Back to Journals » OncoTargets and Therapy » Volume 12
Proton Therapy For Lymphomas: Current State Of The Art
Authors Ricardi U , Maraldo MV, Levis M, Parikh RR
Received 25 June 2019
Accepted for publication 13 September 2019
Published 1 October 2019 Volume 2019:12 Pages 8033—8046
DOI https://doi.org/10.2147/OTT.S220730
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Umberto Ricardi,1 Maja V Maraldo,2 Mario Levis,1 Rahul R Parikh3
1Department of Oncology, University of Torino, Torino, Italy; 2Department of Clinical Oncology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark; 3Department of Radiation Oncology, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ, USA
Correspondence: Mario Levis
Department of Oncology, University of Torino, Via Genova 3, Torino 10125, Italy
Tel +39 011 6334119
Fax +39 011 6336614
Email [email protected]
Abstract: The combination of brief chemo-radiotherapy provides high cure rates and represents the first line of treatment for many lymphoma patients. As a result, a high proportion of long-term survivors may experience treatment-related toxic events many years later. Excess and unintended radiation dose to organs at risk (particularly heart, lungs and breasts) may translate in an increased risk of cardiovascular events and second cancers after a few decades. Minimizing dose to organs at risk is thus pivotal to restrain the risk of long-term complications. Proton therapy, with its peculiar physic properties, may help to better spare organs at risk and consequently to reduce toxicities especially in patients receiving mediastinal radiotherapy. Herein, we review the physical basis of proton therapy and the rationale for its implementation in lymphoma patients, with a detailed description of the clinical data. We also discuss the potential disadvantages and uncertainties of protons that may limit their application and critically review the dosimetric studies comparing the risk of late complications between proton and photon radiotherapy.
Keywords: proton therapy, lymphoma, Hodgkin, radiotherapy
Introduction
Lymphomas are the most common hematologic malignancies worldwide and, despite their different disease processes and histology, have a much more favorable outcome than solid tumors. Particularly, combined chemo-radiotherapy cures most Hodgkin’s lymphoma (HL) patients, with roughly 70–80% of them surviving many decades after treatment.1–4 In contrast to HL, non-Hodgkin lymphomas (NHL) have less favorable outcomes but, in general, survival rates in the long term are better than those of most solid tumors.5 Given their favorable outcome, the reduction of treatment-related toxic effects is the cornerstone of recent advances in the treatment of HL and NHL. Specially, the likelihood of long-term survival raises the issue of long-term complications, mostly related to latent radiation injuries from combined curative treatments.6 In particular, long-term reports of large cohorts and national registries have produced a strong evidence that the benefits from radiation may be counterbalanced, decades later, by increased mortality and morbidity from cardiovascular events and second cancers.7–9 This evidence lead hematologists and clinical oncologists to accept increased relapse rates as a barter for omitting radiotherapy (RT) altogether.10,11 At the same time, efforts have been done from the whole radiation oncology community to minimize RT-related complications to organs adjacent to the target of treatment, particularly to thoracic organs at risk (OARs) as breasts, heart, and lungs by reducing the prescribed RT dose and treatment fields without compromising cure rates.12 In particular, the new concepts of involved-site RT (ISRT) and involved-node RT (INRT) were recently developed for the definition of smaller treatment volumes. Despite marginal differences, in both concepts, the pre-chemotherapy disease involvement determines the clinical target volume, resulting in greater sparing of OARs compared to the older volumes.13 Proton therapy (PT), with its particular ballistic characteristics favoring a low entrance dose and a step fall-off of the dose at the end of the beam range (“Bragg peak”), offers a great opportunity to further minimize the risk of long-term complication related to photon-based radiation while keeping the increased initial cure rate offered by RT. However, PT requires a complex treatment planning, is more expensive than photons and still suffers from some uncertainties. For these reasons, the decision of PT referral should always be driven by a dosimetric comparison with an optimally planned photon treatment in order to demonstrate a clinical benefit for the patient, as already carried out for other tumors.14 In this article, 1) we review the ability of PT in reducing dose to organs at risk with an overview of the current techniques for treatment delivering and of the published clinical data, 2) we describe the actual uncertainties which may limit its application in lymphomas, 3) and we report a detailed summary of the radiation dosimetry literature comparing PT and photons.
Technical Aspects For Lymphomas And Current Techniques In Delivering Proton Therapy
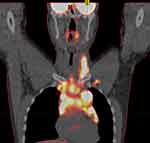
Individualized treatment planning with PT is based on various factors including patient-specific factors such as age, gender, previous treatment, disease location, baseline co-morbidities, and findings from initial disease extension, evaluated with PET/CT scan. Modern radiation planning using ISRT techniques requires appropriate image fusion with functional (usually pretreatment/chemotherapy) imaging to identify the initial sites of involvement.13,15,16
During the CT simulation (ideally with intravenous contrast), for cases involving the mediastinum, a 4-dimensional CT scan is often utilized to determine breathing motion and the appropriate internal target volume (ITV) margin. Also, the deep-inspiration breath-hold (DIBH) technique can be used to reduce the breathing motion of the mediastinum, thus narrowing the mediastinal target, while minimizing the exposure of lung and heart. Modern studies demonstrate that the lowest doses to the nearby organs at risk are obtained for patients treated with PT and DIBH (compared to photons), if clinically available.17 Currently, there are various PT techniques clinically available for cancer patients. This includes passive scattering technique and pencil beam scanning (PBS) techniques.
Passive Scattering
Treatment planning goals for lymphomas with passive-scattered proton beams are to irradiate the target with an adequate dose while reducing the integral dose to the patient, and the commonly utilized technique is the double-scatter (DS) method. The relative size and heterogeneity of the targets can often present a challenge with the DS techniques. Limitations of the passive-scattering delivery technique include the following: field size (maximum), inability to conform the dose proximally to the target, and poor conformality distal to the target (compared to spot scanning). Advantages to passive scattering delivery include increased plan robustness to patient and target motion uncertainties relative to PBS. With appropriate margins and smearing techniques, passive scattering plans are less sensitive to motion and density changes in the beam path.
Most commonly, the treatment planning strategy for passive-scattered PT is to assign the clinical target volume (CTV) or ITV as the beam target. Many treatment planning systems allow that margins be applied for proton range uncertainties, distally and proximally, directly in the properties of each beam. Various institutions use a formula for inherent range uncertainties similar to that described by Moyers et al: (Margin = α % Range + β mm, where α is related to uncertainties in dose calculation).18 This margin accounts for various factors including relative proton stopping power conversion factor, beam-delivery reproducibility, treatment planning system commissioning accuracy, and compensator design. The effects of setup errors on the proton range are compensated by range compensator smearing (thinning) calculated using Urie et al19. Additionally, collimator margins for the lateral penumbra are set to the planning target volume (PTV) or CTV with an adequate expansion for setup variations. Appropriate margins can be set to ensure target coverage along and perpendicular to each beam. Uncertainties due to potential relative biological effectiveness (RBE) variations along the spread-out Bragg peak can be reduced by using multiple treatment fields, rather than single fields. For example, if a single field is used, a single spot of potential high RBE would be delivered to the entire prescription dose rather than a fraction of the full prescription dose.
Plan evaluation, similarly to photons, is based on target coverage goals, OAR dose constraints, and plan quality indices such as integral dose and dose conformality. Frequently, the plans are normalized to the optimal CTV coverage, but PTV coverage requirements certainly help facilitate photon and proton plan comparisons. It is important to note that the treatment time can vary from 30 to 90 mins depending on the number of isocenters and fields being treated each day.20
Below is a summary of the treatment planning specifics of PT planning, specifically DS technique:
- 3D-conformal treatment
- Manually laborious, forward planned
- CTV and normal structures are delineated in the same way as for photons.
- For static geometries, the plan target is the CTV, while when the treatment area is affected by breathing motion an ITV that includes CTV motion is derived from the 4D-CT.
- For lateral beam shaping, expansions for setup uncertainty and inter-fractional anatomy variability are applied to the CTV/ITV.
- Patient-specific beam collimators conform the dose laterally to the CTV/ITV with a margin for penumbra (1 to 10 mm). Range compensators are designed for each beam to conform the dose distally to the CTV/ITV. “Smearing” is applied to compensate for proton range changes due to density changes in the beam path.
- Additionally, along each beam, distal and proximal margins are set to the CTV/ITV to compensate for proton range uncertainties as described above.18
- In the current practice of scatter techniques, margins to the CTV/ITV are assigned per beam:
- Collimator margins for the lateral penumbra are set to the PTV with an expansion for setup variations.
- Distal and proximal margins depend on depth of the distal and proximal edges of the target. This is “beam-specific planning target volume.”
- Beam selection and orientation depend on the unique disease distribution for each patient.
- Whenever possible, the preference is to use anterior or posterior fields, rather than both, to the same targeted area in the mediastinum.
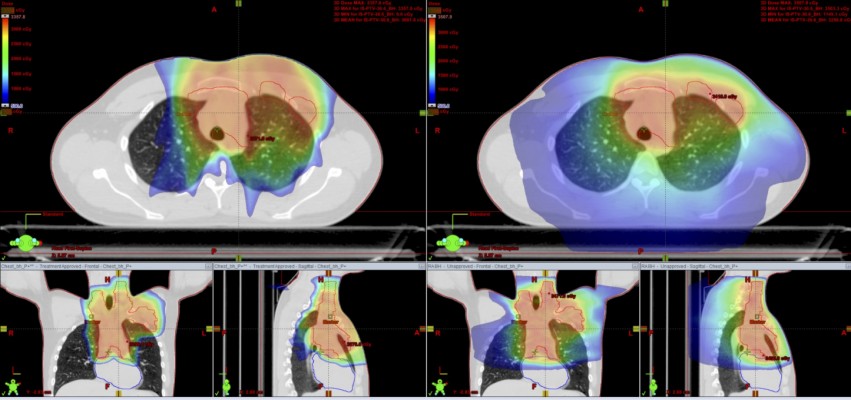
See below examples of treatment planning in a young patient with classical HL using PT with and without DIBH (Figures 1–3).
 |
Figure 2 Comparative planning: proton vs photon using DIBH. Left: protons (DS) +DIBH; right: RapidArc+DIBH. |
 |
Figure 3 Comparative planning proton vs photon in free-breathing. Left: protons (DS); Right: RapidArc. |
Pencil Beam Scanning (PBS)
PBS offers a dosimetric advantage over double scattering technique with regard to conformality around OARs, particularly for irregularly shaped targets. PBS is useful when the target volume varies markedly in depth, and also when the target spans over a large field size. Consequently, PBS solves many of the treatment planning complications of DS by allowing irregular and non-contiguous targets with homogeneous target coverage and improved sparing of OARs. PBS allows for delivery of 3D conformal treatments with 1 to 2 fields without the need for multiple custom-built compensators and apertures, which can be a laborious procedure.
PBS field sizes tend to be larger than DS field sizes, thus larger targets can be easily planned. As noted above, if requiring treatment to a large target with DS, matched fields must be created (with separate apertures and compensators), which can often result in hot or cold dosimetric areas. Additionally, the “beam-on” time for PBS is often longer than for DS technique, making it more difficult to administer a DIBH method (if available). Also, there is more skin sparing with PBS compared to DS, and thus, overlapping beams on the skin are not a major concern. For example, PBS is able to shape the proton beam to conform to the shallow and deep aspects of the target, thus allowing for a “skin-sparing” effect. In DS system, the proton beam can only be shaped to conform to the deeper shape of the target, thus precluding a skin sparing effect for superficial targets. PBS beams are usually angled away from each other to allow for improved robustness. It is also important to note that the above-described margins for both techniques do not protect against unpredictable, uncommon changes that may occur during the course of treatment such as disease progression, pleural effusions, pneumonia (or pulmonary consolidation), or weight loss.
Overall, all of the available PT techniques are used to maximize cure and minimize the morbidity of treatment, and providers are encouraged to follow the current ILROG guidelines for proton beam therapy in adult radiotherapy programs.16 For this reason, a plan comparison between PT and a modern photon solution is strongly recommended before treatment, in order to demonstrate the beneficial role of protons. Indeed, it should be noted that the potential clinical benefit of PT over photon RT is greatly influenced by the disease extension and localization. As an example, some anatomical presentations as the lower anterior mediastinum mostly benefit from a PT solution. It is likely that if one encounters a case such as in Figure 4, in a young patient with curable disease, PT would be of significant dosimetric and clinical benefit.
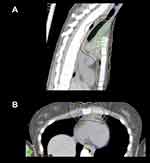 |
Figure 4 When the target spans down in front of the heart, protons can be very useful. (A) Sagittal view; (B) axial view. |
Clinical Reports Of Proton Therapy For Lymphomas
Modern radiotherapy for lymphomas combines smaller fields (involved site radiotherapy, ISRT, and involved node radiotherapy, INRT), lower doses and highly conformal techniques compared to the past. Given the higher conformal dose distribution achieved with protons, many investigators have raised the issue of the potential increased relapse rate, in particular at the field edge.
First, clinical reports were therefore focused on this question, as the main scope was to demonstrate the ability of PT to obtain the same cure rates of modern photon techniques. With this background, University of Florida conducted a phase II pilot study on 15 stage I–III HL patients, treated with involved-node PT between 2009 and 2013.21 With a median follow-up of 37 months, the 3-year relapse-free survival (RFS) rate was 93%, absolutely similar to the outcome obtained with modern photon radiotherapy in HL. Some clinical reports were subsequently published, with most of them focusing on HL patients with mediastinal involvement. The Proton Collaborative Group Registry reported on a cohort of 50 patients treated with consolidation proton ISRT and followed-up prospectively (median follow-up time: 21 months).22 Most patients were adults (64%), with a high prevalence of mediastinal involvement (93%) and of bulky lesions (65%). The overall outcome was good, with a 2-year RFS of 85%. There were only 3 relapses: two were infield, within bulky mediastinal lesions treated with 21 Gy, and one was marginal, superior to the treatment field and to the clinical target volume and for such reason would have been missed with a photon plan as well. Very recently, a collaborative group first reported the clinical results of a cohort of 21 adult HL patients treated with deep inspiration breath-hold PBS-PT.23 All patients were treated at 30 Gy in 15 fractions. With a median follow-up time of 24 months, no patient relapsed and all were alive. Treatment was well tolerated and no severe toxicities were reported.
Although most studies enrolled HL patients, few reports on small series of NHL patients are available. University of Florida first described their outcome with PT in a group of 11 NHLs, which included patients affected with a variety of different histologies.24 Three-year PFS and OS were 91%, with only one case of in-field relapse in a patient treated for a natural killer/T cell lymphoma. No severe toxicities (Grade >2) were reported. Afterward, Plastaras et al25 reported their experience with PT in a cohort of nodal NHLs with mediastinal involvement or primary mediastinal B cell lymphomas. Overall, 24 patients were enrolled, with a high predominance of bulky lesions at baseline (87%). The median follow up was 28 months and 2-year PFS and OS were 87% and 96%, respectively. Only one patient relapsed in-field and none had grade 2 or higher radiation pneumonitis. A recent publication from a German group26 enrolled 20 patients affected with either HL (9) or NHL (11). The outcome was, again, impressive with a 2-year PFS and OS of 95% and 100%, respectively. The toxicity profile was good as well, with a good tolerance to PT and no events of grade 3 or higher.
The adoption of PT is even more promising in the setting of relapsed/refractory disease, when peri-transplant radiation is frequently omitted despite its valuable effect for the concerns related to toxicity – mainly grade 2–3 pneumonitis – after photon RT. Tseng et al27 enrolled in a multi-institutional study 51 patients treated with PT for a relapsed/refractory HL or NHL. All patients were heavily pretreated and one third received peri-transplant PT. With a median PT dose of 36 Gy (range 25.2–54 Gy) and a median follow-up of 21 months, the 2-year progression-free survival (PFS) and overall survival (OS) were 69% and 87%, respectively. Obviously, HL patients had a better outcome compared to NHL patients in terms of both PFS (78% vs 46%) and OS (88% vs 82%). Only 6 patients (12%) developed a symptomatic grade 2 pneumonitis (no grade 3> toxicity event reported), which is lower compared to historical controls treated with photon RT. These preliminary results, that need to be confirmed in larger cohorts, seem to favor PT over photon RT in this setting, given the more conformal dose distribution of protons and ability to better spare fundamental organs at risk as lungs and heart.
Despite the brilliant clinical results, all the studies mentioned above included a small number of patients, mostly within a mono-institutional accrual, and thus a careful extrapolation is mandatory. To date, the largest multi-institutional case series of lymphoma patients treated with PT was published by Hoppe et al28 in 2017. The authors included 138 pediatric and adult HL patients. With a median follow-up of 32 months, the 3-year RFS rate was 92% for all (96% for adults, 87% for pediatric patients), with none marginal relapse. The accompanying editorial for this study29 celebrated the excellent clinical results, similar to those obtained with modern photon radiotherapy in HL. Interestingly, there were no marginal relapses potentially related to the rapid dose fall-off of the Bragg peak. This observation was extremely important, given that the combination of steep dose-gradient techniques with the modern definition of limited target volumes (INRT and ISRT) raises many concerns on the potential increase of relapses. More studies with similar or possibly larger numbers are mandatory to strengthen these preliminary observations and to increase the robustness of the information on the role of PT in lymphoma patients. Table 1 summarizes all clinical studies investigating the role of PT in this setting.
 |
Table 1 Clinical Studies Investigating The Role Of PT In Lymphoma Patients |
Uncertainties Of Proton Therapy
The most relevant challenges associated with PT, compared to photons, are the uncertainties related to the different beam penetration range in tissues and to the changes in the magnitude of the relative biological effect (RBE) along the beam path.16 Herein below, we briefly describe these uncertainties and the possible ways to mitigate them.
Relative Biologic Effectiveness (RBE)
RT prescription and constraints are based on dose parameters and dose–response relationships, which are basically derived by photon therapy. Different radiation modalities may produce different dose responses, and this aspect is relevant also in the comparison between photon (the reference radiation by definition) and protons. This discrepancy is evaluated through the relative biological effect (RBE), which is the ratio of the absorbed dose that produces the same biological effect between photons and protons.34 The currently used RBE in clinical practice is 1.1, but some studies have demonstrated that this parameter depends on many factors as proton energy, dose per fraction, tissue and cell type, oxygenation and other aspects that may influence the radiosensitivity of the tissue.35,36 Moreover, RBE estimation of protons is derived by in vitro studies, but in vivo studies are still missing.37 Furthermore, actual data suggest that RBE may change along the beam path, with a significant increase at the beam tail in the proximity of the Bragg peak. This aspect is related to the increase of the linear energy transfer (LET) of the proton beam at the distal edge and raises many concerns on the estimation of the dose received by the target of treatment and, particularly, by organs at risk located in its close proximity. In fact, the increase in RBE at the beam tail may extend the beam range by 2–4 mm, depending on the depth in the target tissue and on the beam energy.38 This leads to a potential extension of the high-dose region beyond the target volume and may end up in organs at risk located behind the tumor.39 Paradoxically, this aspect may lead to the creation of hotspots in those structures that should be better spared with protons (i.e. heart and breasts) when treating mediastinal lymphomas. Given that RBE-based or LET-based planning is not available to date, precautions should be adopted to protect healthy tissues as the use of multiple fields to dilute the effect, or reduction of the physical dose to organs at risk located in the proximity of the distal edge.16
Uncertainties Due To Density Variations And Organ Motion
Protons are particle therapies with mass and, therefore, density of the tissues and organs encountered by the beam path greatly influences the dose deposited. Any anatomic variation in term of position and size – of both patient and tumor – could affect the dose distribution. Particularly, the axial deviations may translate in range uncertainties; in fact, the range of PT is related to every single voxel of the planning imaging and any minimal change of the patient anatomy (and consequently of the mass density and of the related voxel) will modify the range of all protons crossing that/those specific voxel.16,37 Strategies to mitigate this dosimetric uncertainty are strongly recommended, through the adoption of tracking techniques40 or a 4-dimensional CT scan to compensate for organ motion (mainly lungs).41 Moreover, large margins should be applied to account for the uncertainties due to the inevitable differences in tissue across the proton track. A compensatory margin could be considered also to “protect” very small and critical OARs as the coronary arteries, whose physiologic movement during the cardiac cycle is unavoidable.42 When the sparing of critical organs is mandatory, all these issues should be kept in mind and a comparative planning would be strongly recommended not only between different PT plans, but also with modern and highly conformed photon solutions as intensity-modulated RT (IMRT) and optimized volumetric arc therapy solutions.43 Indeed, in some particular anatomical situations (even in some patients with mediastinal involvement), the gradual fall of the dose around the target volume may favor IMRT, given the high risk of a “full-dose” extension to the organs at risk with PT for all the issues mentioned above.39 Therefore, patient selection is fundamental to estimate who might benefit mostly from the offer of a PT plan. A well-balanced planning comparison with a modern and optimized photon RT solution29 is thus mandatory, taking in to account PTV coverage and doses received by organs at risk. Figure 5 shows a dose-volume histogram, comparing a photon and a proton solution.
“In Silico” Comparison Of The Radiation Late Effects Between PT And Modern Photon RT
In a current radiation treatment, the difference between modern delivery techniques is characterized by a difference in the spatial distribution of radiation dose. Therefore, in lymphoma, there is a large variation in the normal tissue exposure among patients who nominally receive the same form of radiotherapy due to the individual differences in field size as well as in anatomical site of disease. Also, late effects most often occur decades following treatment and the excess risks reported in the literature are consequences of now outdated treatment regimens and should not be extrapolated to patients of today. Instead, the choice of an optimal treatment strategy can be guided by comparative dose planning and modeling studies.
Fourteen studies have evaluated the difference in organ at risk (OAR) exposure with involved node/site PT versus photon therapy in lymphoma (Table 2 for an overview). The studies compare PT delivered with either passive scatter (6 studies) or pencil beam scanning (7 studies) techniques to modern radiotherapy photon planning using 3-dimensional conformal RT (3DCRT, 12 studies) with anteroposterior-posteroanterior fields as well as to more conformal photon planning with IMRT (7 studies), volumetric modulated arc therapy (VMAT, 5 studies), or Helical Tomotherapy (2 studies). Three recent studies44–46 have also compared the benefits of deep inspiration breath-hold (DIBH) in different combinations with PT and IMRT.
 |
Table 2 Studies Comparing Photon Versus Proton Involved Node/site Radiotherapy In Lymphoma |
Importantly, several aspects should be carefully considered in the individual publications: 1) The patient population: what are the no. of patients, HL vs NHL histology, children vs adults, clinical stage, and primary vs relapsed/refractory disease, 2) Where is the target localized and how it is defined? Upper vs lower mediastinal presentation and initial vs residual post-chemotherapy disease, 3) Is information provided on photon planning field set-up, optimization priorities and plan evaluation? and 4) Do the authors have any proton planning experience? Is robustness and dose and range uncertainties accounted for? Among the 14 studies, three include HL and non-HL patients,26,46,47 one report only on pediatric patients,48 three include both primary and relapsed/refractory patients,49–51 five origin from centers without proton facilities,45,52–55 and two provide very limited clinical information.46,56 Also, two studies define the target volume as residual disease following chemotherapy47,57 and cannot be said to adhere to the INRT/ISRT concept. A summary of estimated doses to thoracic OARs in mediastinal lymphoma is provided in Table 3; however, due to the caveats mentioned above, considerable uncertainties apply for the reported absolute values.
 |
Table 3 Mean Dose [Gy] To Thoracic Organs At Risk. Reported Mean Doses Based On Results In42,50,51,53,56 For Early-Stage Mediastinal HL And On Results In24,44,46,54 For Stage I–IV Primary Mediastinal Hodgkin/non-Hodgkin Lymphoma (including Pediatric Patients) Are Normalized To A 30 Gy Prescription Dose And Weighted According To Number Of Patients In Each Publication. Data Do Not Allow For Reporting Of Dosimetric Parameters Other Than Mean Dose |
The following sections focus on primary mediastinal stage I–II HL where data are most robust. In free-breathing (FB), the mean doses to the heart, lungs and female breasts are estimated to decrease with PT compared to 3DCRT and modern RT (mRT). Interestingly, with DIBH-mRT, the estimated mean dose to the heart is even lower, almost similar for the lungs, and higher for female breasts which may be due to the low dose bath associated with rotational therapy and/or that most comparative dose planning studies in PT prioritize the female breasts the highest during planning. When PT is combined with DIBH, a further dose reduction may be achieved, especially for the mean dose to the heart, compared to FB-PT and DIBH-mRT. It is important to mention that this comparison cannot consider the differences in the high- and low-dose distributions between the individual studies which are equally important when choosing the optimal treatment plan. Also, all studies report large individual differences in dose estimates, and the importance of individualized treatment planning is highlighted universally.
The risk of late effects from modern treatment can be estimated through normal tissue complication probability (NTCP) models which describe the probability of a certain endpoint occurring as a function of radiation dose. For INRT/ISRT, only one NTCP study has been published,44 although, Knäusl et al48 calculate the organ equivalent dose which is as a measure of the biological effect of absorbed dose in pediatric patients. However, many photon-based NTCP models have been published to date for lymphoma patients.43,53,59–63 which can be extrapolated to calculate the clinical significance of a dosimetric benefit with PT. In the single PT-based modeling study available to date,44 the estimated reduction in life expectancy attributable to late effects from RT (from heart failure, myocardial infarction, valvular heart disease, lung and breast cancer) is quantified by the Life Years Lost (LYL) measure which accounts for age at exposure, patient sex, and the prognosis of the individual late effects. The authors report a LYL of 2.1, 1.3, 0.9, and 0.7 with FB-mRT, FB-PT, DIBH-mRT, and DIBH-PT, respectively, and the LYL is primarily driven by the risk of death from lung cancer and valvular disease. Interestingly, there is no significant difference in the LYL for FB-PT vs DIBH-mRT and FB-PT vs DIBH-PT, respectively. Again, modeling studies are limited by the same uncertainties as are dose planning studies as well as by the inherent simplifications of the modeling situation.
Conclusion
PT is an attractive modern radiation modality, with unique properties that may significantly reduce the dose to OARs and potentially spare late toxicity compared to modern photon techniques in patients affected with lymphomas, particularly for those patients with a mediastinal involvement. However, the potential benefit is variable and is based on individual factors as gender, age and disease distribution that should be taken into account on a “case-by-case” accurate analysis. Given the limited number of PT facilities, the additional costs of protons compared to photons, the few clinical reports available to date and some pending issues concerning the biological effect, and the physical properties, it seems reasonable to offer a proton treatment to lymphoma patients only after the achievement of a good competency in the field. Lastly, a careful selection of patients who may benefit from PT, after a proper plan comparison with modern photon therapy, might be a significant step towards further optimized and a more safe RT delivery in hematological diseases.
Disclosure
The authors report no conflicts of interest in this work
References
1. Engert A, Schiller P, Josting A, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early- stage unfavorable Hodgkin’s lymphoma: results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2003;21:3601–3608.
2. Eich HT, Diehl V, Görgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early un- favorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28:4199–4206. doi:10.1200/JCO.2010.29.8018
3. Viviani S, Zinzani PL, Rambaldi A, et al. Michelangelo Foundation; Gruppo Italiano di Terapie Innovative nei Linfomi; Intergruppo Italiano Linfomi: ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365:203–212. doi:10.1056/NEJMoa1100340
4. Mounier N, Brice P, Bologna S, et al. Lymphoma Study Association (LYSA): ABVD (8 cycles) versus BEACOPP (4 escalated cycles ≥ 4 baseline): final results in stage III-IV low-risk Hodgkin lymphoma (IPS 0-2) of the LYSA H34 randomized trial. Ann Oncol. 2014;25:1622–1628. doi:10.1093/annonc/mdu189
5. Horner M, Ries L, Krapcho M, et al. SEER cancer statistics review, 1975–2014. National Cancer Institute. 2017 Available from: https://seer.cancer.gov/csr/1975_2014/. Accessed September 21, 2019.
6. Rutenberg MS, Flampouri S, Hoppe BS. Proton therapy for Hodgkin lymphoma. Curr Hematol Malig Rep. 2014;9:203–211. doi:10.1007/s11899-014-0212-7
7. Aleman BM, van Den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. doi:10.1182/blood-2006-07-034405
8. van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. 2015;34:235–243. doi:10.1200/JCO.2015.63.4444
9. Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med. 2015;373:2499–2511. doi:10.1056/NEJMoa1505949
10. Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372:1598–1607. doi:10.1056/NEJMoa1408648
11. Raemaekers JM, André MP, Federico M, et al. Omitting radiotherapy in early positron emission tomography-negative Stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 Trial. J Clin Oncol. 2014;32:1188–1194. doi:10.1200/JCO.2013.51.9298
12. Ho CK, Flampouri S, Hoppe BS. Proton therapy in the management of lymphoma. Cancer J. 2014;20:387–392. doi:10.1097/PPO.0000000000000076
13. Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the international lymphoma radiation oncology group (ILROG). Int J Radiat Oncol Biol Phys. 2014;89:854–862. doi:10.1016/j.ijrobp.2013.05.005
14. Rwigema JM, Lagendijk JA, van der Laan PH, et al. A model-based approach to predict short-term toxicity benefits with proton therapy for oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2019;104:553–562. doi:10.1016/j.ijrobp.2018.12.055
15. Illidge T, Specht L, Yahalom J, et al. Modern radiation therapy for nodal non-Hodgkin lymphoma – target definition and dose guidelines from the international lymphoma radiation oncology group. Int J Radiat Oncol Biol Phys. 2014;89:49–58. doi:10.1016/j.ijrobp.2014.01.006
16. Dabaja BS, Hoppe BS, Plastaras JP, et al. Proton therapy for adults with mediastinal lymphomas: the International Lymphoma Radiation Oncology Group guidelines. Blood. 2018;132:1635–1646. doi:10.1182/blood-2018-03-837633
17. Edvardsson A, Kugele M, Alkner S, et al. Comparative treatment planning study for mediastinal Hosgkin’s lymphoma: impact on normal tissue dose using deep inspiration breath hold proton and photon therapy. Acta Oncol. 2019;58:95–104. doi:10.1080/0284186X.2018.1512153
18. Moyers MF, Miller DW, Bush DA, Slater JD. Methodologies and tools for proton beam design for lung tumors. Int J Radiat Oncol Biol Phys. 2001;49:1429–1438. doi:10.1016/s0360-3016(00)01555-8
19. Urie M, Goitein M, Wagner M. Compensating for heterogeneities in proton radiation therapy. Phys Med Biol. 1984;29:553–566. doi:10.1088/0031-9155/29/5/008
20. Pacelli R, Caroprese M, Palma G, et al. Technological evolution of radiation treatment: implications for clinical applications. Semin Oncol. 2019; in press. doi:10.1053/j.seminoncol.2019.07.004
21. Hoppe BS, Flampouri S, Zaiden R, et al. Involved-node proton therapy in combined modality therapy for Hodgkin lymphoma: results of a Phase 2 study. Int J Radiat Oncol Biol Phys. 2014;89:1053–1059. doi:10.1016/j.ijrobp.2014.04.029
22. Hoppe BS, Tsai H, Larson G, et al. Proton therapy patterns-of-care and early outcomes for Hodgkin lymphoma: results from the Proton Collaborative Group Registry. Acta Oncol. 2016;55:1378–1380. doi:10.1080/0284186X.2016.1197422
23. Ntentas G, Dedeckova K, Andrlik M, et al. Clinical intensity modulated proton therapy for Hodgkin lymphoma: which patients benefit the most? Practical Rad Onc. 2019;9:179–197. doi:10.1016/j.prro.2019.01.006
24. Sachsman S, Flampouri S, Li Z, Lynch J, Mendenhall NP, Hoppe BS. Proton therapy in the management of non-Hodgkin lymphoma. Leuk Lymphoma. 2015;56:2608–2612. doi:10.3109/10428194.2015.1014364
25. Plastaras JP, Maity A, Flampouri S, et al. Bi-institutional report on consolidative proton therapy after initial chemotherapy for mediastinal diffuse large B-cell and primary mediastinal large B-cell lymphomas [abstract]. Int J Radiat Oncol Biol Phys. 2018;102(S3):E350. doi:10.1016/j.ijrobp.2018.07.1061
26. Konig L, Bougatf N, Horner-Rieber J, et al. Consolidative mediastinal irradiation of malignant lymphoma using active scanning proton beams: clinical outcome and dosimetric comparison. Strahlenther Onkol. 2019. doi:10.1007/s00066-019-01460-7
27. Tseng YD, Hoppe BS, Miller D, et al. Rates of toxicity and outcomes after mediastinal proton therapy for relapsed/refractory lymphoma [abstract]. Int J Radiat Oncol Biol Phys. 2017;99(S2):S62. doi:10.1016/j.ijrobp.2017.06.155
28. Hoppe BS, Hill-Kayser CE, Tseng YD, et al. Consolidative proton therapy after chemotherapy for patients with Hodgkin lymphoma. Ann Oncol. 2017;28:2179–2184. doi:10.1093/annonc/mdx287
29. Ricardi U, Dabaja B, Hodgson DC. Proton therapy in mediastinal Hodgkin lymphoma: moving form dosimetric prediction to clinical evidence. Ann Oncol. 2017;28:2049–2050. doi:10.1093/annonc/mdx356
30. Winkfield KM, Gallotto S, Niemierko A, et al. Proton therapy for mediastinal lymphomas: an 8-year single-institution report [abstract]. Int J Radiat Oncol Biol Phys. 2015;93:E461. doi:10.1016/j.ijrobp.2015.07.1725
31. Wray J, Flampouri S, Slayton W, et al. Proton therapy for pediatric Hodgkin lymphoma. Pediatr Blood Cancer. 2016;63:1522–1526. doi:10.1002/pbc.26044
32. Plastaras JP, Vogel J, Elmongy H, et al. First clinical report of pencil beam scanned proton therapy for mediastinal lymphoma [abstract]. Int J Radiat Oncol Biol Phys. 2016;96:E497. doi:10.1016/j.ijrobp.2016.06.1876
33. Dedeckova K, Mocikova H, Markova J, et al. T011: proton radiotherapy for mediastinal Hodgkin lymphoma: single institution experience [abstract]. Haematologica. 2016;101:12–13.
34. Paganetti H, Giantsoudi D. Relative biologic effectiveness uncertainties and implications for beam arrangements and dose constraints in proton therapy. Semin Radiat Oncol. 2018;28:256–263. doi:10.1016/j.semradonc.2018.02.010
35. Paganetti H. Relative biologic effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, linear energy transfer. Phys Med Biol. 2014;59:R419–R472. doi:10.1088/0031-9155/59/22/R419
36. Cuaron JJ, Chang C, Lovelock M, et al. Exponential increase in relative biological effectiveness along distal edges of proton Bragg peak as measured by deoxyribonucleic acid double-strand breaks. Int J Radiat Oncol Biol Phys. 2016;95:62–69. doi:10.1016/j.ijrobp.2016.02.018
37. International Commission on Radiation Units and Measurements. Prescribing, recording, and reporting proton-beam therapy. ICRU Report 78. 1996.
38. Pagnetti H. Relating proton treatments to photon treatments via the relative biological effectiveness – should we revise current clinical practice? Int J Radiat Oncol Biol Phys. 2015;91:892–894. doi:10.1016/j.ijrobp.2014.11.021
39. Dabaja BS, Mikhaeel G. In the battle between protons and photons for hematologic malignancies, the patient must win. Int J Radiat Oncol Biol Phys. 2015;95:43–45. doi:10.1016/j.ijrobp.2015.09.043
40. Eley JG, Newhauser WD, Ritcher D, Luchtenborg R, Saito N, Bert C. Robustness of target dose coverage to motion uncertainties for scanned carbon ion beam tracking therapy of moving tumors. Phys Med Biol. 2015;60:1717–1740. doi:10.1088/0031-9155/60/4/1717
41. Eley JG, Newhauser WD, Luchtenborg R, Graeff C, Bert C. 4D optimization of scanned ion beam tracking therapy for moving tumors. Phys Med Biol. 2014;59:3431–3452. doi:10.1088/0031-9155/59/13/3431
42. Levis M, De Luca V, Fiandra C, et al. Plan optimization for mediastinal radiotherapy: estimation of coronary arteries motion with ECG-gated cardiac imaging and creation of compensatory expansion margins. Radioth Oncol. 2018;127:481–486. doi:10.1016/j.radonc.2018.04.014
43. Levis M, Filippi AR, Fiandra C, et al. Inclusion of heart substructures in the optimization process of volumetric modulated arc therapy techniques may reduce the risk of heart disease in Hodgkin’s lymphoma patients. Radioth Oncol. 2019;138:52–58. doi:10.1016/j.radonc.2019.05.009
44. Rechner LA, Maraldo MV, Vogelius IR, et al. Life years lost attributable to late effects after radiotherapy for early stage Hodgkin lymphoma: the impact of proton therapy and/or deep inspiration breath hold. Radioth Oncol. 2017;125:41–47. doi:10.1016/j.radonc.2017.07.033
45. Baues C, Marnitz S, Engert A, et al. Proton versus photon deep inspiration breath hold technique in pateints with Hodgkin lymphoma and mediastinal radiation. Radiat Oncol. 2018;13:122. doi:10.1186/s13014-018-1066-2
46. Everett AS, Hoppe BS, Louis D, et al. Comparison of techniques for involved-site radiation therapy in patients with lower mediastinal lymphoma. Pract Radiat Oncol. 2019. doi:10.1016/j.prro.2019.05.009
47. Li J, Dabaja BS, Reed V, et al. Rationale for and preliminary results of proton beam therapy for mediastinal lymphoma. Int J Radiat Oncol Biol Phys. 2011;81:167–174. doi:10.1016/j.ijrobp.2010.05.007
48. Knausl B, Lutgendorf-Caucig C, Hopfgartner J, et al. Can treatment of pediatric Hodgkin’s lymphoma be improved by PET imaging and proton therapy? Strahlenther Onkol. 2013;189:54–61. doi:10.1007/s00066-012-0235-8
49. Hoppe BS, Flampouri S, Su Z, et al. Consolidative involved-node proton therapy for stage I-IIIB mediastinal Hodgkin lymphoma: preliminary dosimetric outcomes from a phase II study. Int J Radiat Oncol Biol Phys. 2012;83:260–267. doi:10.1016/j.ijrobp.2011.06.1959
50. Hoppe BS, Flampouri S, Su Z, et al. Effective dose reduction to cardiac structures using protons compared with 3DCRT and IMRT in mediastinal Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2012;84:449–455. doi:10.1016/j.ijrobp.2011.12.034
51. Sachsman S, Hoppe BS, Mendenhall NP, et al. Proton therapy to the subdiaphragmatic region in the management of patients with Hodgkin lymphoma. Leuk Lymphoma. 2015;56:2019–2024. doi:10.3109/10428194.2014.975802
52. Jørgensen AY, Maraldo MV, Brodin NP, et al. The effect on esophagus after different radiotherapy techniques for early stage Hodgkin’s lymphoma. Acta Oncol. 2013;52:1559–1565. doi:10.3109/0284186X.2013.813636
53. Maraldo MV, Brodin NP, Aznar MC, et al. Estimated risk of cardiovascular disease and secondary cancers with modern highly conformal radiotherapy for early-stage mediastinal Hodgkin lymphoma. Ann Oncol. 2013;24:113–118. doi:10.1093/annonc/mdt156
54. Maraldo MV, Brodin NP, Aznar MC, et al. Doses to carotid arteries after modern radiation therapy for Hodgkin lymphoma: is stroke still a late effect of treatment? Int J Radiat Oncol Biol Phys. 2013;87:297–303. doi:10.1016/j.ijrobp.2013.06.004
55. Maraldo MV, Brodin NP, Aznar MC, et al. Doses to head and neck normal tissues for early stage Hodgkin lymphoma after involved node radiotherapy. Radiother Oncol. 2014;110:441–447. doi:10.1016/j.radonc.2013.09.027
56. Zeng C, Plastaras JP, James P, et al. Proton pencil beam scanning for mediastinal lymphoma: treatment planning and robustness assessment. Acta Oncol. 2016;55:1132–1138. doi:10.1080/0284186X.2016.1191665
57. Chera BS, Rodriguez C, Morris CG, et al. Dosimetric comparison of three different involved nodal irradiation techniques for stage II Hodgkin’s lymphoma patients: conventional radiotherapy, intensity-modulated radiotherapy, and three-dimensional proton radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75:1173–1180. doi:10.1016/j.ijrobp.2008.12.048
58. Horn S, Fournier-Bidoz N, Pernin V, et al. Comparison of passive-beam, proton therapy, helical tomotherapy and 3D conformal radiation therapy in Hodgkin’s lymphoma female patients receiving involved-field or involved site radiation therapy. Cancer Radiother. 2016;20:98–103. doi:10.1016/j.canrad.2015.11.002
59. Pinnix CC, Smith GL, Milgrom S, et al. Predictors of radiation pneumonitis in patients receiving intensity modulated radiation therapy for Hodgkin and non-Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2015;92:175–182. doi:10.1016/j.ijrobp.2015.02.010
60. Filippi AR, Ragona R, Piva C, et al. Optimized volumetric modulated arc therapy versus 3d-CRT for early stage mediastinal Hodgkin lymphoma without axillary involvement: a comparison of second cancers and heart disease risk. Int J Radiat Oncol Biol Phys. 2015;92:161–168. doi:10.1016/j.ijrobp.2015.02.030
61. Aznar MC, Maraldo MV, Schut DA, et al. Minimizing late effects for patients with mediastinal Hodgkin lymphoma: deep inspiration breath-hold, IMRT, or both? Int J Radiat Oncol Biol Phys. 2015;92:169–174. doi:10.1016/j.ijrobp.2015.01.013
62. Brodin NP, Maraldo MV, Aznar MC, et al. Interactive decision-support tool for risk-based radiation therapy plan comparison for Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2014;88:433–445. doi:10.1016/j.ijrobp.2013.10.028
63. Pinnix CC, Cella L, Andraos TY, et al. Predictors of hypothyroidism in Hodgkin lymphoma survivors after intensity modulated versus 3-dimensional radiation therapy. Int J Radiat Oncol Biol Phys. 2018;101:530–540. doi:10.1016/j.ijrobp.2018.03.003
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.