Back to Journals » Drug Design, Development and Therapy » Volume 13
Protective effects of five compounds from Livistona chinensis R. Brown leaves against hypoxia/reoxygenation, H2O2, or adriamycin-induced injury in H9c2 cells
Authors Li S , Luo S, Chen H, Zheng Y , Lin L, Yao H , Lin X
Received 16 January 2019
Accepted for publication 25 March 2019
Published 8 May 2019 Volume 2019:13 Pages 1555—1566
DOI https://doi.org/10.2147/DDDT.S201816
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Shaoguang Li, Shaohong Luo, Hao Chen, Yanjie Zheng, Liqing Lin, Hong Yao, Xinhua Lin
Department of Pharmaceutical Analysis, Faculty of Pharmacy, Fujian Medical University, Fuzhou 350122, People’s Republic of China
Purpose: Discovering new antimyocardial ischemia drug candidates that are highly efficient, have low toxicity, and originate from natural products is a popular trend for new cardiovascular drug development at present. The ethanol extract of Livistona chinensis leaves showed a favorable antioxidant activity in our preliminary screening test. This study aims to screen out antioxidants from the herb leaves further and evaluate their efficacy in acute myocardial ischemia treatment at the cellular level.
Materials and methods: Guided with online 1, 1-diphenyl-2-picrylhydrazyl (DPPH)–high-performance liquid chromatography (HPLC) screening, antioxidants were first separated and isolated from the ethanol extract of L. chinensis leaves by preparative-HPLC. Subsequently, offline DPPH approach was used to validate the free radical scavenging activity of the components. Ultimately, the resulting antioxidants were evaluated against the hypoxia/reoxygenation (H/R)-, H2O2-, or adriamycin (ADM)-induced injury in H9c2 cells to verify their cardioprotective effects in vitro.
Results: Five antioxidant ingredients, namely, orientin, isoorientin, vitexin, isovitexin, and tricin, were quickly distinguished and isolated from L. chinensis leaves. The IC50 values of these ingredients were further examined by offline DPPH assay, as follows: 15.51±0.22, 6.64±0.38, 11.86±0.24, 8.89±0.66, and 31.86±0.24 μg/mL, respectively. Out of these ingredients, isoorientin showed the strongest antioxidation, which was equivalent to that of the positive control drug (vitamin C, IC50: 6.99±0.62 μg/mL). Using H/R-, H2O2-, and ADM-induced H9c2 cell injury models, the five ingredients had different extents of cardioprotective effects in vitro. In particular, isoorientin showed the strongest protection. All the five ingredients also showed insignificant cytotoxic effect to normal H9c2 cells.
Conclusion: The ethanol extract of L. chinensis leaves contained five antioxidants with low cardiac cytotoxicity. Isoorientin possessed the strongest antioxidation, which can predominantly account for the myocardial protection effects within the extract.
Keywords: effect-directed analysis, antioxidant, protective effects, flavone, H9c2 cell
Introduction
Livistona chinensis (Jacq.) R. Brown (L. chinensis), which is generally known as Chinese fan or Chinese fountain palm, is a large tree common in the subtropics. This tree is cultivated for ornamental purposes and utilized in herbal remedies in Southern China.1 L. chinensis seeds,2–7 leaves,8–11 and roots12,13 have been traditionally used as folk agents in treating different diseases, such as gastrointestinal cancer and nasopharyngeal carcinoma.13–19 L. chinensis leaves also appear as a main component in few L. chinensis herbal compounds for cardiovascular treatment (patents have been applied for and are being applied for).9–11 At present, studies on the antitumor effects and constituents of seeds are relatively systematic, but no report exists on leaf bioactive constituents. Revealing leaf effective substance basis is an important issue for its development and application in the clinical field.
ROS such as H2O2, superoxide anion radical, and hydroxyl radical (•OH) are associated with various pathological conditions, such as cancer, diabetes, and especially, cardiovascular diseases. Clinically, many myocardial diseases caused by pathological conditions, surgical approach, or drugs (especially anthracycline) are often accompanied by myocardial ischemia and reperfusion, and serious injury after perfusion can be attributed to the increase in oxidative stress. Antioxidants play an important role in the treatment of cardiovascular diseases because they can capture, deactivate, or repair the damage caused by ROS. Therefore, natural antioxidants with cardioprotective potential can be obtained from the plant resources. However, to date, research on the antioxidant components in L. chinensis (Jacq.) R. Brown leaves is limited.18–25
Hence, high-performance liquid chromatography (HPLC) with precolumn 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay (HPLC–DPPH) was performed to screen and orient the active ingredients online, followed by using preparative HPLC (prep-HPLC) to prepare the locked antioxidant components in L. chinensis leaves. The resulting isolated compounds were further identified, and then their antioxidative effects were examined by offline DPPH free radical scavenging. Ultimately, three in vitro cardiovascular protection tests, namely, hypoxia/reoxygenation (H/R)-, H2O2-, and adriamycin (ADM)-induced H9c2 cell injury models, were used to confirm the cardioprotective effects of these antioxidant ingredients further. The present study elucidates the main antioxidant components of L. chinensis leaves and provided evidence in developing excellent antioxidants from this plant resource.
Materials and methods
Reagents and materials
All solvents used to prepare crude samples were of analytical grade and obtained from Sinopharm Chemical Reagents (Shanghai, People's Republic of China). Methanol used for HPLC and prep-HPLC was of chromatographic grade (Merck, Darmstadt, Germany). All aqueous solutions were prepared with ultrapure water.
The L. chinensis leaves were collected in the campus of Fujian Medical University in January 2017. The voucher specimens (no. S171207), which were identified by Professor Hong Yao, were deposited in the Department of Pharmaceutical, Fujian Medical University (Fuzhou, People's Republic of China). The dry leaves were pulverized into coarse powder and passed through a 20 mesh sieve and then extracted two times with an eightfold volume of 75% ethanol under reflux (2 hrs per time in a 90°C water bath). The extracts were merged and concentrated by rotary evaporation at 50°C under reduced pressure. Then, a part of extraction was dissolved in 80% methanol for HPLC analysis.
Antioxidant online screening and target-guided separation
The at-line hyphenation of HPLC–DPPH was used to screen and guide the separation of antioxidants from L. chinensis leaves via prep-HPLC. The scavenging activity of the DPPH free radical was assayed according to the method of Shang-Tse Ho (2010) and Yao Hong (2012).26,27 Briefly, a 0.1 mM DPPH solution was prepared in 100% methanol, and 1 mL of this solution was added to 1 mL of the extract methanol solution (10 mg/mL). The mixture was shaken vigorously and incubated for 30 mins in the dark at room temperature. Then, the reaction mixture and the extract methanol solutions were analyzed by HPLC under the same chromatographic conditions. Online screening was used for HPLC analysis, which was performed on Shimadzu HPLC 20A system (Shimadzu, Japan) equipped with a column temperature controller and a UV detector. The chromatographic conditions are as follows. Chromatographic screening was carried out on a SinoChrom RD C18 column (250 mm×4.6 mm, 5.0 μm, Sino-chromatogram Sci & Tech, Inc., People's Republic of China) at 30°C. The flow rate of mobile phase was maintained at 0.8 mL/min, and the injection volume was 5 μL. Compounds were separated using a stepwise mobile-phase gradient prepared from 0.05% aqueous acetic acid (A) and methanol (B). The gradient was applied as follows: 0.1 min, 33% B in A; 25 mins, 45% B in A; and 60 mins, 75% B in A. The re-equilibrium took 10 mins, thereby resulting in a total run time of 75 mins. The chromatography was recorded using a UV detector at 254 and 340 nm. The HPLC chromatograms of the total extract are shown in Figure 1(A). Subsequently, comparing the chromatograms of the extract showed that some peaks that disappeared or shortened in the chromatogram of the mixture can be considered as the antioxidant peaks, which are indicated in the HPLC chromatograms in Figure 1(B). The components for the five peaks (peaks 1, 2, 3, 4, and 5) showed good DPPH scavenging activity as the peak area reduced after mixing with DPPH. The degree of descent denoted the different free radical scavenging abilities. The results suggested that the antioxidant activity of the components at peak 2 can be extremely strong. Finally, guided by the analysis results, the extraction of leaves was eluted by different volume fraction ethanol solutions (stepwise with 0%, 10%, 30%, 50%, 70%, 90%, and 100% v/v, respectively) with D101 macroporous resin. The eluent of each section, that is, concentrated as fractions I–VII, was screened and selected for further target-guided separation with prep-HPLC. The analysis showed that four targeted components (peaks 1–4) were enriched in fraction IV, and one component (peak 5) was enriched in the fraction VI. The separation procedures were also set, as shown in Table 1. Selected extracts were prepared with a Shimadzu LC-6A HPLC system equipped with a UV detector and a preparative SinoChrom RD C18 column (250 mm×10 mm i.d., 5 μm, Sino-chromatogram Sci & Tech, Inc., China). The prep-HPLC chromatograms of fractions IV and VI are shown in Figure 1(C) and (D).
 | Table 1 Separation procedures of prep-HPLC for fractions IV and VI |
Antioxidant effect offline validation
The potential antioxidants obtained from L. chinensis leaves were further evaluated by offline DPPH assay, as follows. The sample solutions of each compound and ascorbic acid (used as positive control) at different concentrations (0.0625–1 mg/mL) were prepared for measurement. Then, 30 μL of each sample solution and 60 μL of 0.1 mM DPPH in methanol reacted in each well of a 96-well microplate and incubated for 30 mins (n=6). With 30 μL of methanol instead of the sample solution as the control group (n=6), and with 30 and 60 μL of methanol instead of the sample and DPPH solutions, respectively, as the blank group (n=6), their absorbances (OD) were measured at 517 nm. Then, the antioxidative activity (inhibition rate) was calculated using the following equation: antioxidant activity (%)=(ODcontrol−ODsample)/(ODcontrol−ODblank)×100. The percentage of antioxidant activity was plotted against the logarithmic concentration (mg/mL). The antioxidant activity of plant extracts was expressed by half maximum inhibitory concentration (IC50), which indicated that the ODsample concentration can be reduced by half compared with ODcontrol at 517 nm. Every sample was used in six parallel measurements.
Cell culture
Cardiac H9c2 cell line is a subclone of the original clonal cardiomyoblast, which is derived from embryonic rat heart tissue and has numerous cardiomyocyte characteristics.28,29 The cell line purchased from Chinensis Academy of Sciences (Shanghai, People's Republic of China) cell bank was used in the present study. The cells were cultured in DMEM supplemented with 10% fetal bovine serum, 100 units/mL of penicillin, and 100 μg/mL of streptomycin in a humidified incubator containing 95% air and 5% CO2 at 37°C. The media were refreshed every 2–3 days.
The cells cultured at approximately 80% confluence were collected, and the cell suspension concentration was adjusted to 4×103 cells/well. Cells were cultured in a 96-well plate at 37°C for 8 hrs in an incubator containing 5% (v/v) CO2.
Cardiotoxicities of these antioxidants
To investigate the cytotoxicity of these antioxidants on cardiomyocytes, we used H9c2 cells cultured in 96-well plate to suffer high concentrations of these compounds (40 μg/mL) for 24 hrs and then observed and photographed directly by using a microscope (XDS-1B, COIC, Mike Photoelectric Instrument Co., Ltd., Chongqing, People's Republic of China).
Construction of different H9c2 cell injury models and MTT assay
H/R-induced cell injury model construction and MTT assay
H/R cell model was established to simulate H/R injury in vitro. H9c2 cells were switched from high-glucose DMEM to free-glucose DMEM for 1-day treatment. Then, H9c2 cells were placed in an anaerobic condition with 5% (v/v) CO2 and 95% (v/v) N2 for 2 hrs, and culture temperature was controlled at 37±0.5°C. Subsequently, H9c2 cells were recovered to the conventional culture. H9c2 cells were cultured under normal conditions and served as the blank control group. To optimize pretreated time, we pretreated H9c2 cells with different compounds (5 μg/mL) for 0, 24, and 48 hrs before accepting H/R stimulation. The optimized pretreated time was used in subsequent screening. The different concentrations of these compounds (ie, 2.5, 5, 10, 20, and 40 μg/mL) were added to the medium before H/R induction. Compounds were dissolved in dimethyl sulfoxide (DMSO, Sigma).
The effects of five compounds on cell viability were assessed using a cell counting MTT assay. Briefly, the MTT solution was added to a final concentration of 0.5% when the cells were cultured with different compounds 24 hrs later. Then, the cultures were returned to the incubator for 4 hrs, and the culture fluid was removed, followed by adding 150 μL of DMSO to each well. Subsequently, the OD at a wavelength of 570 nm was measured on a microplate spectrophotometer reader (Multiskan GO, Thermo Scientific, MA, USA).30
H2O2-induced cell injury model construction and MTT assay
The three sets of experiments were performed at standard culture conditions, as follows: 1) untreated control cells; 2) cells were treated with the IC50 concentration of H2O2 (100 μmol/L) for 4 hrs, and then medium was changed for another 24 hrs; and 3) cells were treated with the IC50 concentration of H2O2 (100 μM) for 4 hrs, and then the medium was changed. The injured cells were treated with the different concentrations of the five compounds (2.5–40 μg/mL, dissolved in DMSO; Sigma) for 24 hrs.31 Cell viability was analyzed using MTT methods according to the method mentioned above.
ADM-induced cell injury model construction and MTT assay
The use of plant antioxidants is currently being investigated as a possible adjunctive therapy in combination with ADM to protect the myocardium from ADM-induced oxidative stress.32 Plant antioxidants, specifically flavonoids, can prevent ADM-induced cardiotoxicity, largely due to their anticancer and cardioprotective properties.33 The plant antioxidants isolated from L. chinensis leaves were also investigated in this study.
To explore the protective effect of the five compounds on ADM-induced cytotoxicity, we plated H9c2 cells (2×104) on a 96-well plate and allowed them to adhere overnight. After adherence, the cells were treated with ADM at a final concentration of 2.5 μM for 6 hrs. The ADM-induced injured cells were separately treated with the various concentrations of the five compounds (0.625–10 μg/mL, dissolved in DMSO; Sigma) for 24 hrs. Cell viability was analyzed using MTT methods according to the method introduced in “H/R-induced cell injury model construction and MTT assay.”
Results
Antioxidant online screening and target-guided separation
The antioxidants of the five peaks from L. chinensis (Jacq.) R. Brown leaves were identified by electrospray ionization mass spectrometry (Figure S1) and nuclear magnetic resonance (both 1H NMR and 13C NMR). Among these compounds, five were flavonoids, and their 1H NMR and 13C NMR profiles matched with the reported NMR data for orientin, isoorientin, isovitexin, vitexin, and tricin.34–37 The chemical structures of the five antioxidants are shown in Figure 2.
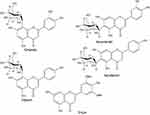 | Figure 2 Chemical structures of five antioxidants found in L. chinensis (Jacq.) R. Brown leaves. |
 | Figure S1 Mass spectrometric data of five compounds. |
Antioxidant effect offline validation
The free radical scavenging activity of the five identified components in L. chinensis leaves was further evaluated by the offline DPPH assay with their standard compounds. We tested the five identified components in L. chinensis leaves on their abilities to scavenge DPPH in a 96-well plate. Most of these compounds can scavenge DPPH compared with ascorbic acid control. The results are listed in Table 2. The table shows that tricin, vitexin, and orientin exhibited a weak DPPH scavenging activity, while isoorientin and isovitexin presented a remarkable free radical scavenging ability. The IC50 values of isoorientin and isovitexin (6.64±0.38 and 8.89±0.66 μg/mL, respectively) were almost equal to that of ascorbic acid (6.99±0.62 μg/mL), which is widely used as antioxidant drugs in clinic setting. The results are in accordance with the findings in online screening with HPLC–DPPH. These results also indicated that the extracts or the derived phytochemicals (isoorientin and isovitexin) from the L. chinensis leaves possessed considerable potential to prevent diseases caused by radical overproduction, and these extract and compounds may be suitable for degenerative disease treatment.
 | Table 2 IC50 values of five compounds and ascorbic acid in scavenging DPPH• (n=6) |
Cardiotoxicities of five flavonoids
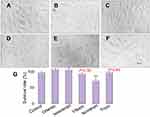
Drug-induced cardiotoxicity is a key safety concern due to the adverse effects on the cardiovascular system, and it has become a major cause for drug failure, thereby limiting the therapeutic efficiency. Hence, knowing whether cardiotoxic side effects are present in the screening of new natural ingredients is extremely important. Given that the toxic effects of natural products may lead to the activation of programmed cell death, we next examined the extent of cell death in cardiomyoblasts.38 Under an inverted microscope, the normal cells were spindle-shaped, and the cells treated with different compounds had different degrees of cell damage and shrinkage, and dead cells appeared (Figure 3D–E). MTT assay was conducted to measure the cytotoxicity of the five compounds with a certain treatment time (24 hrs) and concentration (40 μg/mL) in the H9c2 cardiomyoblasts. The validated MTT data coupled with morphological changes observed under optical microscopy (Figure 3) indicated that orientin, isoorientin, vitexin, and tricin had almost no toxicity, but isovitexin had a certain degree of cytotoxicity in H9c2 cells.
Bioactivity detection of five compounds in H/R-induced myocardial injury model
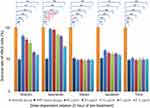
H9c2 cells were pretreated with the five compounds (5 μg/mL) for 48, 24, and 0 hrs and then exposed to H/R-induced myocardial injury conditions for another 24 hrs. The MTT data are shown in Figure 4.
 | Figure 4 Time-dependent relationship of survival rate improvement by pretreating with different compounds (5 μg/mL, **p<0.01 vs control models). |
As shown in the figure above, after H/R injury treatment, the H9c2 cell number decreased to 50.8±1.3% compared with the number in the control group (n=30, p<0.01). The survival rate improvement of the injured H9c2 cells, which were individually pretreated with orientin, isoorientin, ovitexin, isovitexin, and tricin, was different. Our validated MTT data indicated that isoorientin had significantly improved survival rates among the three pretreatment time periods in H/R-induced myocardial injury models, and other compounds only had slightly improved survival rates after 0 hr of pretreatment. However, no effect or even toxicity was noted after 24 and 48 hrs of pretreatment. The data can also enlighten the intervention models of these compounds, which indicated that 0 hr of pretreatment was the most suitable among the pretreatment periods.
Therefore, another groups of H/R injury models were cultured for the dose-dependent relationship of survival rate improvement by 0 hr of pretreatment with different compounds (in H/R injury models, n=6), as shown in Figure 5. In this study, the five compounds (dissolved in DMSO, Sigma) were diluted with culture medium and prepared at a series of concentration ranging from 2.5 to 40 μg/mL. The results showed that isoorientin exhibited the most significantly improved effect on reducing H/R injury and increasing cell survival rate with treatment dose (both in the range of 2.5–40 μg/mL). Orientin also had a certain effect.
Bioactivity detection of five compounds in H2O2-induced myocardial injury model
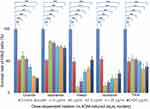
The cells were treated with the IC50 concentration of H2O2 (100 μmol/L) for 4 hrs. Then, the medium was changed and added with different concentrations of the five compounds (2.5–40 μg/mL). As shown in Figure 6, after treatment with H2O2 for 4 hrs, the cell survival rate decreased to 50.27±1.41% compared with the number in the control group (n=30, p<0.01).
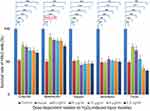 | Figure 6 Dose-dependent relationship of survival rate improvement by treatment with different compounds (in H2O2-induced injury models, n=6), **p<0.01 vs control models. |
The survival rate of model cells treated with orientin, isoorientin, and tricin increased to varying degrees, among which isoorientin exhibited the best effect, followed by orientin and tricin.
Bioactivity detection of five compounds in ADM-induced myocardial injury model
The MTT data shown in Figure 7 reported that after treatment with ADM for 6 hrs, ADM-induced injury decreased the survival rate to 51.07±1.38% compared with the number in the control group (n=30, p<0.01). The model cells were also treated with various concentrations of the five compounds (0.625–10 μg/mL) for 24 hrs. Isoorientin also exhibited a positive effect on the improvement of the survival rates of the injury models when the concentration was in the range of 0.625–10 μg/mL.
Discussion
Plant polyphenols have gained considerable interest for their possible health benefits because they have a rich source of antioxidants that are known to decrease lipid peroxidation induced by increased ROS. This study aimed both to develop an efficient and accurate method on the basis of effect-directed analysis and fast separation of the antioxidants in the crude extract of L. chinensis leaves, and we chose and validated the cardioprotective potential drugs for acute myocardial injury protection through the H9c2 cell models of different injury mechanisms.
As confirmed in the results, effect-directed analysis on the basis of online DPPH–HPLC coupled with prep-HPLC was used to screen and separate five antioxidants efficiently and accurately. The five identified flavones (ie, orientin, isoorientin, vitexin, isovitexin, and tricin) were validated with a certain antioxidant effect by offline DPPH. Therefore, effect-directed analysis is an effective method both in assessing and guiding antioxidant preparation. Three myocardial acute injury models, namely, H/R, H2O2, and ADM, built by optimized modeling conditions, were suitable, stable, and reproducible. Our results showed that the five compounds of L. chinensis (Jacq.) R. Brown had different antioxidant levels and effects in different myocardial protective models. This result suggested that antioxidant capacity was associated with myocardial protection but incompletely. The protective effect may be both related to the cytotoxicity of the ingredients themselves, such as isovitexin, and the different pathogenesis of these models. In reducing the myocardial mortality in the three models injured by H/R, H2O2, and ADM, the myocardial protective effect cannot be achieved by simply reducing the ROS levels but by using other mechanisms. For example, in the injured myocardium, inflammatory reaction, Ca overload, oxidative stress, energy utilization disorder, autophagy, apoptosis, and/or other mechanisms work together to cause cell death.30–33,39–41 The mechanism underlying the cardioprotective effects of the three components has been rarely reported. Among these components, isoorientin with excellent antioxidant capacity and low myotoxicity exhibited the most significant protective effect in acute myocardial injury models induced by H/R, H2O2, and ADM. Orientin had protective effect on acute myocardial injury induced by H/R and H2O2. Tricin was also effective against H2O2-induced injury. According to the results mentioned above, all the components had effects on H2O2-induced models, which indicated that they may quickly reduce the ROS-induced injury due to their antioxidant capacity. H/R- and ADM-induced models may be required for other protective mechanisms first for cardiomyocyte protection. Thus, isoorientin can protect myocardial cells from different injuries because it may be related to the reduction of oxidative stress or through a combination of multiple mechanisms for cardiomyocyte protection to reduce cardiotoxicity in combination with anthracyclines in chemotherapy regimens.
Conclusion
To accomplish the positive long-term outcomes for patients, we explored natural compounds for their extensive benefits. Natural ingredients possessing cardioprotective compounds can be used to supplement the existing chemotherapy regimens to improve their efficacy and reduce drug-induced cardiotoxicity. The method on the basis of the bioassay-guided separation and identification of antioxidant components in L. chinensis R. Brown leaves should be popularized for the development of other natural products and the further pharmacodynamics study. According to the results, three compounds, namely, orientin, isoorientin, and tricin, that showed certain antioxidant effects and cardioprotective effects can be considered as promising cardioprotective components with low cytotoxicity, but they need further development.
Acknowledgments
The authors gratefully acknowledge the financial support of the Fujian Provincial Natural Science Foundation (grant no. 2016J01368), Joint Funds for the Innovation of Science and Technology Fujian Province (grant no. 2017Y9118), and the Natural Science Foundation of Fujian Province in China (grant no. 2016J01767), and Medical Innovation Project of Fujian Province in China (grant no. 2016-CX-44).
Author contributions
Shaoguang Li, Hong Yao, and Xinhua Lin conceived and designed the experiments. Shaohong Luo and Hao Chen performed the experiments. All authors contributed to data analysis, drafting and revising the article, provided final approval of the version to be published, and agreed to be accountable for all the aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1.
2. Lin W, Zhao JY, Cao ZY, et al. Livistona chinensis seeds inhibit hepatocellular carcinoma angiogenesis in vivo via suppression of the Notch pathway. Oncol Rep. 2014;31(4):1723–1728. doi:10.3892/or.2014.3051
3. Cao ZY, Zheng LP, Zhao JY, et al. Anti-angiogenic effect of Livistona chinensis seed extract in vitro and in vivo. Oncol Rep. 2017;14(6):7565–7570.
4. Li CY, Zeng YB, Peng F, et al. In vitro anti-HIV-1 activity of extracts from Livistona chinensis seeds and its mechanism. Chin Tradit Herbal Drugs. 2008;12(39):1833–1838.
5. Yuan T, Yang SP, Zhang HY, et al. Phenolic compounds with cell protective activity from the fruits of Livistona chinensis. J Asian Nat Prod Re. 2009;11(3):243–249. doi:10.1080/10286020802684631
6. Huang WC, Hsu RM, Chi LM, et al. Selective downregulation of EGF receptor and downstream MAPK pathway in human cancer cell lines by active components partially purified from the seeds of Livistona chinensis R. Brown. Cancer Lett. 2007;248(1):137–146. doi:10.1016/j.canlet.2006.06.010
7. Wang CL, Zhang L, Wu MM, et al. Antioxidative and hepatoprotective activities of the ethyl acetate fraction separated from the fruit of Livistona chinensis. J Tradit China Med. 2018;38(4):523–534. doi:10.1016/S0254-6272(18)30884-7
8. Qin WX Chinese medicine preparation for treating asthma and its preparation method. China Patent CN105998498A.201610554506.6. 2016 Oct 12.
9. Ma GF. Granule for treating coronary heart disease. China Patent CN105343735A. 2016 Feb 24.
10. An BH, Han J, Cui JX, et al. Medicine composition for treating myocardial infarction and its application. China Patent CN104398919A. 2015 Mar 11.
11. Wang L Medicine composition for resisting cardiac hypertrophy, preparation method and application thereof. China Patent No. CN105327172A. 2016 Feb 17.
12. Zeng XB, Xiang LM, Li CY, et al. Cytotoxic ceramides and glycerides from the roots of Livistona chinensis. Fitoterapia. 2012;83(3):609–616. doi:10.1016/j.fitote.2012.01.008
13. Zhong ZG, Zhang FF, Zhang WY, et al. Study on the anticancer effects of extracts from roots of Livistona chinensis in vitro. J Chin Med Mater. 2007;30(1):60–63.
14. Cheng XS, Zhong F, He K, et al. A novel phenolic natural product from Livistona chinensis, induces autophagy-related apoptosis in hepatocellular carcinoma cells. Oncol Lett. 2016;12(5):3739–3748. doi:10.3892/ol.2016.5178
15. Zeng XB, Wang YH, Qiu Q, et al. Bioactive phenolics from the fruits of Livistona chinensis. Fitoterapia. 2012;83(1):104–109. doi:10.1016/j.fitote.2011.09.020
16. Chen Y, Lin XH, Li SG, et al. Anti-tumor activity of the extracts of Livistona chinensis in vitro. J Fujian Med Univ. 2008;42(2):93–95.
17. Lin W, Zhao JY, Cao ZY, et al. Livistona chinensis seed suppresses hepatocellular carcinoma growth through promotion of mitochondrial-dependent apoptosis. Oncol Lett. 2013;29(5):1859–1866.
18. Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell B. 2007;39(5):44–84. doi:10.1016/j.biocel.2006.07.001
19. Murphy MP, Holmgren A, Larsson NG, et al. Unraveling the biological roles of reactive oxygen species. Cell Metabol. 2011;13(4):361–366. doi:10.1016/j.cmet.2011.03.010
20. Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi:10.1152/physrev.00018.2001
21. Djukic M, Ninkovic M, Jovanovic M. Oxidative stress-clinical diagnostic significance. J Mol Biol. 2008;27(4):409–425.
22. Jian J, Xuan FF, Qin FZ, Huang R. The antioxidant, anti-inflammatory and anti-apoptotic activities of the bauhinia championii flavone are connected with protection against myocardial ischemia/reperfusion injury. Cell Physiol Biochem. 2016;38(4):1365–1375. doi:10.1159/000443080
23. Lu CC, Xu YQ, Wu JC, et al. Vitexin protects against cardiac hypertrophy via inhibiting calcineurin and CaMKII signaling pathways. N-S Arch Pharmacol. 2013;386(8):747–755. doi:10.1007/s00210-013-0873-0
24. Woodman OL, Chin KY, Thomas CJ, et al. Flavonols and flavones – protecting against myocardial ischemia/reperfusion injury by targeting protein kinases. Curr Med Chem. 2018;25:34. doi:10.2174/0929867325666180326161730
25. Yuan Y, Pan S, Yang SL, et al. Antioxidant and cardioprotective effects of Ilex cornuta on myocardial ischemia injury. Chin J Nat Med. 2017;15(2):94–104. doi:10.1016/S1875-5364(17)30025-0
26. Ho ST, Tung YT, Cheng KC, et al. Screening, determination and quantification of major antioxidants from Balanophora laxiflora flowers. Food Chem. 2010;122(3):584–588. doi:10.1016/j.foodchem.2010.03.014
27. Yao H, Chen Y, Shi P-Y, et al. Screening and quantitative analysis of antioxidants in the fruits of Livistona chinensis R. Br using HPLC-DAD-ESI/MS coupled with pre-column DPPH assay. Food Chem. 2012;135(4):2802–2807. doi:10.1016/j.foodchem.2012.07.076
28. Hescheler J, Meyer R, Plant S, et al. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 1991;69(6):1476–1486.
29. Karlsson M, Kurz T, Brunk UT, et al. What does the commonly used DCF test for oxidative stress really show? Biochem J. 2010;428(2):183–190. doi:10.1042/BJ20100208
30. Sun Y, Xun LR, Jin G, Shi L. Salidroside protects renal tubular epithelial cells from hypoxia/reoxygenation injury in vitro. J Pharmacol Sci. 2018;137(2):170–176. doi:10.1016/j.jphs.2018.05.011
31. Yang DK, Kim SJ. Cucurbitacin I protects H9c2 cardiomyoblasts against H2O2-induced oxidative stress via protection of mitochondrial dysfunction. Oxid Med Cell Long. 2018;2018:1–11. doi:10.1155/2018/9579262
32. Wang XY, Chen LL, Wang T, et al. Ginsenoside Rg3 antagonizes adriamycin-induced cardiotoxicity by improving endothelial dysfunction from oxidative stress via upregulating the Nrf2-ARE pathway through the activation of akt. Phytomedicine. 2015;22(10):875–884. doi:10.1016/j.phymed.2015.06.010
33. Yi XJ, Zhu JF, Zhang JH, et al. Investigation of the reverse effect of Danhong injection on doxorubicin-induced cardiotoxicity in H9c2 cells: insight by LC–MS based non-targeted metabolomic analysis. J Pharmaceut Biomed. 2018;152:264–270. doi:10.1016/j.jpba.2018.02.012
34. Liu J, Luo JG, Ye H, Zeng X. Preparation, antioxidant and antitumor activities in vitro of different derivatives of levan from endophytic bacterium Paenibacillus polymyxa EJS-3. Food Chem Toxicol. 2012;50(3–4):767–772. doi:10.1016/j.fct.2011.11.016
35. Yu XX, Huang JY, Xu D, et al. Isolation and purification of orientin and vitexin from Trollius chinensis Bunge by high-speed counter-current chromatography. Nat Prod Res. 2014;28(9):674–676. doi:10.1080/14786419.2014.891111
36. Farag MA, Otify A, Porzel A, et al. Comparative metabolite profiling and fingerprinting of genus Passiflora leaves using a multiplex approach of UPLC-MS and NMR analyzed by chemometric tools. Anal Bioanal Chem. 2016;408(12):3125–3143. doi:10.1007/s00216-016-9376-4
37. Chung I, Ali M, Ahmad A. Oryzasesterpenolide from the hulls of Oryza sativa and complete NMR assignments of momilactones A, B and Tricin. Asian J Chem. 2005;17(4):2467–2478.
38. Jain A, Rani V. Anti-hypotensive drug induced cardiotoxicity: an in vitro study. In Vitro Cell Dev Biol Anim. 2018;54:92–98. doi:10.1007/s11626-017-0222-6
39. Jiang YQ, Chang G, Wang Y, et al. Geniposide prevents hypoxia/reoxygenation-induced apoptosis in H9c2 cells: improvement of mitochondrial dysfunction and activation of GLP-1R and the PI3K/AKT signaling pathway[J]. Cell Physiol Biochem. 2016;39(1):407–421. doi:10.1159/000445634
40. Sun B, Sun GB, Xiao J, et al. Isorhamnetin inhibits H2O2‐induced activation of the intrinsic apoptotic pathway in H9c2 cardiomyocytes through scavenging reactive oxygen species and ERK inactivation. J Cell Biochem. 2012;113(2):473–485. doi:10.1002/jcb.23371
41. Cheung KG, Cole LK, Xiang B, et al. Sirtuin-3 (SIRT3) protein attenuates doxorubicin-induced oxidative stress and improves mitochondrial respiration in H9c2 cardiomyocytes. J Biol Chem. 2015;290(17):10981–10993. doi:10.1074/jbc.M114.607960
Supplementary Material
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.