Back to Journals » International Journal of General Medicine » Volume 16
Psittacosis Pneumonia: Diagnosis, Treatment and Interhuman Transmission
Received 3 November 2022
Accepted for publication 22 December 2022
Published 4 January 2023 Volume 2023:16 Pages 1—6
DOI https://doi.org/10.2147/IJGM.S396074
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
ZiQi Cui,1 Ling Meng2
1Shandong First Medical University & Shandong Academy of Medical Sciences, Taian, 271000, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, The Second Affiliated Hospital, Shandong First Medical University & Shandong Academy of Medical Sciences, Taian, 271000, People’s Republic of China
Correspondence: Ling Meng, Department of Respiratory and Critical Care Medicine, The Second Affiliated Hospital, Shandong First Medical University & Shandong Academy of Medical Sciences, No. 366 Taishan Street, Taian, 271000, People’s Republic of China, Tel +86-18505386929, Email [email protected]
Abstract: Psittacosis pneumonia is a zoonosis caused by Chlamydia psittacosis infection, mainly resulting from contact with aerosols of birds or poultry’s urine, feces, and excrement. The clinical manifestations range from general symptoms of infection to severe acute respiratory syndrome and systemic diseases, currently diagnosed using metagenomic next-generation sequencing (mNGS) to improve diagnostic accuracy. To date, most reports have only discussed human exposure to poultry disease. However, the latest studies have shown that human-to-human transmission of Chlamydia psittaci occurs not only between infected patients and their close contacts but also between secondary contacts. After looking back on relevant literature at home and abroad in the past ten years, this paper reviews the diagnosis, diagnosis and treatment, and progress in epidemiological research of Psittacosis pneumonia.
Keywords: Chlamydia psittaci, psittacosis, human-to-human transmission, re-emerging pathogen, mNGS
Chlamydia psittaci is a Gram-negative bacterium, and the infection of humans and poultry with this pathogen mainly manifests as community-acquired pneumonia (CAP). According to a meta-analysis,1 approximately 1–2% of the community-acquired pneumonia population is hospitalized for psittacosis every year, which is considered a rare infectious disease.2 Humans are mainly affected by direct contact with birds and poultry excrement, feathers, nasal secretions, etc.3 In previous studies, the human-to-human transmission was considered to be rare. However, the latest research4 shows that psittacosis Chlamydia has a variety of human-to-human transmission routes, not only between infected persons and contacts but also between three generations of human-to-human transmission, and asymptomatic carriers can also transmit the pathogen. In particular, the fetal mortality rate caused by psittacosis during pregnancy 22 was 82.6%, and the maternal mortality rate was 8.7%. In recent years, with the continuous development of diagnosis and treatment technology, metagenomic next-generation sequencing (mNGS). This detection method can quantitatively, rapidly, and accurately detect the pathogen of psittacosis,5 which makes the diagnosis clear and is conducive to guiding precise treatment. This article, mainly through the literature review, systematically expounds on the research progress of the parrot fever pneumonia epidemic and diagnosis and treatment technology.
Etiology
Chlamydia psittaci belongs to the Chlamydia family and is a gram-negative pathogen between viruses and rickettsia. Among them, Chlamydia psittaci can infect not only poultry but also the feathers and feces of infected poultry. To mammals, including humans. In addition, gram-negative pathogens that host humans include Chlamydia trachomatis and Chlamydia pneumoniae.19 According to statistics, more than 400 species of birds are susceptible to Chlamydia psittaci.20 Parrot fever pneumonia is a particular type of atypical pneumonia. The clinical manifestations are dry cough, fever, muscle aches, and gastrointestinal symptoms at first (Table 1). The symptoms are similar to common infections, so clinicians often ignore them and miss the best treatment time. The disease progresses rapidly, and severe cases can develop into systemic diseases and even die from meningitis, severe pneumonia, and dyspnea. Therefore, identifying the infection caused by the pathogen is essential to disease diagnosis and treatment to avoid public health risks and hospital society loss.
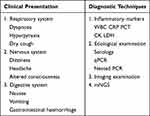 |
Table 1 Clinical Characteristics of Psittacine Pneumonia |
Epidemiology
C psittaci was initially recognized as a bird pathogen. In studies prior to 2013, the majority of transmission modes of psittacine pneumonia were restricted to human exposure to contaminated birds. Some literature11 mentioned the possibility of human-to-human transmission, but no precise study data was recorded. With sporadic outbreaks of psittacine pneumonia, there are increasing numbers of case reports in various countries, including developing and developed countries, such as Australia,6 Japan,7 Sweden,8 and Belgium.9 The earliest literature reporting an outbreak of psittacine pneumonia in humans dates back to 1876 (Ratter,1876). Until the late 19th century and early 20th century, psittacine pneumonia erupted again in Europe and America due to foreign bird trading (Hegler, 1930). Since 2020, the number of birds raised in families and farms has increased, so the mass aggregation of psittacine pneumonia has frequently occurred. Reports on the mode of transmission of Chlamydia psittaci are continuously updated. For example, studies in 197712 suggested that psittacine pneumonia could cause nosocomial infections. Eight people were secondary infections of C psittaci, and one was a patient in the same ward who was exposed to infected individuals. However, due to the limitations of diagnostic conditions at that time, serological tests could not definitively diagnose C psittaci, thus reducing the persuasion of epidemiology. In the same year, in another report, Hughes et al13 proposed that there may be the person-to-person transmission of psittacine pneumonia in hospitals. Subsequently, in the 2011 outbreak of psittacosis in Scotland,14 four family members and one healthcare worker exposed to the patient also developed community-acquired pneumonia (CAP) symptoms, some manifested as mild influenza symptoms, and others realized severe pneumonia. C psittaci latency varies between 7 and 28 days.15 Because the contact became ill within 7–28 days and the infecting agent was confirmed to be C psittaci by complement fixation test (CFT), it suggests that there is a possibility of human-to-human transmission. Given the possibility of human-to-human transmission of psittacine pneumonia has been reported successively, an outbreak occurred in Sweden in 2013,8 and infection was confirmed by polymerase chain reaction (PCR) in patients with the primary disease based on epidemiological investigation studies. The study showed that 10 ICU nurses, doctors, ward workers, wives, and children who came into contact with them were also ill, and two of them who had a history of bird contact, such as parrots, were excluded. One nurse out of the remaining eight patients was confirmed never to have direct contact with the patient, and this nurse is a nurse helper in charge of patients with primary disease. This suggests that there is a small range of human-to-human transmission of psittacine pneumonia and that secondary human-to-human transmission by infected individuals cannot be excluded. However, limited to the low number of cases collected at that time, similar reports of human-to-human transmission are particularly rare, and no clear literature data have been found to confirm them before 2020.
In recent years, domestic and foreign scholars have focused on the diagnostic methods and clinical features of psittacine pneumonia. Whether there are reports of interpersonal transmission in the second and third generations has not yet reached a definite conclusion. Only five cases of interpersonal transmission4,8,12–14 have been reported in the domestic and foreign literature so far (Table 2). Of concern, Zhang et al in found4 that all six infected individuals were admitted with intermittent fever and cough by studying six duck meat processing factory workers, 61 close receivers, and five close medical staff families, and all were diagnosed with Chlamydia psittaci infection by NGS. Among them, five cases of secondary infection were detected by nested PCR and qPCR using C psittaci DNA as primers, as well as three confirmed cases of contact with these five close contacts in the same space-time and space. In conclusion, comparing the previous literature, it was found that the most recent literature addresses its epidemiology differently than previously reported cases. On the one hand, the exposed poultry were ducks, including duck meat, duck feathers, and duck feces. Most of the previously reported cases, however, have a history of contact with poultry, such as birds (Allen et al, 1988), parrots, and pigeons (Gu et al, 2020). Since 2007, 3 cases16–18 related to closed ducks have been mentioned. According to statistics, the infection rate of poultry in China is as high as 6% −8%. On the other hand, this is the first published literature in China on the existence of a mass outbreak of human-to-human transmission of Chlamydia psittaci and has clearly documented data. Most importantly, it was demonstrated that C psittaci is transmitted through multiple routes, not only between infected individuals and contacts but also between subclause and third-generation infected individuals, and asymptomatic carriers can also transmit the pathogen.
 |
Table 2 Case Reports of Human-to-Human Transmission |
Diagnostic Techniques
According to records, cases of psittacosis have been reported for more than 40 years. Diagnostic techniques are constantly updated and changed. According to relevant retrospective studies published after 1986, psittacine pneumonia cannot be diagnosed by laboratory tests and chest CT alone. The diagnosis of community-acquired pneumonia21 mainly relies on X-ray examination showing patchy, patchy, or interstitial changes, elevated clinical symptoms, and infection indicators to confirm the diagnosis. There are no specific clinical manifestations of psittacine pneumonia, so non-specific examination and imaging alone are insufficient to diagnose Chlamydia psittaci infection. It is often missed or misdiagnosed, and how to confirm the pathogen is the focus and difficulty in its research progress.
Laboratory Tests
Non-Specific Test
Inflammatory markers such as elevated white blood cells, C-reactive protein, neutrophils, and PCT are typical indicators of infection and are not specific in terms of diagnosis. But it helps monitor the severity of the condition. At the same time, most patients are accompanied by hyponatremia, hypokalemia, and elevated transaminases. A retrospective study22 shows patients have elevated creatine kinase isoenzymes and lactate dehydrogenase (LDH). These indicators can also be indirectly measured in clinical work to monitor disease severity.
Etiological Examination
Accurate identification of pathogens, timely symptomatic treatment, and prevention of rapid disease progression are essential links in clinical diagnosis. Empirical use alone is not effective in community-acquired pneumonia. Pathological examination of psittacine pneumonia is difficult, so more and more clinicians want further study.
In serology,23 these include pathogen isolation, enzyme-linked immunosorbent assay (ELISA), specific complement fixation experiments, and MIF, the most commonly used of which is MIF. Serum antibody titers greater than a 4-fold increase in convalescent and acute phases, or IgM > 1:32, are diagnostic.24 However, the detection time is long; paired sera need to be collected at intervals of several weeks,25 which is not conducive to early diagnosis and treatment and is only suitable for retrospective diagnosis. PCR technique has 100% specificity and sensitivity to distinguish C psittaci from C. abortus.24 Although pathogens can be precisely detected, detection takes a long time (1–2 hours). In 2021, Pang et al26 proposed recombinant polymerase amplification (RPA), a rapid and effective novel method for detecting Chlamydia psittaci.
Real-time fluorescence PCR is a relatively novel detection technique that has the advantages of high specificity, high sensitivity, and rapidity. It has an absolute advantage in detecting C psittaci. The application of real-time PCR technology has a 1000-fold increase in sensitivity over conventional PCR technology in detecting chlamydial genome sensitivity. From 2005 to 2010,33 qPCR technology has been continuously improving and improving, and it has also been shown to have the advantages of high specificity and good reproducibility.
Imaging Examination
For chest CT with inconspicuous features, it has been documented that the lesion was initially located in the upper lobe and then gradually progressed to involve bilateral or multilobar infiltrates.27 Su et al analyzed 39 patients with C psittaci infection and found consolidation on chest CT in all 39 patients. Bronchial inflation sign and ground-glass opacity were observed in most of these patients, and paving stone sign was observed in a small proportion of patients.9,27,28 Seventy percent of these patients have pleural effusion manifestations, which are directly proportional to the severity of the disease, and severe pneumonia may present with bilateral pleural effusion. Hochhegger et al29 proposed that chest CT in psittacine pneumonia can present as nodular shadows in both lower lungs or peripheral halo signs, but imaging diagnosis always lacks specificity. It can be combined with medical history, laboratory tests, and clinical manifestations, but the final diagnosis depends on etiology.
mNGS
With the development of sequencing technology and biological information technology, metagenomic next-generation sequencing (mNGS) technology has a high clinical value in the diagnosis of infectious diseases. This technique does not rely on traditional microbial culture methods, but non-targeted sequencing of DNA/RNA from all microorganisms including bacteria, fungi, viruses, and parasites for a site. It is of great significance for clinical “precise diagnosis and treatment”, especially for the rapid identification of acute and critical diseases and difficult pathogens.33 Michael et al first reported mNGS technology for the detection of rare pathogens in 2014,34 which sheds new light and plays a decisive role in the diagnosis of psittacine pathogens. In 2022, Zhang et al first reported the existence of interpersonal transmission in the second and third generations of psittacine pneumonia by nested PCR, qPCR and mNGS combined detection techniques.4 Compared to PCR technology and antigen-antibody immunology, mNGS does not require known pathogen gene sequences and is therefore suitable for broad screening. In addition, compared with traditional cultures such as sputum culture, mNGS is time-consuming and efficient and can accurately grasp the optimal timing of treatment. Retrospective studies showed that the positive rate and sensitivity of mNGS detection in lavage fluid were increased by 44.6% and 15% compared with the traditional method, especially for detecting special pathogens, fungi, and viruses.30–32 mNGS is less affected by antibiotics than traditional cultures, and studies have mentioned that C psittaci can still be detected if quinolone antibiotics have been used before mNGS testing. In conclusion, as an efficient detection method for confirming pathogens and guiding medication, lavage fluid mNGS not only shortens the diagnosis and treatment time but also improves the screening efficiency and, more accurately, improves patients’ quality of life.
Summary
C psittaci has not been included in routine clinical laboratory screening, which is easily misdiagnosed and missed, with a misdiagnosis rate as high as 50%.10 Reported cases of psittacine pneumonia are increasing every year. Psittacine pneumonia, which, once infected, can result in extremely high mortality rates, is causing more cases, according to reports. In this paper, by reviewing the domestic and foreign literature, it is concluded that the outbreak is followed by secondary and tertiary human-to-human transmission, and there is a hidden chain of transmission between people. With the development of diagnosis and treatment technology, serological tests and imaging cannot confirm the diagnosis. Today emerging mNGS combined with Nest-PCR can significantly improve the specificity and sensitivity of psittacosis detection. It not only saves time but also improves the detection rate and facilitates clinicians to treat the cause promptly. However, for psittacine pneumonia, prevention is not yet mature, and new prevention methods or vaccine development need to be explored to prevent disease transmission. At the same time, society and the medical industry need to pay much attention to the potential threat of Chlamydia psittaci. It is recommended to be used as a routine screening for respiratory pathogens.
Funding
This work was supported by Shandong Provincial Traditional Chinese Medicine Science and Technology Project, China [Grant/Award Number: 2020Z21].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hogerwerf L, De Gier B, Baan B, Van Der Hoek W. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2017;145(15):3096–3105. doi:10.1017/S0950268817002060
2. Hogerwerf L, Roof I, de Jong MJK, Dijkstra F, van der Hoek W. Animal sources for zoonotic transmission of psittacosis: a systematic review. BMC Infect Dis. 2020;20(1):192. doi:10.1186/s12879-020-4918-y
3. Balsamo G, Maxted AM, Midla JW, et al. Compendium of measures to control chlamydia psittaci infection among humans (psittacosis) and pet birds (avian chlamydiosis), 2017. J Avian Med Surg. 2017;31(3):262–282. doi:10.1647/217-265
4. Zhang Z, Zhou H, Cao H, et al. Human-to-human transmission of Chlamydia psittaci in China, 2020: an epidemiological and aetiological investigation. Lancet Microbe. 2022;3(7):e512–e520. doi:10.1016/S2666-5247(22)00064-7
5. Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. 2019;14:319–338. doi:10.1146/annurev-pathmechdis-012418-012751
6. Polkinghorne A, Weston KM, Branley J. Recent history of psittacosis in Australia: expanding our understanding of the epidemiology of this important globally distributed zoonotic disease. Intern Med J. 2020;50(2):246–249. doi:10.1111/imj.14726
7. Iijima Y, Akiyoshi K, Tanaka S, et al. Psittacosis outbreak at an avian exhibition. Kansenshogaku zasshi. J Jpn Assoc Infect Dis. 2009;83(5):500–505.
8. Wallensten A, Fredlund H, Runehagen A. Multiple human-to-human transmission from a severe case of psittacosis, Sweden, January-February 2013. Euro Surveill. 2014;19(42):20937. doi:10.2807/1560-7917.ES2014.19.42.20937
9. Rybarczyk J, Versteele C, Lernout T, Vanrompay D. Human psittacosis: a review with emphasis on surveillance in Belgium. Acta Clin Belg. 2020;75(1):42–48. doi:10.1080/17843286.2019.1590889
10. Gorman J, Cook A, Ferguson C, van Buynder P, Fenwick S, Weinstein P. Pet birds and risks of respiratory disease in Australia: a review. Aust N Z J Public Health. 2009;33(2):167–172. doi:10.1111/j.1753-6405.2009.00365.x
11. Ojeda Rodriguez JA, Modi P, Brady MF. Psittacosis pneumonia. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
12. Broholm KA, Böttiger M, Jernelius H, Johansson M, Grandien M, Sölver K. Ornithosis as a nosocomial infection. Scand J Infect Dis. 1977;9(4):263–267. doi:10.3109/inf.1977.9.issue-4.02
13. Hughes C, Maharg P, Rosario P, et al. Possible nosocomial transmission of psittacosis. Infect Control Hosp Epidemiol. 1997;18(3):165–168. doi:10.2307/30141976
14. McGuigan CC, McIntyre PG, Templeton K. Psittacosis outbreak in Tayside, Scotland, December 2011 to February 2012. Euro Surveill. 2012;17(22):20186. doi:10.2807/ese.17.22.20186-en
15. Heymann DL. Control of Communicable Diseases Manual.
16. Haas WH, Swaan CM, Meijer A, et al. A Dutch case of atypical pneumonia after culling of H5N1 positive ducks in Bavaria was found infected with Chlamydophila psittaci. Euro Surveill. 2007;12(11):E071129. doi:10.2807/esw.12.48.03320-en
17. Hulin V, Bernard P, Vorimore F, et al. Assessment of chlamydia psittaci shedding and environmental contamination as potential sources of worker exposure throughout the mule duck breeding process. Appl Environ Microbiol. 2015;82(5):1504–1518. doi:10.1128/AEM.03179-15
18. Tiong A, Vu T, Counahan M, Leydon J, Tallis G, Lambert S. Multiple sites of exposure in an outbreak of ornithosis in workers at a poultry abattoir and farm. Epidemiol Infect. 2007;135(7):1184–1191. doi:10.1017/S095026880700790X
19. Elwell C, Mirrashidi K, Engel J. Chlamydia cell biology and pathogenesis. Nat Rev Microbiol. 2016;14(6):385–400. doi:10.1038/nrmicro.2016.30
20. Huminer D, Pitlik S, Kitayin D, Weissman Y, Samra Z. Prevalence of Chlamydia psittaci infection among persons who work with birds. Isr J Med Sci. 1992;28(10):739–741.
21. Prina E, Ranzani OT, Torres A. Community-acquired pneumonia. Lancet. 2015;386(9998):1097–1108. doi:10.1016/S0140-6736(15)60733-4
22. Kong CY, Zhu J, Lu JJ, Xu ZH. Clinical characteristics of Chlamydia psittaci pneumonia. Chin Med J. 2021;134(3):353–355. doi:10.1097/CM9.0000000000001313
23. Souriau A, Rodolakis A. Rapid detection of Chlamydia psittaci in vaginal swabs of aborted ewes and goats by enzyme linked immunosorbent assay (ELISA). Vet Microbiol. 1986;11(3):251–259. doi:10.1016/0378-1135(86)90027-1
24. Opota O, Jaton K, Branley J, et al. Improving the molecular diagnosis of Chlamydia psittaci and Chlamydia abortus infection with a species-specific duplex real-time PCR. J Med Microbiol. 2015;64(10):1174–1185. doi:10.1099/jmm.0.000139
25. Nieuwenhuizen AA, Dijkstra F, Notermans DW, van der Hoek W. Laboratory methods for case finding in human psittacosis outbreaks: a systematic review. BMC Infect Dis. 2018;18(1):442. doi:10.1186/s12879-018-3317-0
26. Pang Y, Cong F, Zhang X, et al. A recombinase polymerase amplification-based assay for rapid detection of Chlamydia psittaci. Poult Sci. 2021;100(2):585–591. doi:10.1016/j.psj.2020.11.031
27. Chen X, Cao K, Wei Y, et al. Metagenomic next-generation sequencing in the diagnosis of severe pneumonias caused by Chlamydia psittaci. Infection. 2020;48(4):535–542. doi:10.1007/s15010-020-01429-0
28. Beeckman DS, Vanrompay DC. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin Microbiol Infect. 2009;15(1):11–17. doi:10.1111/j.1469-0691.2008.02669.x
29. Hochhegger B, Marchiori E, Irion KL, Santos de Melo G, Mendes F, Zanetti G. Psittacosis presenting as a halo sign on high-resolution computed tomography. J Thorac Imaging. 2009;24(2):136–137. doi:10.1097/RTI.0b013e318191987e
30. Miao Q, Ma Y, Wang Q, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(suppl_2):S231–S240. doi:10.1093/cid/ciy693
31. Langelier C, Zinter MS, Kalantar K, et al. Metagenomic sequencing detects respiratory pathogens in hematopoietic cellular transplant patients. Am J Respir Crit Care Med. 2018;197(4):524–528. doi:10.1164/rccm.201706-1097LE
32. Parize P, Muth E, Richaud C, et al. Untargeted next-generation sequencing-based first-line diagnosis of infection in immunocompromised adults: a multicentre, blinded, prospective study. Clin Microbiol Infect. 2017;23(8):574.e1–574.e6. doi:10.1016/j.cmi.2017.02.006
33. Kawada J, Okuno Y, Torii Y, et al. Identification of viruses in cases of pediatric acute encephalitis and encephalopathy using next-generation sequencing. Sci Rep. 2016;6:33452. doi:10.1038/srep33452
34. Wilson MR, Naccache SN, Samayoa E, et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370(25):2408–2417. doi:10.1056/NEJMoa1401268
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
