Back to Journals » Infection and Drug Resistance » Volume 9
Pseudomonas aeruginosa ventilator-associated pneumonia management
Authors Ramirez Estrada S, Borgatta B, Rello J
Received 22 June 2015
Accepted for publication 15 October 2015
Published 20 January 2016 Volume 2016:9 Pages 7—18
DOI https://doi.org/10.2147/IDR.S50669
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Sergio Ramírez-Estrada,1 Bárbara Borgatta,1,2 Jordi Rello3,4
1Critical Care Department, Vall d'Hebron University Hospital, 2CRIPS, Vall d'Hebron Institute of Research (VHIR), 3Department of Medicine, Universitat Autònoma de Barcelona (UAB), Barcelona, 4Centro de Investigación Biomédica en Red Enfermedad Respiratoria – CIBERES, Madrid, Spain
Abstract: Ventilator-associated pneumonia is the most common infection in intensive care unit patients associated with high morbidity rates and elevated economic costs; Pseudomonas aeruginosa is one of the most frequent bacteria linked with this entity, with a high attributable mortality despite adequate treatment that is increased in the presence of multiresistant strains, a situation that is becoming more common in intensive care units. In this manuscript, we review the current management of ventilator-associated pneumonia due to P. aeruginosa, the most recent antipseudomonal agents, and new adjunctive therapies that are shifting the way we treat these infections. We support early initiation of broad-spectrum antipseudomonal antibiotics in present, followed by culture-guided monotherapy de-escalation when susceptibilities are available. Future management should be directed at blocking virulence; the role of alternative strategies such as new antibiotics, nebulized treatments, and vaccines is promising.
Keywords: multidrug-resistant, ICU, new-antibiotics, adjunctive-therapies, care-bundles
Background
Ventilator-associated pneumonia (VAP) is the most common infection among the critically ill and the first cause of antibiotic prescription in intensive care units (ICUs), with an incidence of five to 20 cases per 1,000 mechanical ventilation (MV)-days and a global prevalence of 15.6%1–5 that has not changed significantly despite the implementation of care bundles. Episodes caused by multidrug-resistant (MDR) organisms, such as Pseudomonas aeruginosa are associated with significant attributable mortality;3,6 VAP represents a major clinical and economical problem in critically ill patients due to its associated morbidity, prolonged MV-days, and ICU length of stay (LOS), which translates to elevated health care costs as high as US$40,000 per episode.7,8
P. aeruginosa (with Staphylococcus aureus) is one of the most common bacteria causing VAP,5,9 with a prevalence of approximately 4%,2 and its attributable mortality is as high as 13.5%, even with adequate antibiotic treatment.3 In MDR strains, mortality rises up to 35.8%, and the presence of MDR strains has been identified as an independent predictor of hospital death (adjusted odds ratio [AOR] 1.634, 95% confidence interval [CI]: 1.124–2.374) and is the single strongest predictor of initial inadequate antibiotic therapy (AOR 5.706, 95% CI: 3.587–9.077).5,9 A recent study by Micek et al demonstrated that P. aeruginosa VAP mortality has increased to 41.9%, with increased age and Charlson comorbidity score, inappropriate initial antibiotic therapy, and vasopressor use as independent predictors of mortality.10 Antibiotic resistance has been on the rise in the last decade,5,11–13 which is worrisome since P. aeruginosa is one of the three top microorganisms causing health care respiratory infection and is resistant to carbapenem,14 and, even in patients with early-onset VAP and no risk factors, MDR P. aeruginosa is frequent.15,16 Among known risk factors for MDR P. aeruginosa in MV patients, the most frequent are antimicrobial therapy within 90 days (51.9%) and current hospitalization of more than or equal to 5 days (45.3%).2 Infection by MDR P. aeruginosa is associated with worse outcomes with an excess mortality rate of 12 with a more than twofold increased risk of mortality (relative risk [RR] 2.34, 95% CI: 1.53–3.57) and ICU LOS, compared to susceptible strains.11 In VAP caused by MDR P. aeruginosa,10,17 both prior antibiotic use and delayed effective antibiotic therapy in infection also negatively affect mortality and cost.5,18,19
P. aeruginosa serotypes causing VAP have different behavior; O6 and O11, the most common, are associated with a clinical resolution of 60%, and serotypes O1 and O2, represent less common strains, with higher mortality.16 Vallés et al performed an analysis of pulsed-field electrophoresis on more than 1,700 isolates of P. aeruginosa in ICU patients, identifying different genotypes. Clones that were responsible for colonization (skin, gut, and respiratory) least frequently caused pneumonia, and VAP’s resolution was frequent and uncomplicated. However, clones that were not related to prior colonization were associated with very high mortality rates.20 This observation may be associated with the expression of virulence factors in P. aeruginosa, such as type III secretory proteins.21
Most clonally related isolates caused gastric colonization before skin or respiratory tract colonization, suggesting an association with instillation of tap water used for medication by the oral route. A similar study conducted in two different ICUs in a single hospital in France4 identified an MDR clone of P. aeruginosa in the sinks of 12 rooms. As a whole, from 26 cases of colonization/infection by P. aeruginosa, five were related to an exogenous colonization (environmental colonization in four patients and cross-infection in one). These findings emphasize the fact that different risk factors may be implicated depending on whether the clone is from exogenous contamination or carried as endogenous colonization. Therefore, different infection control strategies should be applied to prevent colonization of patients with P. aeruginosa, including strategies to limit the potential of sinks to act as potential reservoirs.
Risk factors
Risk factors for P. aeruginosa in VAP are mainly prior antibiotic exposure and MV longer than 5 days.22–24 Patients with chronic obstructive pulmonary disease and other chronic respiratory diseases may carry endogenous colonization and can develop a severe respiratory infection following intubation and MV. Interestingly, risk factors in patients with P. aeruginosa and prior antibiotic exposure are different.25 P. aeruginosa is the first cause of pneumonia in the postoperative period of lung transplant26 and in intubated patients with a prior episode of pneumonia.27 P. aeruginosa is also the most common pathogen in patients with health care-associated pneumonia who required ICU admission and further MV.28
Current management
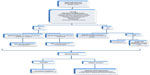
Latest guidelines for the antibiotic treatment of P. aeruginosa VAP are the 2005 American Thoracic Society/Infectious Diseases Society of America guidelines, which recommend combination therapy with antipseudomonal cephalosporin (cefepime, ceftazidime) or carbapenem (imipenem, meropenem, or β-lactam/β-lactamase inhibitor [piperacillin–tazobactam]) plus antipseudomonal fluoroquinolone (ciprofloxacin or levofloxacin) or aminoglycoside.29 However, since their publication a decade ago, many findings have been made in the field of antibiotic management in the critically ill, highlighting inappropriate treatment due to insufficient dosing and suboptimal antibiotic exposure, which are associated with increased mortality and worse outcomes.30–33 Furthermore, the rise of MDR strains in nosocomial pneumonia renders this approach outdated.12,34 It is important to bear in mind that it is critical to avoid antibiotics to which the patient has been exposed over the last 30 days, since the new episodes usually are relapses of a strain with phenotypic variations and not reinfection. Also, recently, a multicenter study has shed some light regarding treatment failure in P. aeruginosa VAP. With an occurrence rate of approximately 30% of episodes, the study identified risk factors for failure, including age, chronic illness, limitation of life support, severity of illness, previous use of a fluoroquinolone, and bacteremia. Interestingly, neither antibiotic susceptibility patterns nor combination therapy influenced failure rates; on the other hand, treatment with a fluoroquinolone did decrease it.35 Figure 1 outlines initial P. aeruginosa VAP management.
To avoid suboptimal antibiotic management, we believe that a composite approach has to be made, taking into account variables other than the classic microbiological paradigm of appropriate antibiotic therapy based only in minimum inhibitory concentration (MIC)’s susceptibility patterns and tailoring treatment to each patient, assessing specific risk factors especially for MDR (Figure 1).36 The cornerstone for improving outcomes is timing; early effective therapy as soon as possible might be the difference between death and successful treatment, especially when shock is present.37,38 Appropriate empirical choice of agent is fundamental, as is the use of a broad-spectrum antibiotic based on local ecology followed by reassessment of clinical response and microbiological data at 48–72 hours.39,40 In P. aeruginosa VAP, empiric combination therapy with a β-lactam plus an aminoglycoside has proved to be superior to monotherapy, reducing mortality up to 50% in many studies and meta-analyses, mainly due to appropriate initial therapy.40–42 However, there is no difference between one or two effective antibiotics, which is the rationale for de-escalating to monotherapy once microbiological results are available.42 De-escalation is a safe strategy and has to be done when possible, even in neutropenic patients.43 Regarding duration of therapy, many studies have demonstrated that 8 days of antibiotic for VAP is safe, reduces emergence of MDR and costs, and avoids unnecessary toxicity to the patient.44–47 However, in VAP caused by gram-negative bacilli, 8 vs 15 days of antibiotic is associated with increased pulmonary infection recurrence.45 Since the aim of antibiotic therapy is pneumonia resolution and not P. aeruginosa eradication from the endotracheal tube/tracheostomy biofilm, antibiotic courses longer than 10 days in patients with clinical cure only add MDR-strain selection. In P. aeruginosa VAP, patients with inappropriate empirical antibiotic therapy, clinical resolution (fever and hypoxemia) is delayed 8 days (median), as happens with other MDR bacteria.46 Furthermore, longer antibiotic courses may be recommended for immunosupressed patients with initial inappropriate empirical therapy VAP caused by MDR/extensively drug-resistant strains without clinical resolution.47 Recently, biomarkers’ roles in antibiotic duration guidance have been the subject of multiple studies, with procalcitonin being the only one that has proved to be safe and reduce antibiotic days in VAP. When procalcitonin concentration is <0.5 ng/mL or has decreased by ≥80% (compared with the first peak concentration), antibiotics can be discontinued even in very short-course therapy (3 days), irrespective of the severity of the infectious episode; however, in bacteremic patients, at least 5 days of therapy is recommended.48–50
Another point to consider is optimizing the choice of antimicrobial according to pharmacokinetic (PK)/pharmacodynamic parameters. It is important to bear in mind that the antibiotic we choose has to reach therapeutic concentrations at the site of infection, where the bacteria–antibiotic interaction takes place, in order to obtain bacterial clearance as soon as possible.51 Also, administration of a loading dose and administration of β-lactams in extended and continuous infusions increases antibiotic exposure and the probability of PK target attainment, which is essential in cases of septic shock, obesity, burn patients, and intermediate-resistant P. aeruginosa strains,32 and it is associated with decreased 14-day mortality, faster recovery, and shorter ICU LOS and duration of treatment.52–64 With this in mind, nebulized antibiotic administration in MV may increase alveolar penetration compared with IV administration.47 Nebulized colistin (high dose) in monotherapy has been studied in a small-randomized trial and a retrospective study, and noninferiority to IV combination therapy has been reported.65–67 This approach is very interesting since it enables delivery of high concentrations of the antibiotic with minimal absorption and marginal systemic levels, which could be a turning point in cases of MDR strains where available drugs are highly toxic. Effective treatment of VAP caused by MDR organisms such as P. aeruginosa and Acinetobacter baumannii has been reported with high-dose nebulized colistin, even achieving airway eradication.65 Currently, a few agents are available for nebulization (colistin, tobramycin, aztreonam, ceftazidime, and amikacin) but are required to be tested in randomized clinical trials to know the safety and what adds to standard therapy. Further research and evidence-based guidelines are required. Other nebulized agents such as hypertonic saline and N-acetylcysteine, sometimes used as coadjutant therapy in the treatment of P. aeruginosa lung infection in cystic fibrosis patients, are still controversial, without strong evidence supporting or advice against its use in VAP treatment.68–72
New antibiotic treatments
Cephalosporins
Proven efficacy, broad spectrum (some of them including P. aeruginosa), and a well-characterized PK/pharmacodynamic profile, in addition to a favorable safety profile, make this antimicrobial class play an important role in nosocomial infection treatment, including VAP.73 In response to the emergence of nosocomial infections due to β-lactam-resistant gram-negative bacteria in recent years, two strategies have been developed to improve their coverage: the development of new β-lactam molecules with the capacity to evade some mechanisms expressed by resistant bacteria and the addition of novel compounds capable of inactivating β-lactamases.74
Ceftobiprole (BAL9141)
Ceftobiprole medocaril has enhanced activity against gram-negative pathogens, including Escherichia coli, Klebsiella pneumoniae, A. baumannii, and other Enterobacteriaceae; its antipseudomonal in vitro activity is similar to that of cefepime, and P. aeruginosa cross-resistance between ceftobiprole and other antipseudomonal cephalosporins has been reported.75,76 Also, it is inactive against bacteria expressing extended-spectrum β-lactamase (ESBL).75,76 Its bactericidal activity also acts against gram-positive bacteria, including resistant Streptococcus pneumoniae, methicillin-resistant S. aureus, and Enterococcus faecalis, but not against Enterococcus faecium.78 Its activity against some of the ESKAPE pathogens and its stability against a wide range of β-lactamases (not KPC) make it an attractive option for hospital-acquired pneumonia treatment. A total of 781 patients were included in a Phase III study, 210 of whom had VAP. Clinical cure rates overall were 49.9% and 52.8% for ceftobiprole and ceftazidime/linezolid, respectively. However, while the cure rates were not different in nosocomial pneumonia, ceftobiprole performed worse on VAP (23.1% vs 36.5 cure rate). In contrast, those patients who had to be ventilated because of worsening of the pneumonia had a better outcome with ceftobiprole than with ceftazidime/linezolid (Table 1).77 These findings might be associated with increases in distribution volume in septic patients receiving sedation to start MV, which cannot be anticipated using Monte Carlo simulation.
Ceftazidime–avibactam
Ceftazidime is a well-known antipseudomonal cephalosporin, also active against other gram-negative bacilli and gram-positive cocci and playing an important role in the treatment of nosocomial infections; however, it is susceptible to degradation due to β-lactamases, especially those of Ambler class A and C. Avibactam (NXL 104), recently added to the three approved β-lactamase inhibitors, is a molecule capable of avoiding the activity from A-, B-, and some D-class β-lactamases, including AmpC, KPC (Klebsiella pneumoniae carbapenemase), and ESBL.73,74,78,79 Despite not having antibacterial activity, its union with ceftazidime protects it from degradation from β-lactamases, enhancing its activity against Enterobacteriaceae producing β-lactamases, including P. aeruginosa.79,80 In a murine model, ceftazidime–avibactam has shown good penetration of epithelial lining fluid and effectiveness against P. aeruginosa with an MIC up to 32 μg/mL.81 Ceftazidime–avibactam exhibits a great in vitro MIC50/90 reduction against P. aeruginosa producing β-lactamases compared with ceftazidime alone and also shows activity against some meropenem-non-susceptible strains in catheter-associated urinary tract infection.74,82,83 Phase II trials with ceftazidime avibactam have shown favorable results, a good safety profile, and have been well tolerated when used alone for complicated urinary infections, and when used with metronidazol for intra-abdominal infections.84,85 Its role in nosocomial pneumonia is actually being analyzed in a Phase III study (Table 1).86 Caution should be taken into account in countries/institutions where the main resistance problem is OXA-48, and consideration given to the need for initial loading dose, to avoid the potential risk of initial underdosing, particularly in those patients with decreased creatinine clearance.
Ceftolozane–tazobactam (CXA-201)
Like other cephalosporins, ceftolozane develops its bactericidal activity by inhibiting the cell wall synthesis via penicillin-binding proteins; particularly, ceftolozane has shown an enhanced affinity for these proteins in comparison with β-lactams.87 In vitro studies suggest it is not affected by some β-lactam resistance mechanisms expressed by P. aeruginosa, such as efflux pumps or reduced wall permeability due to porin channel mutations,88,89 making it the most active antipseudomonal β-lactam.90,91 However, by itself it does not have activity against β-lactamase-producing strains. Tazobactam’s activity against β-lactamases bring to ceftolozane the potential to eliminate many resistant strains of P. aeruginosa and other β-lactamase-producing gram-negative bacteria.92 A Phase III trial has shown ceftolozane–tazobactam’s efficacy in complicated intra-abdominal infections in combination with metronidazole, including those caused by MDR pathogens,93 and a Phase II trial also demonstrated its efficacy in complicated urinary tract infection treatment.94 Currently, a Phase III study is evaluating its safety and efficacy in VAP (Table 1).95
Arbekacin
Arbekacin is an aminoglycoside discovered in the 1970s and has been used in many countries for more than 2 decades. Usually indicated in the treatment of infections caused by methicillin-resistant S. aureus, it has also shown activity against gram-negative pathogens, including Pseudomonas spp. Its capacity to be unaltered by many of the aminoglycoside-modifying enzymes, one of the most frequent ways by which aminoglycosides are inactivated, confers to arbekacin enhanced activity against P. aeruginosa resistant to amikacin, gentamicin, and tobramycin.93,96 In vitro analysis suggests that arbekacin in combination with aztreonam is an effective regimen against MDR P. aeruginosa, including metallo-β-lactamase-producing strains;93 however, further studies are needed to show its applicability and safety in clinical practice. In PK studies, arbekacin has shown acceptable pulmonary tissue distribution and an adequate safety profile;97 however, therapeutic plasma level monitoring is recommended to optimize its efficacy and minimize adverse effects, mainly nephrotoxicity.93
POL7080
POL7080 is a novel peptidomimetic antibiotic with proven activity against P. aeruginosa in murine models.98 Its mechanism of action is not totally clear, but it is known that it modifies the lipopolysaccharide-assembling of the bacterial outer membrane via the lipopolysaccharide-assembling protein LptD.98 A Phase I study has shown POL7080 to be safe and well tolerated,99 and actually a Phase II study is evaluating its safety and efficacy in patients with VAP due to P. aeruginosa (Table 1).100 Nephrotoxicity is a major concern with this drug.
Pathogenicity and newer adjunctive therapies
Pathogenicity
P. aeruginosa’s pathogenicity is very complex,101–103 and a detailed analysis is far from the objective of this report. During a host’s infection process, P. aeruginosa uses pili, flagella, and fimbriae, a series of functional elements, to move and adhere on living and nonliving surfaces, such as different tissues and medical devices,104,105 and also employs these mobile elements to form bacterial communities based on an intricate intercellular communication mechanism (ie, quorum sensing), many times surrounded by a polysaccharide-based structure known as biofilm. This structure is produced by the bacterial colony and acts as a barrier against different chemical factors and physical forces (eg, immune system response and antibiotics), providing a favorable environment for colony survival and playing an important role in its permanency and in the chronic colonization/infection process.21,104,106,107
Many of the steps in the biofilm formation process are being highly investigated as treatment targets, with many others not being completely understood yet.21
Alginate is a very important virulence variable, affecting children with cystic fibrosis.108,109 However, cystic fibrosis patients carry mucosal strains110 which are uncommon in patients with VAP, requiring different therapeutic considerations.
Quorum sensing
Quorum sensing is an evolved adaptive strategy expressed in several gram-negative and gram-positive bacteria species, based on a highly complex cell-to-cell communication mechanism, which allows a group of bacteria to exchange information and make dynamic and coordinated changes in response to different environmental stimuli, thus playing an important role in host infection and the bacterial permanence.111 This system is based on signal molecules expressed by bacteria in a density-dependent way and released to the environment; these molecules are called autoinducers and are recognized by other cells, in some cases from different species (eg, between P. aeruginosa and Burkholderia cepacia), inducing genomic changes and giving to a population of bacteria the ability to deploy coordinated responses to affront different environmental assaults.111–113 With three known autoinducers from the acyl-homoserine lactone (AHL) family, Las, Rhl, and the P. aeruginosa quinolone signal, P. aeruginosa has one of the most classical and understood quorum sensing models, involved in many defense mechanisms such as biofilm formation and production of antimicrobial substances and bacterial virulence factors.21,111,112,114 This communication system facilitates host infection, ensures the permanency of colonies, and makes eradication of these colonies difficult, making it a highly attractive target for novel treatments. Three targets in this communication circuit have been identified as susceptible to pharmacological intervention: the inhibition of both Las and Rhl synthesis, the autoinducers’ degradation, and the blockage of AHL receptor function,21,111,115 with several in vitro and animal model trials demonstrating the blockade of the quorum sensing as a feasible strategy to reduce the bacterial virulence and restore some P. aeruginosa susceptibility to classical antibiotics. However, further investigations are needed to evaluate its role in the treatment of human infections due to MDR P. aeruginosa.
Monoclonal antibodies
Current research in the management of P. aeruginosa infection has been directed toward prevention of infection in high-risk patients with vaccines and modulation of virulence with monoclonal antibodies instead of focusing on bacterial clearance attainment. Its main appeal relies on multidrug therapy with one molecule targeting mechanisms of action of bacteria covering MDR strains and probably active in different infection models.
Monoclonal anti-type three secretion system antibodies
Type three secretion system, known as TTSS or T3SS, is a complex system expressed by some bacteria which allows intoxication of host cells. This system is present in many gram-negative bacteria, including P. aeruginosa, and is based in several groups of proteins (more than 20) exhibited in the bacterial wall, which acts as a syringe, making the bacteria capable of injecting modulation factors and cytotoxins into other eukaryotic organisms, including the immune host apparatus and epithelial cells, inducing cellular death and playing an important role in P. aeruginosa virulence and in the inflammatory response.116–119 TTSS is a marker of virulence in P. aeruginosa penumonia110 and its presence in patients with VAP is associated with worse outcomes.119,120 TTSS plays an important role in VAP, since worse clinical outcomes are seen when TTSS is present. An obvious implication of this is that adjunctive therapies targeting these proteins, such as antibodies, may improve outcomes of patients under MV and P. aeruginosa respiratory isolation (both colonization and infection).119,121 The PcrV is a needle-tip protein involved in many steps of the TTSS-mediated infection process, sensing the outside environment and helping bacteria to recognize the strange cells. It also plays a role in translocation and secretion control of some proteins involved in functional molecular syringe assembling and facilitating the union into the molecular needle and the host membrane, which makes an attractive target in TTSS-mediated virulence control, with studies showing loss of virulence capacity in bacteria with an unfunctional PcrV, both in in vitro and in vivo animal models.116,121 Based on this idea, antibodies have been developed for the blockage of PcrV protein function, with many studies reporting a decrease in blood bacterial colonies and a less severe inflammatory response in various animal models treated with anti-PcrV immunoglobulins.117,122–124 One of the most successful is the KB001, a high-affinity PEGylated Fab antibody, which, in a Phase II study, has been well tolerated and showed a safety profile in mechanically ventilated patients colonized by P. aeruginosa, also showing a nonstatistically significant tendency to reduce P. aeruginosa pneumonia episodes in the intervention group (Table 2).125
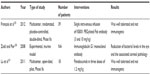  | Table 2 Studies regarding the effect of adjunctive therapies on Pseudomonas aeruginosa infection |
Monoclonal anti-alginate antibodies
Alginate is involved in many processes during P. aeruginosa infection, providing protection against a variety of host defense mechanisms and environmental factors such as antimicrobial agents; it also is highly present in mucoid biofilms and facilitates medical device colonization.105,107,126 This exopolysaccharide, principally exhibited by mucoid strains of P. aeruginosa, is capable of reducing the host immune response by interfering with the activation of complements and polymorphonuclear chemotaxis, and also was shown to play a role in decreasing the phagocytosis of Pseudomonas spp., both those that are planktonic and those that form biofilm structure guaranteeing the P. aeruginosa survival during the first steps of primary infection, its permanency, and its chronic colonization development.105,107,127
Different monoclonal antibodies against alginate have been developed, showing an increase in P. aeruginosa phagocytosis. In some cases, as with the monoclonal antibody F429, this improvement in immune response against P. aeruginosa infection was also reported in different models of infection such as pneumonia, sepsis, and keratitis in animal models,109,128 being promising as an adjunctive strategy in P. aeruginosa infection management (Table 2).
Panobacumab (AR-101)
Panobacumab is an IgM-type human monoclonal antibody that is directed against IATS 011 serotype P. aeruginosa, one of the most prevalent serotypes associated with nosocomial pneumonia.16,129,130 A multicenter Phase II study using panobacumab in combination with different antipseudomonal antibiotics in critical patients with nosocomial pneumonia due to P. aeruginosa serotype O11, almost all with VAP, showed a good safety profile with good PKs (Table 1).131,132
Vaccines
P. aeruginosa’s infection mechanism and its interaction with the host immunity is highly studied and well known. With the advances in antimicrobial therapy and many sites identified as possible targets to improve the acquired immunity response and block the P. aeruginosa infection and biofilm formation, different types of vaccines are being designed to improve the immune response against many substances involved in this process. The most common targets are components of the bacterial surface, such as outer membrane proteins (Opr) and different polysaccharides (lipopolysaccharides, mucoid exopolysaccharide, and O-polysaccharides), structures involved in P. aeruginosa adhesion and movement, such as flagella, pili, and several virulence factors, such as TTSS, exotoxin A, or proteases.133,134 Development of an effective vaccine is difficult due to the high variability between Pseudomonas species and the complexity of its infection process and its interaction with the host immune response. In many cases during phase I, II and III studies, some molecules failed to provide an adequate coverage against different P. aeruginosa strains, or showed a low inmunogenicity capacity or an unsecure profile.133–135
One of the most promising targets to induce an acquired immune response are the Opr, showing an improved immune response against P. aeruginosa infection in murine models previously exposed to modified epitopes from Opr.133,135,136 From this group, the Opr-based vaccine IC43 has been used in healthy individuals and in different groups with increased risk to develop P. aeruginosa infection, including critical patients under MV, showing a good safety profile and being well tolerated,137–140 and there is an ongoing Phase II/III study designed to show its effect on mortality in mechanically ventilated ICU patients.141
Conclusion
P. aeruginosa VAP management requires prompt and adequate antibiotic exposure. Initial empiric therapy should be done with broad-spectrum antibiotics in combination therapy followed by de-escalation with one effective antibiotic since its effectiveness equals two antibiotics. Immunotherapy, including strategies with monoclonal antibodies, might be a new approach to treat (and perhaps prevent) P. aeruginosa infections. Future research should focus on optimizing outcomes with strategies of blocking virulence and vaccination.
Disclosure
Jordi Rello has served in the Advisory Boards and Speakers Bureau of Cubist. The authors report no other conflicts of interest in this work.
References
Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867–903. | |
Kollef MH, Chastre J, Fagon JY, et al. Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Crit Care Med. 2014;42(10):2178–2187. | |
Rello J, Jubert P, Vallés J, Artigas A, Rué M, Niederman MS. Evaluation of outcome for intubated patients with pneumonia due to Pseudomonas aeruginosa. Clin Infect Dis. 1996;23(5):973–978. | |
Berthelot P, Grattard F, Mahul P, et al. Prospective study of nosocomial colonization and infection due to Pseudomonas aeruginosa in mechanically ventilated patients. Intensive Care Med. 2001;27(3):503–512. | |
Tumbarello M, De Pascale G, Trecarichi EM, et al. Clinical outcomes of Pseudomonas aeruginosa pneumonia in intensive care unit patients. Intensive Care Med. 2013;39(4):682–692. | |
Melsen WG, Rovers MM, Groenwold RH, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13(8):665–671. | |
Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33(10):2184–2193. | |
Bouadma L, Sonneville R, Garrouste-Orgeas M, et al; OUTCOMEREA Study Group. Ventilator-associated events: prevalence, outcome, and relationship with ventilator-associated pneumonia. Crit Care Med. 2015;43(9):1798–1806. | |
Micek S, Johnson MT, Reichley R, Kollef MH. An institutional perspective on the impact of recent antibiotic exposure on length of stay and hospital costs for patients with gram-negative sepsis. BMC Infect Dis. 2012;12:56. | |
Micek ST, Wunderink RG, Kollef MH, et al. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: impact of multidrug resistance. Crit Care. 2015;19:219. | |
Nathwani D, Raman G, Sulham K, Gavaghan M, Menon V. Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2014; 3(1):32. | |
Vincent JL, Rello J, Marshall J, et al; EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. | |
Wang CY, Jerng JS, Chen KY, et al. Pandrug-resistant Pseudomonas aeruginosa among hospitalised patients: clinical features, risk-factors and outcomes. Clin Microbiol Infect. 2006;12(1):63–68. | |
Leroy O, d’Escrivan T, Devos P, Dubreuil L, Kipnis E, Georges H. Hospital-acquired pneumonia in critically ill patients: factors associated with episodes due to imipenem-resistant organisms. Infection. 2005;33(3):129–135. | |
Martin-Loeches I, Deja M, Koulenti D, et al; EU-VAP Study Investigators. Potentially resistant microorganisms in intubated patients with hospital-acquired pneumonia: the interaction of ecology, shock and risk factors. Intensive Care Med. 2013;39(4):672–681. | |
Lu Q, Eggimann P, Luyt CE, et al. Pseudomonas aeruginosa serotypes in nosocomial pneumonia: prevalence and clinical outcomes. Crit Care. 2014;18(1):R17. | |
Sandiumenge A, Lisboa T, Gomez F, Hernandez P, Canadell L, Rello J. Effect of antibiotic diversity on ventilator-associated pneumonia caused by ESKAPE organisms. Chest. 2011;140(3):643–651. | |
Peña C, Gómez-Zorrilla S, Oriol I, et al. Impact of multidrug resistance on Pseudomonas aeruginosa ventilator-associated pneumonia outcome: predictors of early and crude mortality. Eur J Clin Microbiol Infect Dis. 2013;32(3):413–420. | |
Rello J, Borgatta B, Lisboa T. Risk factors for Pseudomonas aeruginosa pneumonia in the early twenty-first century. Intensive Care Med. 2013;39(12):2204–2206. | |
Vallés J, Mariscal D, Cortés P, et al. Patterns of colonization by Pseudomonas aeruginosa in intubated patients: a 3-year prospective study of 1,607 isolates using pulsed-field gel electrophoresis with implications for prevention of ventilator-associated pneumonia. Intensive Care Med. 2004;30(9):1768–1775. | |
Veesenmeyer JL, Hauser AR, Lisboa T, Rello J. Pseudomonas aeruginosa virulence and therapy: evolving translational strategies. Crit Care Med. 2009;37(5):1777–1786. | |
Rello J, Ausina V, Ricart M, et al. Risk factors for infection by Pseudomonas aeruginosa in patients with ventilator-associated pneumonia. Intensive Care Med. 1994;20(3):193–198. | |
Rello J, Lisboa T, Koulenti D. Respiratory infections in patients undergoing mechanical ventilation. Lancet Respir Med. 2014;2(9):764–774. | |
Rello J, Borgatta B, Lagunes L. Management of Pseudomonas aeruginosa pneumonia: one size does not fit all. Crit Care. 2014;18(2):136. | |
Rello J, Allegri C, Rodriguez A, et al. Risk factors for ventilator-associated pneumonia by Pseudomonas aeruginosa in presence of recent antibiotic exposure. Anesthesiology. 2006;105(4):709–714. | |
Riera J, Caralt B, López I, et al; Vall d’Hebron Lung Transplant Study Group. Ventilator-associated respiratory infection following lung transplantation. Eur Respir J. 2015;45(3):726–737. | |
Rello J, Mariscal D, March F, et al. Recurrent Pseudomonas aeruginosa pneumonia in ventilated patients: relapse or reinfection? Am J Respir Crit Care Med. 1998;157(3 Pt 1):912–916. | |
Vallés J, Mesalles E, Mariscal D, et al. A 7-year study of severe hospital-acquired pneumonia requiring ICU admission. Intensive Care Med. 2003;29(11):1981–1988. | |
American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. | |
Roberts JA, Abdul-Aziz MH, Lipman J, et al; International Society of Anti-Infective Pharmacology and the Pharmacokinetics and Pharmacodynamics Study Group of the European Society of Clinical Microbiology and Infectious Diseases. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14(6):498–509. | |
Taccone FS, Laterre PF, Dugernier T, et al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care. 2010;14(4):R126. | |
Roberts JA, Paul SK, Akova M, et al; DALI Study. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072–1083. | |
Blot S, Koulenti D, Akova M, et al. Does contemporary vancomycin dosing achieve therapeutic targets in a heterogeneous clinical cohort of critically ill patients? Data from the multinational DALI study. Crit Care. 2014;18(3):R99. | |
Kett DH, Cano E, Quartin AA, et al; Improving Medicine through Pathway Assessment of Critical Therapy of Hospital-Acquired Pneumonia (IMPACT-HAP) Investigators. Implementation of guidelines for management of possible multidrug-resistant pneumonia in intensive care: an observational, multicentre cohort study. Lancet Infect Dis. 2011;11(3):181–189. | |
Planquette B, Timsit JF, Misset BY, et al; OUTCOMEREA Study Group. Pseudomonas aeruginosa ventilator-associated pneumonia. Predictive factors of treatment failure. Am J Respir Crit Care Med. 2013;188(1):69–76. | |
Borgatta B, Rello J. How to approach and treat VAP in ICU patients. BMC Infect Dis. 2014;14:211. | |
Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. | |
Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42(8):1749–1755. | |
Sandiumenge A, Diaz E, Bodí M, Rello J. Therapy of ventilator-associated pneumonia. A patient-based approach based on the ten rules of “The Tarragona Strategy”. Intensive Care Med. 2003;29(6):876–883. | |
Garnacho-Montero J, Corcia-Palomo Y, Amaya-Villar R, Martin-Villen L. How to treat VAP due to MDR pathogens in ICU patients. BMC Infect Dis. 2014;14:135. | |
Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519–527. | |
Garnacho-Montero J, Sa-Borges M, Sole-Violan J, et al. Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: an observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit Care Med. 2007;35(8):1888–1895. | |
Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41–49. | |
Capellier G, Mockly H, Charpentier C, et al. Early-onset ventilator-associated pneumonia in adults randomized clinical trial: comparison of 8 versus 15 days of antibiotic treatment. PLoS One. 2012;7(8):e41290. | |
Chastre J, Wolff M, Fagon JY, et al; PneumA Trial Group. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588–2598. | |
Vidaur L, Planas K, Sierra R, et al. Ventilator-associated pneumonia: impact of organisms on clinical resolution and medical resources utilization. Chest. 2008;133(3):625–632. | |
Goldstein I, Chastre J, Rouby JJ. Novel and innovative strategies to treat ventilator-associated pneumonia: optimizing the duration of therapy and nebulizing antimicrobial agents. Semin Respir Crit Care Med. 2006;27(1):82–91. | |
Luyt CE, Combes A, Trouillet JL, Chastre J. Value of the serum procalcitonin level to guide antimicrobial therapy for patients with ventilator-associated pneumonia. Semin Respir Crit Care Med. 2011;32(2):181–187. | |
Quenot JP, Luyt CE, Roche N, et al. Role of biomarkers in the management of antibiotic therapy: an expert panel review II: clinical use of biomarkers for initiation or discontinuation of antibiotic therapy. Ann Intensive Care. 2013;3(1):21. | |
Pugh R, Grant C, Cooke RP, Dempsey G. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev. 2011;(10):CD007577. | |
Vazquez-Grande G, Kumar A. Optimizing antimicrobial therapy of sepsis and septic shock: focus on antibiotic combination therapy. Semin Respir Crit Care Med. 2015;36(1):154–166. | |
Crandon JL, Bulik CC, Kuti JL, Nicolau DP. Clinical pharmacodynamics of cefepime in patients infected with Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2010;54(3):1111–1116. | |
Rafati MR, Rouini MR, Mojtahedzadeh M, et al. Clinical efficacy of continuous infusion of piperacillin compared with intermittent dosing in septic critically ill patients. Int J Antimicrob Agents. 2006;28(2):122–127. | |
Chytra I, Stepan M, Benes J, et al. Clinical and microbiological efficacy of continuous versus intermittent application of meropenem in critically ill patients: a randomized open-label controlled trial. Crit Care. 2012; 16(3):R113. | |
Lodise TP Jr, Lomaestro B, Drusano GL. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis. 2007;44(3):357–363. | |
Lorente L, Lorenzo L, Martín MM, Jiménez A, Mora ML. Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to gram-negative bacilli. Ann Pharmacother. 2006;40(2):219–223. | |
Lorente L, Jiménez A, Palmero S, et al. Comparison of clinical cure rates in adults with ventilator-associated pneumonia treated with intravenous ceftazidime administered by continuous or intermittent infusion: a retrospective, nonrandomized, open-label, historical chart review. Clin Ther. 2007;29(11):2433–2439. | |
Dulhunty JM, Roberts JA, Davis JS, et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trial. Clin Infect Dis. 2013;56(2):236–244. | |
Kasiakou SK, Sermaides GJ, Michalopoulos A, Soteriades ES, Falagas ME. Continuous versus intermittent intravenous administration of antibiotics: a meta-analysis of randomised controlled trials. Lancet Infect Dis. 2005;5(9):581–589. | |
Roberts JA, Webb S, Paterson D, Ho KM, Lipman J. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit Care Med. 2009;37(6):2071–2078. | |
Mohamed AF, Karaiskos I, Plachouras D, et al. Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob Agents Chemother. 2012;56(8):4241–4249. | |
Pea F, Brollo L, Viale P, Pavan F, Furlanut M. Teicoplanin therapeutic drug monitoring in critically ill patients: a retrospective study emphasizing the importance of a loading dose. J Antimicrob Chemother. 2003;51(4):971–975. | |
Wang JT, Fang CT, Chen YC, Chang SC. Necessity of a loading dose when using vancomycin in critically ill patients. J Antimicrob Chemother. 2001;47(2):246. | |
Oparaoji EC, Siram S, Shoheiber O, Cornwell EE 3rd, Mezghebe HM. Appropriateness of a 4 mg/kg gentamicin or tobramycin loading dose in post-operative septic shock patients. J Clin Pharm Ther. 1998; 23(3):185–190. | |
Lu Q, Yang J, Liu Z, Gutierrez C, Aymard G, Rouby JJ; Nebulized Antibiotics Study Group. Nebulized ceftazidime and amikacin in ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2011;184(1):106–115. | |
Lu Q, Luo R, Bodin L, et al; Nebulized Antibiotics Study Group. Efficacy of high-dose nebulized colistin in ventilator-associated pneumonia caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Anesthesiology. 2012;117(6):1335–1347. | |
Arnold HM, Sawyer AM, Kollef MH. Use of adjunctive aerosolized antimicrobial therapy in the treatment of Pseudomonas aeruginosa and Acinetobacter baumannii ventilator-associated pneumonia. Respir Care. 2012;57(8):1226–1233. | |
Safdar A, Shelburne SA, Evans SE, Dickey BF. Inhaled therapeutics for prevention and treatment of pneumonia. Expert Opin Drug Saf. 2009;8(4):435–449. | |
Wark P, McDonald VM. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst Rev. 2009;(2):CD001506. | |
Michon AL, Jumas-Bilak E, Chiron R, Lamy B, Marchandin H. Advances toward the elucidation of hypertonic saline effects on Pseudomonas aeruginosa from cystic fibrosis patients. PLoS One. 2014;9(2):e90164. | |
Boe J, Dennis JH, O’Driscoll BR, et al; European Respiratory Society Task Force on the use of nebulizers. European Respiratory Society Guidelines on the use of nebulizers. Eur Respir J. 2001;18(1):228–242. | |
Duijvestijn YC, Brand PL. Systematic review of N-acetylcysteine in cystic fibrosis. Acta Paediatr. 1999;88(1):38–41. | |
Bassetti M, Merelli M, Temperoni C, Astilean A. New antibiotics for bad bugs: where are we? Ann Clin Microbiol Antimicrob. 2013;12:22. | |
Sader HS, Castanheira M, Flamm RK, Farrell DJ, Jones RN. Antimicrobial activity of ceftazidime-avibactam against Gram-negative organisms collected from US medical centers in 2012. Antimicrob Agents Chemother. 2014;58(3):1684–1692. | |
Bustos C, Del Pozo JL. Emerging agents to combat complicated and resistant infections: focus on ceftobiprole. Infect Drug Resist. 2010;3:5–14. | |
Farrell DJ, Flamm RK, Sader HS, Jones RN. Ceftobiprole activity against over 60,000 clinical bacterial pathogens isolated in Europe, Turkey, and Israel from 2005 to 2010. Antimicrob Agents Chemother. 2014;58(7):3882–3888. | |
Awad SS, Rodriguez AH, Chuang YC, et al. A phase 3 randomized double-blind comparison of ceftobiprole medocaril versus ceftazidime plus linezolid for the treatment of hospital-acquired pneumonia. Clin Infect Dis. 2014;59(1):51–61. | |
Lahiri SD, Mangani S, Durand-Reville T, et al. Structural insight into potent broad-spectrum inhibition with reversible recyclization mechanism: avibactam in complex with CTX-M-15 and Pseudomonas aeruginosa AmpC β-lactamases. Antimicrob Agents Chemother. 2013;57(6):2496–2505. | |
Lagacé-Wiens P, Walkty A, Karlowsky JA. Ceftazidime-avibactam: an evidence-based review of its pharmacology and potential use in the treatment of Gram-negative bacterial infections. Core Evid. 2014;9:13–25. | |
Curcio D. Multidrug-resistant Gram-negative bacterial infections: are you ready for the challenge? Curr Clin Pharmacol. 2014;9(1):27–38. | |
Housman ST, Crandon JL, Nichols WW, Nicolau DP. Efficacies of ceftazidime-avibactam and ceftazidime against Pseudomonas aeruginosa in a murine lung infection model. Antimicrob Agents Chemother. 2014;58(3):1365–1371. | |
Levasseur P, Girard AM, Claudon M, et al. In vitro antibacterial activity of the ceftazidime-avibactam (NXL104) combination against Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother. 2012;56(3):1606–1608. | |
Crandon JL, Schuck VJ, Banevicius MA, et al. Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2012;56(12):6137–6146. | |
Vazquez JA, González Patzán LD, Stricklin D, et al. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr Med Res Opin. 2012;28(12):1921–1931. | |
Lucasti C, Popescu I, Ramesh MK, Lipka J, Sable C. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind, Phase II trial. J Antimicrob Chemother. 2013;68(5):1183–1192. | |
AstraZeneca. A Study Comparing Ceftazidime-Avibactam Versus Meropenem in Hospitalized Adults With Nosocomial Pneumonia. Available from: https://clinicaltrials.gov/ct2/show/NCT01808092. NLM identifier: NCT01808092. Accessed February 26, 2015. | |
Hong MC, Hsu DI, Bounthavong M. Ceftolozane/tazobactam: a novel antipseudomonal cephalosporin and β-lactamase-inhibitor combination. Infect Drug Resist. 2013;6:215–223. | |
Takeda S, Nakai T, Wakai Y, Ikeda F, Hatano K. In vitro and in vivo activities of a new cephalosporin, FR264205, against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2007;51(3):826–830. | |
Walkty A, Karlowsky JA, Adam H, et al. In vitro activity of ceftolozane-tazobactam against Pseudomonas aeruginosa isolates obtained from patients in Canadian hospitals in the CANWARD study, 2007 to 2012. Antimicrob Agents Chemother. 2013;57(11):5707–5709. | |
Moya B, Zamorano L, Juan C, Pérez JL, Ge Y, Oliver A. Activity of a new cephalosporin, CXA-101 (FR264205), against beta-lactam-resistant Pseudomonas aeruginosa mutants selected in vitro and after antipseudomonal treatment of intensive care unit patients. Antimicrob Agents Chemother. 2010;54(3):1213–1217. | |
Chandorkar G, Huntington JA, Gotfried MH, Rodvold KA, Umeh O. Intrapulmonary penetration of ceftolozane/tazobactam and piperacillin/tazobactam in healthy adult subjects. J Antimicrob Chemother. 2012;67(10):2463–2469. | |
Solomkin J, Hershberger E, Miller B, et al. Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: results from a randomized, double-blind, phase 3 trial (ASPECT-cIAI). Clin Infect Dis. 2015;60(10):1462–1471. | |
Matsumoto T. Arbekacin: another novel agent for treating infections due to methicillin-resistant Staphylococcus aureus and multidrug-resistant Gram-negative pathogens. Clin Pharmacol. 2014;6:139–148. | |
Calixa Therapeutics, Inc. Safety and Efficacy of IV CXA-101 and IV Ceftazidime in Patients With Complicated Urinary Tract Infections. Available from: https://clinicaltrials.gov/ct2/show/study/NCT00921024. NLM identifier: NCT00921024. Accessed February 28, 2015. | |
Cubist Pharmaceuticals Holdings LLC. Study of Intravenous Ceftolozane/Tazobactam Compared to Piperacillin/Tazobactam in Ventilator-Associated Pneumonia. Available from: https://clinicaltrials.gov/ct2/show/NCT01853982. NLM identifier: NCT01853982. Accessed February 28, 2015. | |
Araoka H, Baba M, Tateda K, et al; ABX Combination Therapy Study Group. In vitro combination effects of aztreonam and aminoglycoside against multidrug-resistant Pseudomonas aeruginosa in Japan. Jpn J Infect Dis. 2012;65(1):84–87. | |
Funatsu Y, Hasegawa N, Fujiwara H, et al. Pharmacokinetics of arbekacin in bronchial epithelial lining fluid of healthy volunteers. J Infect Chemother. 2014;20(10):607–611. | |
Srinivas N, Jetter P, Ueberbacher BJ, et al. Peptidomimetic antibiotics target outer-membrane biogenesis in Pseudomonas aeruginosa. Science. 2010;327(5968):1010–1013. | |
Antibiotic POL7080 [webpage on the Internet]. Allschwil: Polyphor Ltd; [updated March 25, 2015]. Available from: http://www.polyphor.com/products/pol7080. Accessed March 29, 2015. | |
Polyphor Ltd. Pharmacokinetics, Safety and Efficacy of POL7080 in Patients With Ventilator Associated Pseudomonas Aeruginosa Pneumonia. Available from: https://clinicaltrials.gov/ct2/show/NCT02096328. NLM identifier: NCT02096328. Accessed March 1, 2015. | |
Huston WM, Potter AJ, Jennings MP, Rello J, Hauser AR, McEwan AG. Survey of ferroxidase expression and siderophore production in clinical isolates of Pseudomonas aeruginosa. J Clin Microbiol. 2004;42(6):2806–2809. | |
Battle SE, Meyer F, Rello J, Kung VL, Hauser AR. Hybrid pathogenicity island PAGI-5 contributes to the highly virulent phenotype of a Pseudomonas aeruginosa isolate in mammals. J Bacteriol. 2008;190(21):7130–7140. | |
Battle SE, Rello J, Hauser AR. Genomic islands of Pseudomonas aeruginosa. FEMS Microbiol Lett. 2009;290(1):70–78. | |
Laverty G, Gorman SP, Gilmore BF. Biomolecular mechanisms of Pseudomonas aeruginosa and Escherichia coli biofilm formation. Pathogens. 2014;3(3):596–632. | |
Sharma G, Rao S, Bansal A, Dang S, Gupta S, Gabrani R. Pseudomonas aeruginosa biofilm: potential therapeutic targets. Biologicals. 2014;42(1):1–7. | |
Leid JG, Willson CJ, Shirtliff ME, Hassett DJ, Parsek MR, Jeffers AK. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J Immunol. 2005;175(11):7512–7518. | |
Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis. 2013;67(3):159–173. | |
Mai GT, McCormack JG, Seow WK, Pier GB, Jackson LA, Thong YH. Inhibition of adherence of mucoid Pseudomonas aeruginosa by alginase, specific monoclonal antibodies, and antibiotics. Infect Immun. 1993;61(10):4338–4343. | |
Pier GB, Boyer D, Preston M, et al. Human monoclonal antibodies to Pseudomonas aeruginosa alginate that protect against infection by both mucoid and nonmucoid strains. J Immunol. 2004;173(9):5671–5678. | |
Sordé R, Pahissa A, Rello J. Management of refractory Pseudomonas aeruginosa infection in cystic fibrosis. Infect Drug Resist. 2011;4:31–41. | |
Juhas M, Eberl L, Tümmler B. Quorum sensing: the power of cooperation in the world of Pseudomonas. Environ Microbiol. 2005;7(4):459–471. | |
de Kievit TR. Quorum sensing in Pseudomonas aeruginosa biofilms. Environ Microbiol. 2009;11(2):279–288. | |
Whiteley M, Lee KM, Greenberg EP. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1999;96(24):13904–13909. | |
Lesprit P, Faurisson F, Join-Lambert O, et al. Role of the quorum-sensing system in experimental pneumonia due to Pseudomonas aeruginosa in rats. Am J Respir Crit Care Med. 2003;167(11):1478–1482. | |
Hraiech S, Hiblot J, Lafleur J, et al. Inhaled lactonase reduces Pseudomonas aeruginosa quorum sensing and mortality in rat pneumonia. PLoS One. 2014;9(10):e107125. | |
Sato H, Frank DW. Multi-functional characteristics of the Pseudomonas aeruginosa type III needle-tip protein, PcrV; comparison to orthologs in other gram-negative bacteria. Front Microbiol. 2011;2:142. | |
Sawa T, Ito E, Nguyen VH, Haight M. Anti-PcrV antibody strategies against virulent Pseudomonas aeruginosa. Hum Vaccin Immunother. 2014;10(10):2843–2852. | |
Lynch SV, Flanagan JL, Sawa T, et al. Polymorphisms in the Pseudomonas aeruginosa type III secretion protein, PcrV – implications for anti-PcrV immunotherapy. Microb Pathog. 2010;48(6):197–204. | |
Hauser AR, Cobb E, Bodi M, et al. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med. 2002;30(3):521–528. | |
Schulert GS, Feltman H, Rabin SD, et al. Secretion of the toxin ExoU is a marker for highly virulent Pseudomonas aeruginosa isolates obtained from patients with hospital-acquired pneumonia. J Infect Dis. 2003;188(11):1695–1706. | |
Goure J, Pastor A, Faudry E, Chabert J, Dessen A, Attree I. The V antigen of Pseudomonas aeruginosa Is required for assembly of the functional PopB/PopD translocation pore in host cell membranes. Infect Immun. 2004;72(8):4741–4750. | |
Wang Q, Li H, Zhou J, et al. PcrV antibody protects multi-drug resistant Pseudomonas aeruginosa induced acute lung injury. Respir Physiol Neurobiol. 2014;193:21–28. | |
Warrener P, Varkey R, Bonnell JC, et al. A novel anti-PcrV antibody providing enhanced protection against Pseudomonas aeruginosa in multiple animal infection models. Antimicrob Agents Chemother. 2014;58(8):4384–4391. | |
Shime N, Sawa T, Fujimoto J, et al. Therapeutic administration of anti-PcrV F(ab’)(2) in sepsis associated with Pseudomonas aeruginosa. J Immunol. 2001;167(10):5880–5886. | |
François B, Luyt CE, Dugard A, et al. Safety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: a randomized, double-blind, placebo-controlled trial. Crit Care Med. 2012;40(8):2320–2326. | |
Nakagawa A, Hosoyama T, Chubachi K, Takahashi S, Ohkubo T, Iyobe S. A search for Pseudomonas alginate biosynthesis inhibitors from microbial metabolites. J Antibiot (Tokyo). 1997;50(3):286–288. | |
Oliver AM, Weir DM. The effect of Pseudomonas alginate on rat alveolar macrophage phagocytosis and bacterial opsonization. Clin Exp Immunol. 1985;59(1):190–196. | |
Zaidi T, Pier GB. Prophylactic and therapeutic efficacy of a fully human immunoglobulin G1 monoclonal antibody to Pseudomonas aeruginosa alginate in murine keratitis infection. Infect Immun. 2008;76(10):4720–4725. | |
de Kievit TR, Lam JS. Monoclonal antibodies that distinguish inner core, outer core, and lipid A regions of Pseudomonas aeruginosa lipopolysaccharide. J Bacteriol. 1994;176(23):7129–7139. | |
Lazar H, Horn MP, Zuercher AW, et al. Pharmacokinetics and safety profile of the human anti-Pseudomonas aeruginosa serotype O11 immunoglobulin M monoclonal antibody KBPA-101 in healthy volunteers. Antimicrob Agents Chemother. 2009;53(8):3442–3446. | |
Lu Q, Rouby JJ, Laterre PF, et al. Pharmacokinetics and safety of panobacumab: specific adjunctive immunotherapy in critical patients with nosocomial Pseudomonas aeruginosa O11 pneumonia. J Antimicrob Chemother. 2011;66(5):1110–1116. | |
Summary of AR-101 clinical data [webpage on the Internet]. San Jose: Aridis Pharmaceuticals. Available from: http://www.aridispharma.com/ar101clinicaldata.html. Accessed June 7, 2015. | |
Sharma A, Krause A, Worgall S. Recent developments for Pseudomonas vaccines. Hum Vaccin. 2011;7(10):999–1011. | |
Pier G. Application of vaccine technology to prevention of Pseudomonas aeruginosa infections. Expert Rev Vaccines. 2005;4(5):645–656. | |
Baumann U, Mansouri E, von Specht BU. Recombinant OprF-OprI as a vaccine against Pseudomonas aeruginosa infections. Vaccine. 2004;22(7):840–847. | |
von Specht BU, Knapp B, Muth G, et al. Protection of immunocompromised mice against lethal infection with Pseudomonas aeruginosa by active or passive immunization with recombinant P. aeruginosa outer membrane protein F and outer membrane protein I fusion proteins. Infect Immun. 1995;63(5):1855–1862. | |
Sorichter S, Baumann U, Baumgart A, Walterspacher S, von Specht BU. Immune responses in the airways by nasal vaccination with systemic boosting against Pseudomonas aeruginosa in chronic lung disease. Vaccine. 2009;27(21):2755–2759. | |
Mansouri E, Blome-Eberwein S, Gabelsberger J, Germann G, von Specht BU. Clinical study to assess the immunogenicity and safety of a recombinant Pseudomonas aeruginosa OprF-OprI vaccine in burn patients. FEMS Immunol Med Microbiol. 2003;37(2–3):161–166. | |
Mansouri E, Gabelsberger J, Knapp B, et al. Safety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein F-I vaccine in human volunteers. Infect Immun. 1999;67(3):1461–1470. | |
Valneva Austria GmbH. Study Assessing Immunogenicity and Safety of IC43 In Intensive Care Patients. Available from: https://clinicaltrials.gov/ct2/show/NCT00876252. NLM identifier: NCT00876252. Accessed April 6, 2015. | |
Intercell. A confirmatory phase II/III study assesing efficacy, immunogenecity and safety of IC43 recombinant Pseudomonas vaccie in intensive care patients. Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2011-004771-36/ES. EudraCT identifier: 2011-004771-36. Accessed April 6, 2015. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.