Back to Journals » Infection and Drug Resistance » Volume 16
Pseudolaric Acid A: A Promising Antifungal Agent Against Prevalent Non-albicans Candida Species
Authors Li Z , Zhu B, Chen W , Hu J, Xue Y, Yin H, Hu X, Liu W
Received 2 May 2023
Accepted for publication 18 August 2023
Published 7 September 2023 Volume 2023:16 Pages 5953—5964
DOI https://doi.org/10.2147/IDR.S419646
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Zhen Li,1,* Bin Zhu,2,* Weiqin Chen,1 Jun Hu,1 Yingjun Xue,1 Hongmei Yin,1 Xiaobo Hu,3 Weiwei Liu1
1Department of Laboratory Medicine, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, 200032, People’s Republic of China; 2Department of Rehabilitation, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, 200025, People’s Republic of China; 3General Office, Centre for Clinical Laboratory, Shanghai, 200126, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Weiwei Liu, Department of Laboratory Medicine, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, 200032, People’s Republic of China, Email [email protected] Xiaobo Hu, General Office, Centre for Clinical Laboratory, Shanghai, 200126, People’s Republic of China, Email [email protected]
Purpose: The non-albicans Candida (NAC) species have recently gained great importance worldwide due to the increasing proportion in candida causing bloodstream infections. This investigation aimed to explore the efficacy of Pseudolaric acid A (PAA, a diterpenoid derived from Pseudolarix kaempferi) and its synergistic effect with fluconazole (FLC) against NAC species, including C. tropicalis, C. parapsilosis complex, and C. glabrata.
Methods: The microdilution checkerboard assay and time-killing curves were performed to detect the antifungal efficiency. To examine the integrity of cell walls and membranes, calcofluor white stain and propidium iodide stain were used. The changes of intracellular ultrastructure in Candida cells after treatment were observed using transmission electron microscopy. Changes in cell viability with the autophagy inhibitor 3-MA were assessed by the XTT method.
Results: It was revealed that PAA alone is effective on C. tropicalis, C. parapsilosis sensu stricto, C. orthopsilosis, and C. metapsilosis (MIC 8– 128 μg/mL). Strong synergism against FLC-resistant C. tropicalis was observed (FICI 0.07– 0.281), when PAA and FLC were combined. PAA had dose-dependently detrimental effects on C. tropicalis cell membranes. Moreover, increased vacuoles and autophagosome formation were found in C. tropicalis exposed to PAA. And the inhibitory effect of PAA against C. tropicalis can be relieved by autophagy inhibitor 3-MA in a certain concentration range. Ultrastructural alterations of C. tropicalis were more pronounced under the combination of PAA and FLC, including separation of the cell membrane from the cell wall, increased number of vacuoles, and degradation of organelles.
Conclusion: These observations indicated that PAA and its combination with FLC could be a promising therapeutic candidate for treating infections caused by NAC species.
Keywords: pseudolaric acid A, fluconazole, antifungal, combination, non-albicans Candida, synergy, autophagy
Introduction
Candida species are major human opportunistic fungi with the remarkable property of shifting from commensal microbes to pathogens. Infections caused by Candida range from superficial mucosal and skin lesions to serious invasive candidiasis.1 In recent decades, an important epidemiological transition in global distributions of Candida that cause candidiasis has taken place. Even while C. albicans has long been the predominant aetiology of invasive candidiasis, the incidence of NAC species has dramatically increased.2–4 The three most prevalent pathogenic NAC species that have been published are C. tropicalis, C. glabrata, and C. parapsilosis complex, according to multicenter surveys conducted by reputable organizations like the Centers for Disease Control and Prevention (CDC), the China Hospital Invasive Fungal Surveillance Net (CHIF-NET), and the global SENTRY Antifungal Surveillance Program.5–7 Moreover, the proportion of candidemia induced by these three NAC species has crossed that of C. albicans as reported by the CDC and CHIF-NET.6,7
The increasing resistance of NAC species to current anticandidal drugs poses challenging problems in the clinic. In particular, C. tropicalis and C. glabrata have been observed to exhibit intrinsic or acquired resistance to triazoles. In the USA and China, C. glabrata’s resistance to FLC was 7% and 10.2%, respectively.6,7 While a recent study on C. glabrata in Germany found that the resistance rate to FLC was 38%, and cross-resistance rate to FLC and echinocandin was 14%.8 The antifungal resistance of C. tropicalis is also very serious, owing to high rates of fluconazole resistance that have been reported in KSA (13.5%), Australia (16.7%), China (22.2%), and Japan (30.2%).9–12
It is essential to explore innovative anti-candidal compounds and treatment approaches due to increased infection incidence and resistance rates. Combinatorial treatment is a valid strategy to increase the effectiveness of therapeutic drugs. Some substances derived from medicinal plants have been found to exhibit synergistic activities on Candida species when combined with clinically used antifungal agents.13–17 Pseudolaric acid A (PAA) is a diterpenoid isolated from a Chinese herbal named “Tu-jing-pi/Tu-jin-pi”, which is the processed and dried root bark of Pseudolarix kaempferi.18 “Tu-jing-pi/Tu-jin-pi” has been traditionally applied to treat cutaneous fungi infections. Some researches have proven the inhibitory activity of PAA against C. albicans.18,19 It has not yet been established if PAA alone or in combination with fluconazole is effective on NAC species.
The current study investigated the anti-candidal capacity of PAA and its synergistic effect with FLC on the prevalent NAC species. Furthermore, the integrity of cell walls and membranes, as well as alterations in intracellular ultrastructure, were observed to preliminarily clarify the anticandidal mechanism of the combined treatment. It is hoped that the current work will contribute to overcoming infections caused by NAC species.
Materials and Methods
Fungal Strains and Agents
In this study, the three most prevalent pathogenic NAC species were involved, including 16 strains of C. tropicalis, 9 strains of C. glabrata sensu stricto, and 17 strains of the C. parapsilosis complex (10 C. parapsilosis sensu stricto, 5 C. metapsilosis, and 2 C. orthopsilosis). Candida species were identified using the CHROMagar (bioMe´rieux, Marcy l’E´ toile, France) and the VITEK 2 system. Further identification was performed by molecular and proteomic methods. Molecular identification was conducted using sequence-based method for the internal transcribed spacer (ITS) region. Proteomic identification was confirmed by matrix-assisted laser desorption ionization-time of flight mass spectrometry (Bruker, Microflex LT/SH). For quality control, the reference strains ATCC 750, ATCC 22019, and ATCC 2001 were used. Prior to each assay, the strains were cultivated twice onto Sabouraud dextrose agar (SDA). The drugs used in this study include PAA (Tauto Biotech Co., Ltd., Shanghai) and FLC (Sigma-Aldrich). The autophagy inhibitor 3-Methyladenine (3-MA) was obtained from Sigma-Aldrich. Cell viability assay was performed using Cell Proliferation Kit II (XTT, Roche).
Antifungal Susceptibility Testing
The microdilution method was applied to detect the anticandidal potential of the drug alone, and the microdilution checkerboard assay was carried out to determine the interaction of two drugs.16 Briefly, serial twofold dilutions of the drugs (FLC and PAB) were prepared in RPMI 1640. The final concentrations ranged from 0.125 to 128 µg/mL for PAA and 0.125 to 512 µg/mL for FLC, with some minor adjustments for different Candida species.100 μL of Candida suspensions (about 1.5×103 CFU/mL) and 100 μL of drug serial dilutions were added into the 96-well plate. For the microdilution checkerboard assay, 50 μL FLC and 50 μL PAB with different concentrations were added into each well. Then, 100μL of the Candida suspensions (about 1.5×103 CFU/mL) were added. The well only containing 200 μL medium was served as a negative control, and the well containing 100 μL of Candida suspensions and 100μL medium was regarded as growth control. After 24h-incubation at 37°C, the MICs were assigned as the lowest concentrations at which no discernible cell growth occurred. We calculated the fractional inhibitory concentration index (FICI) as follows:  . FICI ≤0.5 indicates synergism, 0.5 < FICI ≤ 4, indifference, and FICI > 4, antagonism, respectively.
. FICI ≤0.5 indicates synergism, 0.5 < FICI ≤ 4, indifference, and FICI > 4, antagonism, respectively.
Time–Kill Curves
In order to further confirm the synergism of drugs, the time–kill curves test was conducted as previously reported.20 FLIC-resistant strain 302 was used. With a starting inoculum of either 105 CFU/mL or 103 CFU/mL, two distinct groups were involved. For the group with 103 CFU/mL starting inoculum, the drug concentrations were set as follows: 2 µg/mL PAA (MIC in combination), 2 µg/mL FLC (MIC in combination), 2 µg/mL PAA+2 µg/mL FLC. When the initial inoculum was 105 CFU/mL, the drugs used were 8µg/mL PAA (1/2MIC alone), 256µg/mL FLC (1/2MIC alone), 8 µg/mL PAA+256 µg/mL FLC. The growth control did not contain any drugs. The cells were cultured with shaking (120 rpm) at 37◦C. At predestined time intervals (12, 24, 36, 48 h), 100 µL Candida suspensions was aspirated and 10-fold serially diluted with sterile saline. The diluted suspensions were plated on SDA, after which colony counts were performed. When the Candida was cultured in RPMI1640 medium for 48 h, 20 μL of suspensions was aspirated and put into a fast-counting plate, so as to detect the morphology of cells with optical microscope. Synergism or antagonism were considered as an increase or decrease of 2 log10 CFU/mL in antifungal activity produced after 24 h by the combination group compared with the most active drug alone. If the change was less then 2 log10 CFU/mL, it is indifferent.
Fluorescence Microscopy Analysis
Calcofluor white staining was performed to observe the cell wall of Candida since it can bind to chitin.21 Propidium iodide (PI) stain was used to evaluate membrane integrity. At 37◦ C for 24 h, Candida cells (ATCC750, about 1.5×103 CFU/mL) were cultured without and with agents (2 µg/mL PAA, 8 µg/mL PAA, 2 µg/mL FLC, and 2µg/mL PAA + 2µg/mL FLC). Then, the suspensions were centrifuged, washed, and resuspended with PBS buffer. For Calcofluor white staining, 500 µL of the suspensions were incubated with 50 μL Calcofluor White stain (Calcofluor White M2R 1 g/L and Evans Blue 0.5 g/L). For PI staining, the centrifuged cells were rsuespended with 500 µL PI staining solution (1 μg/mL). After being stained for 20 min in the dark, the cell suspensions were centrifuged and washed once more. The cells were visualized by fluorescence microscope (Nikon Eclipse Ti-E).
Transmission Electron Microscopy (TEM)
The Candida suspensions (ATCC750, about 5×103 CFU/mL) containing drugs (2 µg/mL PAA, 8 µg/mL PAA, 2 µg/mL FLC, and 2µg/mL PAA + 2µg/mL FLC) were cultured with shaking (120 rpm) at 37◦ C. The control group was free of agents. After being washed with PBS, the cells were fixed for 24 h at 4°C in 2.5% glutaraldehyde. Then they were washed in 0.1 M sodium phosphate buffer (pH 7.4) for 3 times and pre-embedded in 1% agarose solution. Thereafter, they were post-fixed for 2h with 1% osmium acid and dehydrated sequentially by ethanol with different gradients. They were then embedded in EMBed 812 and polymerized, after which ultrathin sections were prepared. Finally, sections of sample were doubly stained with 2% uranyl acetate and 2.6% lead citrate before being observed by TEM (Hitachi-HT7700, Tokyo, Japan).
Cell Viability Assay
To evaluate the effects of agents (PAA and FLC) on Candida autophagy, an autophagy inhibitor (3-Methyladenine, 3-MA) was used. The change in cell viability was detected using the XTT method. For the single-drug groups, 100 μL of drug serial dilutions were added to the 96-well plate. For the combination group, 50 μL FLC and 50 μL PAB with different concentrations were added into each well. Then, 100μL of the Candida (ATCC 750) suspensions (about 1.5×103 CFU/mL) with 5 mM 3MA were added. The corresponding comparison wells were added with the same amount of suspension without 3-MA. The well only containing medium served as a negative control, and the well containing 100 μL of Candida suspensions and 100μL medium was a positive control. After incubation at 37°C for 24 h, 50 µL of XTT solution was added to each well. Pipette gently up and down to mix well. The XTT solution was obtained by mixing the electron-coupling reagent with the XTT labeling reagent (1:50 dilution) prior to each test. The plates were further incubated in the dark at 37°C for 1 h. Then the absorbance was measured at 492/620 nm. The percentage of cell viability was calculated for each well by dividing the result of the positive control after subtracting the background absorbance of the negative control.
Statistical Analysis
Graphs and statistical analyses were performed with GraphPad Prism 9 software. The data are shown as the mean ± SE and the p < 0.05 was considered statistically significant.
Results
In vitro Antifungal Activity of the PAA Alone
The antifungal effects of PAA alone on C. tropicalis, C. glabrata, and C. parapsilosis complex were assessed. A total of 16 C. tropicalis strains were involved in our study, including 8 FLC-resistant strains and 8 FLC- susceptible strains. As presented in Table 1, PAA exhibited equivalent inhibitory activity on both sensitive and resistant C. tropicalis isolates, with MICs varying from 8 to 16 µg/mL. The C. parapsilosis complex consists of three different species, including C. parapsilosis sensu stricto, C. metapsilosis, and C. orthopsilosis. The agent of PAA was also effective against the tested C. parapsilosis complex isolates, showing a similar MIC range (64–128 µg/mL) among these three species (Table 2). However, PAA alone did not show any inhibitory effect on C. glabrata even at higher drug concentrations (512 µg/mL).
 |
Table 1 Drug Interactions of PAA and FLC Against C. tropicalis in vitro |
 |
Table 2 Drug Interactions of PAA and FLC Against C. parapsilosis Complex in vitro |
In vitro Antifungal Activity of the Combination
The interactions between PAA and FLC on the NAC species mentioned in our research were determined by using the microdilution checkerboard assay. It was discovered that the combinatorial treatment of PAA and FLC generated potent synergism on C. tropicalis isolates resistant to fluconazole, with FICI ranging between 0.07 and 0.281 (Table 1). Compared with vehicles alone, the MICs of PAA and FLC both decreased sharply when they were used in combination. The method of combinatorial treatment (PAA+FLC) has no synergistic activity in FLC-susceptible C. tropicalis and C. parapsilosis complex, even though PAA alone possesses fungal killing capacity.
The assay of Time–kill curves was performed to validate the synergism of PAA and FLC. When the initial inoculum was 103 CFU/mL, the antifungal efficacy of 2µg/mL FLC was inferior to that of 2 µg/mL PAA within the tested time span. In comparison to FLC alone, the combinatorial treatment of PAA (2 µg/mL) and FLC (2 µg/mL) yielded decreases of 2.70 log10 CFU/mL and 3.12 log10 CFU/mL at 24 and 48 hours, respectively, demonstrating synergistic inhibitory activity on FLC-resistant C. tropicalis (Figure 1). Similarly, synergistic activity was also observed, when the starting inoculum was increased to 105 CFU/mL and drug concentrations were adjusted to 1/2 MIC alone. As is presented in Figure 1, the inhibitory effect of FLC alone (256 µg/mL, 1/2 MIC) on C. tropicalis with 105 CFU/mL starting inoculum was very close to that of PAA alone (8 µg/mL, 1/2 MIC). Meanwhile, the value in the combination was reduced by more than 2 log10 CFU/mL compared to that in the FLC-alone treatment (2.45) and PAA-alone treatment (2.41) at 24 h. What’s more, we observed the morphology and growth of cells with optical microscope at 48 h. In the combinatorial treatment, the quantity of yeast cells was much lower than in the control and drug-alone treatments, further supporting the synergistic antifungal effect. Notably, most cells of C. tropicalis underwent apparent shape alterations from round shapes to elongated shapes, after 48 hours of continuous treatment with 8 µg/mL PAA (Figure 1).
The Integrity of Cell Walls and Membranes
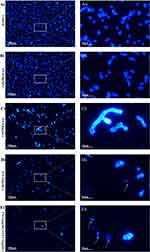
Propidium iodide (PI) can only penetrate compromised cell membranes to stain DNA and RNA, which is considered to be an indicator of membrane integrity. Fluconazole impairs the fungal cell membrane by inhibiting ergosterol synthesis. This was also confirmed in our analysis. Compared to the control, the proportion of damaged C. tropicalis cells increased obviously in the 2 µg/mL FLC-treated group (Figure 2). As for PAA treatment, no considerable change was observed when using a lower drug concentration (2 µg/mL). However, impaired cells increased along with the increased concentration of PAA (8 µg/mL), indicating that PAA had dose-dependently detrimental effects on C. tropicalis cell membranes. Calcofluor white mostly stains chitin, which is a substance of the fungal cell wall. As can be seen in Figure 3, Calcofluor White staining showed that the cell wall treated with PAA / FLC did not differ significantly from the control. Furthermore, large amounts of cellular debris without intact cell structure were found in the groups FLC alone and in combination.
The Changes in Intracellular Morphology
The intracellular ultrastructures of Candida cells were observed by TEM after treatment with these two drugs. The untreated control cells displayed normal morphology, including the intact cell wall, the normally shaped cell membrane and the homogeneous cytoplasm. C. tropicalis exposed to 2 µg/mL FLC appeared to have broken cell walls and plasma membranes. While cells treated with 2 µg/mL PAA displayed different changes from those to FLC, showing increased vacuoles and autophagosome formation. When the PAA concentration was raised to 8 µg/mL, oversized vacuoles containing autophagosomes began to develop in the cells. When C. tropicalis cells were treated with PAA and FLC together, alterations in ultrastructure were more pronounced, including the the separation of cell membrane from cell wall, the increased number of vacuoles, and the degradation of organelles and cytoplasmic content (Figure 4).
The Changes in Cell Viability with 3-MA
The agent of 3-MA is an autophagic flux inhibitor, which is commonly applied to block autophagy by inhibiting class III phosphoinositide 3‐kinase. We used the autophagy inhibitor 3-MA to assess the effect of PAA on Candida autophagy by detecting the change in cell viability. As shown in Figure 5, 8µg/mL PAA exhibited an inhibitory effect on C. tropicalis, cell viability decreased to 36.07% compared with the untreated control. However, the cell viability raised to 83.73% in the presence of PAA (8µg/mL) and 3-MA (2.5mM). This change in cell viability was statistically significant (P < 0.01). In a certain concentration range, the inhibitory effect of PAA against C. tropicalis can be relieved by 3-MA. However, no significant change in cell viability was found in the tested combination group containing 3MA, which may be due to the fact that the cells were seriously damaged with combinatorial treatment.
Discussion
In recent years, NAC species have developed into significant opportunistic pathogens, specifically C. tropicalis, C. parapsilosis complex, and C. glabrata. In some studies from India, Egypt, Turkey, KSA, and China, C. tropicalis or C. parapsilosis is the most commonly encountered NAC species, while C. glabrata is reported as the most frequently separated species following C. albicans in European countries.6,9,22–24 Despite the geographical differences in distribution, the total percentage of these three Candida species has even reached more than 50% in recent surveys from the United States (51%) and China (57.8%).6,7 Moreover, reduced susceptibility to current antifungal agents among NAC species has been increasingly reported, which highlights the importance of finding novel antifungal agents.6,8
PAA is the main component of Chinese herbal medicine“Tu-jing-pi/Tu-jin-pi”. The inhibitory effect of PAA on C. albicans has been described.18 While few published reports have examined the antifungal activity of PAA on NAC species. In this investigation, we assessed the capacity of PAA and its synergistic activity with FLC against NAC species in vitro.
Except for C. glabrata, all Candida strains examined were susceptible to PAA (MIC 8–128µg/mL), including C. tropicalis, C. orthopsilosis, C. metapsilosis, and C. parapsilosis sensu stricto. When used alone, PAA seemed to be more potent against C. tropicalis with a lower MIC (8–16µg/mL) than other NAC species. It is noteworthy that PAA exhibited equivalent inhibitory activity to both FLC-sensitive and FLC-resistant C. tropicalis isolates, indicating that PAA has diverse anticandidal targets from fluconazole.
The combinatorial treatment of PAA and FLC produced potent synergistic activity on resistant C. tropicalis strains rather than those susceptible to fluconazole. This trend has also been described in several previous studies related to the combination treatment of medicinal plant extract and fluconazole. For example, Guo et al20 found that pseudolaric acid B, another component from Pseudolarix kaempferi, displayed stronger synergism on FLC-resistant C. albicans when used together with fluconazole. Moreover, gypenosides were also found to be synergistic with fluconazole on resistant C. albicans, which are triterpenoid saponins derived from Gynostemma pentaphyllum Makino.15
In the present study, oversized vacuoles containing autophagosomes appeared in C. tropicalis when treated with PAA. And the inhibitory effect of PAA against C. tropicalis can be relieved by autophagy inhibitor 3-MA in a certain concentration range. Autophagy is a highly conserved biological process in which excessive or damaged organelles, proteins, and invading pathogens are packed into autophagosomes and then degraded via the vacuole in yeast.25 It plays an important role in recycling intracellular materials and maintaining cellular homeostasis.26–28 Although autophagy is important for cells to survive poor living conditions, it also destroys cells when it is out of balance. At present, the mechanism responsible for PAA exerting antifungal effects remains unclear. In an anti-tumor study, PAA was found to be a novel heat shock protein 90 (Hsp90) inhibitor that causes cell cycle arrest in HeLa cells.29 Moreover, it has been demonstrated that Hsp90, a molecular chaperon, plays a significant role in autophagy by regulating the activity and stability of signaling proteins, and some Hsp90 inhibitors may induce autophagy in cancer therapy.30
As is well known, azoles exert fungistatic activity by inhibiting lanosterol 14α-demethylase (Erg11), which prevents the production of ergosterol and causes the loss of membrane structural properties.31 Fluconazole, one of the azole antifungal agents, has been widely utilized in the treatment and prophylaxis of Candida infections. However, resistance to FLC is becoming more and more common, especially in NAC species.6 It is possible that PAA may possess alternative modes of action against Candida that differ from fluconazole, considering its comparable inhibitory activity to FLC-sensitive and FLC-resistant C. tropicalis. PAA combined with FLC may target several molecular targets involved in diverse cellular processes at the same time, so as to enhance the antifungal effect and display synergistic activity.
There were several limitations in the present study. The number of Candida isolates in our study was not large enough, especially resistant ones. Moreover, further molecular research should be carried out to elucidate the detailed mechanism of synergistic therapy.
Conclusions
In conclusion, Pseudolaric acid A is an effective antifungal agent against C. tropicalis, C. orthopsilosis, C. parapsilosis sensu stricto, and C. metapsilosis in vitro. When PAA was applied with fluconazole on FLC-resistant C. tropicalis, superior synergistic effects were observed. It seemed that autophagy may be activated in PAA-treated C. tropicalis. Moreover, PAA has damaging effects on C. tropicalis cell membranes in a dose-dependent manner. Our research supports the potential application of PAA as one promising anticandidal agent with different mechanisms from fluconazole. The combinatorial treatment of PAA and fluconazole could be a useful strategy for combating infections caused by resistant C. tropicalis.
Acknowledgments
We are grateful to our laboratory colleagues (Tiantian Liu, Guanyi Zhang, Xueting Yao, Jia Chen) for their support in the development of this study.
Funding
This work was financially supported by the National Natural Science Foundation of China (grant number 82003817).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Bilal H, Shafiq M, Hou B, et al. Distribution and antifungal susceptibility pattern of Candida species from mainland China: a systematic analysis. Virulence. 2022;13(1):1573–1589. doi:10.1080/21505594.2022.2123325
2. Kotey FA-O, Dayie NT, Tetteh-Uarcoo PB, Donkor EA-O. Candida bloodstream infections: changes in epidemiology and increase in drug resistance. Infect Dis. 2021;14:1–5. doi:10.1177/11786337211026927.
3. Zhang W, Song X, Wu H, and Zheng R. Epidemiology, risk factors and outcomes of Candida albicans vs non- albicans candidaemia in adult patients in Northeast China. Epidemiol Infect. 2019;147:e277. doi:10.1017/S0950268819001638
4. Gómez-Gaviria M, Ramírez-Sotelo U, Mora-Montes HA-O. Non-albicans Candida species: immune response, evasion mechanisms, and new plant-derived alternative therapies. J Fungi. 2023;9(1):11. doi:10.3390/jof9010011
5. Pfaller MA, Carvalhaes CG, DeVries S, Huband MD, Castanheira M. Elderly versus nonelderly patients with invasive fungal infections: species distribution and antifungal resistance, SENTRY antifungal surveillance program 2017–2019. Diagn Microbiol Infect Dis. 2022;102(4):115627. doi:10.1016/j.diagmicrobio.2021.115627
6. Xiao M, Chen SC, Kong F, et al. Distribution and antifungal susceptibility of Candida species causing candidemia in China: an update from the CHIF-NET study. J Infect Dis. 2020;221(Suppl 2):S139–S147. doi:10.1093/infdis/jiz573
7. Tsay SV, Mu Y, Williams S, et al. Burden of candidemia in the United States, 2017. Clin Infect Dis. 2020;71(9):e449–e453. doi:10.1093/cid/ciaa193
8. Aldejohann AM, Herz M, Martin R, Walther G, Kurzai O. Emergence of resistant Candida glabrata in Germany. JAC Antimicrob Resist. 2021;3(3):dlab122. doi:10.1093/jacamr/dlab122
9. Al-Musawi TS, Alkhalifa WA, Alasaker NA, Rahman JU, Alnimr AM. A seven-year surveillance of Candida bloodstream infection at a university hospital in KSA. J Taibah Univ Med Sci. 2021;16(2):184–190. doi:10.1016/j.jtumed.2020.12.002.
10. Chapman B, Slavin M, Marriott D, et al. Changing epidemiology of candidaemia in Australia. J Antimicrob Chemother. 2017;72(4):1103–1108. doi:10.1093/jac/dkx047
11. Wang Y, Fan X, Wang H, et al. Continual decline in azole susceptibility rates in Candida tropicalis over a 9-year period in China. Front Microbiol. 2021;12:702839. doi:10.3389/fmicb.2021.702839
12. Khalifa HO, Watanabe A, Kamei K. Azole and echinocandin resistance mechanisms and genotyping of Candida tropicalis in Japan: cross-boundary dissemination and animal-human transmission of C. tropicalis infection. Clin Microbiol Infect. 2022;28(2):302 e305–302 e308. doi:10.1016/j.cmi.2021.10.004
13. Canturk Z. Evaluation of synergistic anticandidal and apoptotic effects of ferulic acid and caspofungin against Candida albicans. J Food Drug Anal. 2018;26(1):439–443. doi:10.1016/j.jfda.2016.12.014
14. Behbehani JM, Irshad M, Shreaz S, Karched M. Synergistic effects of tea polyphenol epigallocatechin 3-O-gallate and azole drugs against oral Candida isolates. J Mycol Med. 2019;29(2):158–167. doi:10.1016/j.mycmed.2019.01.011
15. Liu Y, Ren H, Wang D, Zhang M, Sun S, and Zhao Y. The synergistic antifungal effects of gypenosides combined with fluconazole against resistant Candida albicans via inhibiting the drug efflux and biofilm formation. Biomed Pharmacother. 2020;130:110580. doi:10.1016/j.biopha.2020.110580
16. Pan M, Wang Q, Cheng T, et al. Paeonol assists fluconazole and amphotericin B to inhibit virulence factors and pathogenicity of Candida albicans. Biofouling. 2021;37(8):922–937. doi:10.1080/08927014.2021.1985473
17. Xu Y, Quan H, Wang Y, et al. Requirement for ergosterol in berberine tolerance underlies synergism of fluconazole and berberine against fluconazole-resistant Candida albicans isolates. Front Cell Infect Microbiol. 2017;7:491. doi:10.3389/fcimb.2017.00491
18. Chiu P, Leung LT, Ko BC. Pseudolaric acids: isolation, bioactivity and synthetic studies. Nat Prod Rep. 2010;27(7):1066–1083. doi:10.1039/b906520m
19. Zhu B, Li Z, Yin H, et al. Synergistic antibiofilm effects of pseudolaric acid A combined with fluconazole against Candida albicans via inhibition of adhesion and Yeast-To-Hypha transition. Microbiol Spectr. 2022;10(2):e0147821. doi:10.1128/spectrum.01478-21
20. Guo N, Ling G, Liang X, et al. In vitro synergy of pseudolaric acid B and fluconazole against clinical isolates of Candida albicans. Mycoses. 2011;54(5):e400–e406. doi:10.1111/j.1439-0507.2010.01935.x
21. Kollu NV, LaJeunesse DR. Cell rupture and morphogenesis control of the dimorphic yeast Candida albicans by nanostructured surfaces. ACS Omega. 2021;6(2):1361–1369. doi:10.1021/acsomega.0c04980
22. Chakraborty M, Banu H, Gupta MK. Epidemiology and antifungal susceptibility of Candida Species causing blood stream infections: an Eastern India perspective. J Assoc Physicians India. 2021;69(8):11–12.
23. Reda NM, Hassan RM, Salem ST, Yousef RHA. Prevalence and species distribution of Candida bloodstream infection in children and adults in two teaching university hospitals in Egypt: first report of Candida kefyr. Infection. 2022;51(2):389–395. doi:10.1007/s15010-022-01888-7
24. Dogan O, Yesilkaya A, Menekse S, et al. Effect of initial antifungal therapy on mortality among patients with bloodstream infections with different Candida species and resistance to antifungal agents: a multicentre observational study by the Turkish Fungal Infections Study Group. Int J Antimicrob Agents. 2020;56(1):105992. doi:10.1016/j.ijantimicag.2020.105992
25. Li W, He P, Huang Y, et al. Selective autophagy of intracellular organelles: recent research advances. Theranostics. 2021;11(1):222–256. doi:10.7150/thno.49860
26. Xu DD, Du LL. Fission yeast autophagy machinery. Cells. 2022;11(7):1086. doi:10.3390/cells11071086
27. Shimamura S, Miyazaki T, Tashiro M, et al. Autophagy-Inducing Factor Atg1 Is Required for Virulence in the Pathogenic Fungus Candida glabrata. Front Microbiol. 2019;10:27. doi:10.3389/fmicb.2019.00027
28. Hu Y, Reggiori F. Molecular regulation of autophagosome formation. Biochem Soc Trans. 2022;50(1):55–69. doi:10.1042/BST20210819
29. Liu J, Wu XD, Li W, Yuan Z, Yang K, Zhao QS. Discovery of pseudolaric acid A as a new Hsp90 inhibitor uncovers its potential anticancer mechanism. Bioorg Chem. 2021;112:104963. doi:10.1016/j.bioorg.2021.104963
30. Wang B, Chen Z, Yu F, et al. Hsp90 regulates autophagy and plays a role in cancer therapy. Tumour Biol. 2016;37(1):1–6. doi:10.1007/s13277-015-4142-3
31. Pais P, Califórnia R, Galocha M, et al. Candida glabrata transcription factor Rpn4 mediates fluconazole resistance through regulation of ergosterol biosynthesis and plasma membrane permeability. Antimicrob Agents Chemother. 2020;64(9):e00554. doi:10.1128/AAC.00554-20
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.