associated and cytoplasmic epithelial PSMA expression. The observed endoscopic active disease of the distal 20 cm from the anal verge was not detected on the PET scan, although PSMA protein upregulation was detectable by IHC in surgically resected tissue. Relative to the terminal ileum, however, there were differences in both the magnitude and localization of this PSMA expression, both of which likely contributed to the nondetectable PET signal in the colon. In inflamed colon, PSMA was only detected in the cytoplasm of epithelial cells, where it was heterogeneously upregulated in a portion of epithelia (~20%). At this time, it is unclear if the reduced magnitude of expression in the colon vs ileum may have been below the limit of detection for [18F]DCFPyL or if the target may not have been accessible to the radiotracer, possibly due to alterations in blood flow in this region due to chronic inflammation-associated changes in tissue architecture such as scarring and/or fibrosis. Regardless, this will need to be further investigated in larger patient cohorts to determine factors that affect PSMA-targeted PET uptake in IBD. Notably, these findings suggest that PSMA-PET scanning can identify inflammation patterns that are currently not distinguishable with clinical grade endoscopic and histologic analyses. This observation introduces the intriguing possibility of novel patient stratification and/or inclusion in personalized treatment algorithms.
For our other two patients with colonic involvement of their IBD, [18F]DCFPyL uptake was increased in the descending colon, sigmoid colon, and rectum (ulcerative colitis), and increased in the sigmoid colon and rectum (colonic CD), indicative of regions of endoscopic active disease. In agreement with the observed radiotracer uptake, IHC identified numerous PSMA-positive cells in inflamed colon biopsies collected from these sites. Further studies to characterize PSMA localization as a function of IBD subtype and disease site are of high interest and remain ongoing in our laboratory.
In summary, abnormal gastrointestinal [18F]DCFPyL uptake was detected in all three IBD patients, and in all disease phenotypes, the sites of increased [18F]DCFPyL uptake were consistent with endoscopically and histologically inflamed regions. These findings support the continued evaluation of [18F]DCFPyL PET/CT imaging as an adjunct diagnostic tool for IBD flares in both Crohn’s disease and ulcerative colitis. Future studies are warranted to evaluate [18F]DCFPyL uptake in patients as a function of disease severity, to longitudinally examine [18F]DCFPyL uptake in patients during periods of treatment, remission, and flares, and to potentially identify patients with high PSMA expression for inclusion in clinical trials using therapeutic PSMA inhibitors, which are in active development and have shown promising efficacy in preclinical models.8–10,33
Limitations
Our study has limitations, including a small sample size. We also recognize that PET/CT imaging is generally more expensive than CT imaging alone, and PSMA targeted [18F]DCFPyL PET/CT is likely to be available in large academic centres and not as widely available as other radiological modalities. Another drawback of PET/CT is the ionizing radiation, which is higher than that of CT imaging alone.
Conclusion
This paper demonstrates the important utility of PSMA targeted [18F]DCFPyL PET/CT as a promising non-invasive imaging agent to assess location, extent, and disease activity in IBD. If these findings are demonstrated consistently in larger studies, this will potentially address the unmet need to noninvasively detect gastrointestinal inflammation and assess the location, extent, and activity of IBD using a noninvasive modality. In addition, this novel modality of detecting inflammation may allow sub-classification of IBD based on molecular features, bringing us closer to individualized therapies and the goal of furthering precision medicine.
Disclosure
M.P. is a coinventor on a US patent covering [18F]DCFPyL and as such is entitled to a portion of any licensing fees and royalties generated by this technology. B.S.S. and D.E.P. are coinventors on Johns Hopkins patent applications covering the use and novel compositions of PSMA inhibitors as IBD therapeutics. These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict-of-interest policies. D.E.P. also reports NIH T32 training grant (T32 OD011089). S.P.R. is a consultant to Progenics Pharmaceuticals, the licensee of [18F]DCFPyL. S.P.R. and M.P. receive research funding from Progenics Pharmaceuticals. M.P. is a founder of Precision Molecular, Inc. S.P.R. and M.P. are consultants to Precision Molecular, Inc. The authors report no other conflicts of interest in this work.
References
1. Liverani E, Scaioli E, Digby RJ, Bellanova M, Belluzzi A. How to predict clinical relapse in inflammatory bowel disease patients. World J Gastroenterol. 2016;22(3):1017–1033. doi:10.3748/wjg.v22.i3.1017
2. Colombel JF, Narula N, Peyrin-Biroulet L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology. 2017;152(2):351–361 e355. doi:10.1053/j.gastro.2016.09.046
3. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324–1338. doi:10.1038/ajg.2015.233
4. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) Initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583. doi:10.1053/j.gastro.2020.12.031
5. Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55(3):426–431. doi:10.1136/gut.2005.069476
6. Bryant RV, Winer S, Travis SP, Riddell RH. Systematic review: histological remission in inflammatory bowel disease. Is ‘complete’ remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis. 2014;8(12):1582–1597. doi:10.1016/j.crohns.2014.08.011
7. Vornov JJ, Peters D, Nedelcovych M, et al. Looking for drugs in all the wrong places: use of GCPII Inhibitors Outside the Brain. Neurochem Res. 2020;45(6):1256–1267. doi:10.1007/s11064-019-02909-y
8. Rais R, Jiang W, Zhai H, et al. FOLH1/GCPII is elevated in IBD patients, and its inhibition ameliorates murine IBD abnormalities. JCI Insight. 2016;1(12). doi:10.1172/jci.insight.88634.
9. Slusher RR, Li X. Methods for Treating Inflammatory Bowel Disease Using Prostate Specific Membrane Antigen (PSMA) Inhibitors (US); 2015.
10. Peters DE, Norris LD, Slusher BS. Spontaneous loss-of-function Dock2 mutation alters murine colitis sensitivity and is a confounding variable in inflammatory bowel disease research. Crohn Colitis. 2019;360:1.
11. Vornov JJ, Hollinger KR, Jackson PF, et al. Still NAAG’ing after all these years: the continuing pursuit of GCPII Inhibitors. Adv Pharmacol. 2016;76:215–255.
12. de Galiza Barbosa F, Queiroz MA, Nunes RF. Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings. Cancer Imaging. 2020;20(23). doi:10.1186/s40644-020-00300-7
13. Troyer JK, Beckett ML, Wright GL. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int J Cancer. 1995;62(5):552–558. doi:10.1002/ijc.2910620511
14. Mhawech-Fauceglia P, Zhang S, Terracciano L, et al. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology. 2007;50(4):472–483. doi:10.1111/j.1365-2559.2007.02635.x
15. Haffner MC, Kronberger IE, Ross JS, et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum Pathol. 2009;40(12):1754–1761. doi:10.1016/j.humpath.2009.06.003
16. Kinoshita Y, Kuratsukuri K, Landas S, et al. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J Surg. 2006;30(4):628–636. doi:10.1007/s00268-005-0544-5
17. Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3(1):81–85.
18. Zhang T, Song B, Zhu W, et al. An ileal crohn’s disease gene signature based on whole human genome expression profiles of disease unaffected ileal mucosal biopsies. PLoS One. 2012;7(5):e37139. doi:10.1371/journal.pone.0037139
19. Foss CA, Mease RC, Cho SY, Kim HJ, Pomper MG. GCPII imaging and cancer. Curr Med Chem. 2012;19(9):1346–1359. doi:10.2174/092986712799462612
20. Chen Y, Pullambhatla M, Foss CA, et al. 2-(3-{1-Carboxy-5-[(6-[18F]Fluoro-Pyridine-3-Carbonyl)-Amino]-Pentyl}-Ureido)-Pentanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res. 2011;17(24):7645–7653. doi:10.1158/1078-0432.CCR-11-1357
21. Szabo Z, Mena E, Rowe SP, et al. Initial evaluation of [(18)F]DCFPyL for prostate-specific membrane antigen (PSMA)-Targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17(4):565–574. doi:10.1007/s11307-015-0850-8
22. Chandekar KR, Tanigassalam S, Kavanal AJ, et al. [68 Ga]Ga-PSMA-11 small bowel uptake in crohn’s disease: revisiting the “Non-specificity” of PSMA ligands. Nucl Med Mol Imaging. 2022;56(2):102–104. doi:10.1007/s11307-015-0850-8
23. Nováková Z, Foss CA, Copeland BT, et al. Novel monoclonal antibodies recognizing human prostate-specific membrane antigen (PSMA) as research and theranostic tools. Prostate. 2017;77(7):749–764. doi:10.1002/pros.23311
24. Ince MN, Elliott DE. Effective use of the laboratory in the management of patients with inflammatory bowel diseases. Gastroenterol Clin North Am. 2019;48(2):237–258. doi:10.1016/j.gtc.2019.02.006
25. Chang S. Disease monitoring in inflammatory bowel disease. World J Gastroenterol. 2015;21(40):11246–11259. doi:10.3748/wjg.v21.i40.11246
26. D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(12):2218–2224. doi:10.1002/ibd.22917
27. Garcia-Sanchez V, Iglesias-Flores E, González R, et al. Does fecal calprotectin predict relapse in patients with Crohn’s disease and ulcerative colitis? J Crohns Colitis. 2010;4(2):144–152. doi:10.1016/j.crohns.2009.09.008
28. Annese V, Daperno M, Rutter MD, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7(12):982–1018. doi:10.1016/j.crohns.2013.09.016
29. Kothari ST, Huang RJ, Shaukat A, et al. ASGE review of adverse events in colonoscopy. Gastrointest Endosc. 2019;90(6):863–876 e833. doi:10.1016/j.gie.2019.07.033
30. Panés J, Bouzas R, Chaparro M, et al., Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Alimentary Pharmacology & Therapeutics. 2011;34(2):125–145. doi:10.1111/j.1365-2036.2011.04710.x
31. Loftus EV.Using CT and MR enterography to diagnose and monitor IBD. Gastroenterol Hepatol. 2010;6(12):754–756.
32. Lapp RT, Spier BJ, Perlman SB, et al. Clinical utility of positron emission tomography/computed tomography in inflammatory bowel disease. Mol Imaging Biol. 2011;13(3):573–576. doi:10.1007/s11307-010-0367-0
33. Date AA, Rais R, Babu T, et al. Local enema treatment to inhibit FOLH1/GCPII as a novel therapy for inflammatory bowel disease. J Control Release. 2017;263:132–138. doi:10.1016/j.jconrel.2017.01.036
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.