Back to Journals » Journal of Inflammation Research » Volume 13
Protective Effects of Curcumin and its Nano-Phytosome on Carrageenan-Induced Inflammation in Mice Model: Behavioral and Biochemical Responses
Authors Baradaran S, Hajizadeh Moghaddam A, Khanjani Jelodar S, Moradi-kor N
Received 4 October 2019
Accepted for publication 10 December 2019
Published 21 January 2020 Volume 2020:13 Pages 45—51
DOI https://doi.org/10.2147/JIR.S232462
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Saeideh Baradaran, 1 Akbar Hajizadeh Moghaddam, 1 Sedigheh Khanjani Jelodar, 1 Nasroallah Moradi-kor 2
1Department of Biology, Faculty of Basic Sciences, University of Mazandaran, Babolsar, Iran; 2Research Center of Physiology, Semnan University of Medical Sciences, Semnan, Iran
Correspondence: Nasroallah Moradi-kor
Research Center of Physiology, Semnan University of Medical Sciences, Damghan Road, P.O. Box 35195-163, Semnan, Iran
Email [email protected]
Akbar Hajizadeh Moghaddam
Department of Biology, Faculty of Basic Sciences, University of Mazandaran, P.O. Box 57416-13534, Babolsar, Iran
Email [email protected]
Background and purpose: Natural compounds are used for prevention of inflammation. Curcumin has antioxidant and anti-inflammatory properties, and loading it into nano-phytosomes may improve its efficiency. The present study investigates the effects of curcumin and its nano-phytosome on behavioral and biochemical responses in carrageenan-induced inflammation in the mice model.
Methods: The mice were divided into six groups and received oral administration of curcumin or its nano-phytosome at a dose of 15 mg/kg for seven days before the administration of carrageenan. Acute inflammation in the mice was induced by administration of carrageenan (1%) into the subplantar region of the left paw. Antioxidant activity and behavioral responses were then evaluated.
Results: The results showed that the serum concentrations of antioxidant enzymes were significantly higher in the sal+sal group compared to the cara+sal group (P< 0.05). Using nanophytosome, separately and in combination with indomethacin, increased the levels of antioxidant enzymes compared to the cara+sal group (P< 0.05). Latency was significantly lower in the cara+sal group compared to the cara+sal group (P< 0.05), but it was considerably higher in other groups, especially in the cara+nano.ph.cur+indo group (P< 0.05).
Conclusion: It can be stated that the nano-phytosome of curcumin could improve antioxidant and behavioral responses in inflamed mice.
Keywords: antioxidant activity, inflammation, mice model, nano-phytosome
Introduction
Inflammation is a defensive response that is marked with increased pro-inflammatory cytokines.1 Some compounds are extensively used for inducing inflammation. Carrageenan, a sulfated polysaccharide, is broadly applied as a food additive and induces inflammation in animal models.2 Carrageenan is used for induction of inflammation, evaluation of inflammation mediators, and some assessment of anti-inflammation compounds.3 Inflammation induced by carrageenan comprises cascade from Toll-like receptor, activates B-cell leukemia/lymphoma (BCL)10-dependent and increases the production of IL-8.2 It was reported that inflammation and oxidative stress mutually boost each other's effect in the vasculature system and visceral fat.4 Inflammation also increases the production of leukocytes.4 Activated leukocytes produce an enzyme and a biomarker that increases reactive oxygen species (ROS).5 Increased ROS causes tissue damage, lipid peroxidation, and inflammatory cycle.5 There is a relationship between inflammation and oxidative stress. Natural compounds are used for prevention of inflammation. One of these natural compounds is curcumin which is an active ingredient in turmeric and is responsible for its yellow color.6 Curcumin has antioxidant and anti-inflammatory properties. Anti-inflammatory activity of curcumin is due to its mediator effect that regulates the action of some signaling mediators such as downregulation of COX-2 properties, mitogen-activated and Janus kinases, and preventing the production of the TNF-alpha and interleukins.7,8 Antioxidant activity of curcumin can be due to its ability in scavenging radicals produced in peroxidation processes and also protecting the cell membrane against oxidative damage.9,10 However, active compounds are volatile and may be degraded during utilization. It is thus required to design structures that help absorption of the phytonutrients. Phytosome is a novel technology that is used for production of lipid-compatible molecular complexes and increases absorption and bioavailability of the nutrients.11 Phytosome is produced by combining the soy phospholipids with the selected derivatives in a convenient solvent. Chemophysical and spectroscopic properties were observed in the phytosome.12 Inflammation has a strong relation with antioxidant properties, and antioxidant agents can seemingly be used as safe agents for improving antioxidant properties in mice that are suffering from inflammation. The aim of the present study is to investigate the effects of curcumin and its nano-phytosomes on behavioral and biochemical responses in carrageenan-induced inflammation in mice.
Materials and Methods
Reagents
Curcumin (EC No. 2072805) and carrageenan (EC No. 1001198493) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Indomethacin was prepared from Temad Pharmaceutical Company (Tehran, Karaj, Iran).
Animals
Swiss albino mice with initial weights of 25±5 g, were prepared from the Pasteur Institute (Amole, Iran). The study was conducted in the Faculty of Basic Sciences, University of Mazandaran (Mazandaran, Iran). All the experimental procedures were performed on the basis of the local Ethics Committee of the University of Mazandaran (IR.UMZ.REC.1397.048). The animals were grouped in a spacious indoor cage and kept in the animal room under standard conditions (12 h light-dark cycle; temperature 22 ± 2°C; moderate humidity 60 ± 5%) with ad libitum access to standard pellets. The mice were adapted with laboratory conditions for 10 days.
Preparation of Curcumin Nano-Phytosome
To load curcumin into phytosom, ethanol was used as reported by previous studies.13
Summary, 6 g curcumin was dissolved in 120 mL of warm ethanol at 55°C with 6 g of EYPC and 4.32 g of Tween 80. A syringe was used for administration of the ethanol solution into 120 mL of hydration media (0.01 M phosphate buffer solution, 150 mM NaCl, PBS, pH 7.4) in 55°C in a water bath with a magnetic stirrer. Aqueous phase was milky color that is a marker for production of liposome. The liposomal system was agitated for 0.5 h and then moved into a round-bottom flask attached to a rotary evaporator at 55°C. To remove ethanol, the pressure was decreased. To prepare the nanophytosom, the phytosom suspension was then exposed to a probe sonication process in an ice bath for 0.5 h in 240 W with a sequence of 1 s of sonication and 1 s of rest by a sonicator (Sonics & Materials, Inc., 20 kHz, Newtown, Connecticut, USA). The final sample was packed in vials and maintained in the refrigerator (about 4°C in the dark). A scanning electron microscope (SEM; KYKY- EM3200, KYKY Technology Development Ltd, Zhongguancun Beijing, China) was used for evaluation of the size and morphology of samples. Images were taken under a voltage of 26 kV.
Experimental Design
Animals received oral administrations of indomethacin, curcumin, or nano-phytosome of curcumin at a dose of 15 mg/kg for one week before administration of carrageenan. In many studies, curcumin was administered in different doses (10–50 mg/kg), but we tried to use curcumin in a lower dosage and the dose of curcumin and its nano-phytosome were selected on the basis of our previous study (15 mg/kg). To induce inflammation, 0.1 mL carrageenan 1% was administrated into the sub-plantar region of the left hind paw of each mouse.14,15 A total number of 48 mice were divided into six groups as follows; 1) negative control that received saline (sal+sal); 2) positive control that administrated with carrageenan and received saline (cara+sal); 3) indomethacin group that received carrageenan and indomethacin (cara+indo); 4) curcumin group that administrated with carrageenan and received curcumin (cara+cur); 5) nano-phytosome group that administrated with carrageenan and received nano-phytosome of curcumin (cara+nano.ph.cur); and 6) indomethacin+nano-phytosome group that administrated with carrageenan and received nano-phytosome of curcumin+indomethacin (cara+nano.ph.cur+indo). The diagram of groups and administration of carrageenan is illustrated in Figure 1.
 |
Figure 1 Experimental design and stages. |
Behavioral Responses
Behavioral responses were measured at 0.5, 2, and 24 h after administration of carrageenan. All the mice were tested for behavioral responses. To conduct the hot plate test, an aluminum hot plate surface was heated up to 55°C. A glass cylinder was used to ensure that animals stayed in the heated region but the mice had free movement in the space.16 In this test, latency to withdrawal of the paw was recorded. Paw withdrawal latencies were assessed in 0.5, 2, and 24 h after the treatment. To perform the tail-pinch test, the animals’ tail was marked by a felt tip pen in a diameter by 4 mm. A standard metal caliper was used for this work. The mice were then positioned in the test chamber and the tail-pinch latency was recorded.
Biochemical Responses
Twenty-four hours after administration of carrageenan, the mice (n=4 mice per group) were euthanized, blood samples were collected, and the serums were separated. The samples were used for evaluation of the activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX) and glutathione reductase (GRx). The activities of SOD and CAT were evaluated by using Genet procedure as reported by Genet et al.17 Summary, a mixture of 50 mM sodium phosphate with EDTA (0.0018 mM), pyrogallol (0.003 mM), and 20 μL enzymatic extract was prepared. Decreased absorbance was then continued in 420 nm for 180 s in 25°C against a blank containing all the compounds without the tissue homogenate. To evaluate the CAT, the mixture consisted of 50 mM sodium phosphate buffer, 10 mM hydrogen peroxide and 20 μL of the enzymatic extract. A spectrophotometer was used for evaluation of the absorbance of the supernatant in 240 nm for 5 min at 25°C against a blank without tissue homogenate. Enzymatic activity was reported as μmole/H2O2 consumed per min per mg protein.17 GPX activity was assessed as reported by Rotruck.18 Summary, the reaction mixture was made up of phosphate buffer (0.4 M, pH 7.0) containing EDTA (0.4 mm), NaN3 (5 mM) and GSH (4 mM) and 200 μL of supernatant was placed in 37°C for 5 min. H2O2 was added it and incubated at 37°C for a further one min. The absorbance was investigated at 340 nm. GRx activity was evaluated as reported by Pinto and Bartley.19 Summary, the reaction mixture was containing 0.1 mM phosphate buffer (pH 7.0), 125 mM NADPH, and 20 μL of the enzyme solution in a final part of 1 mL in 30°C. The absorbance was assessed in 340 nm. One unit of the enzyme was reported as 1 μmol of NADPH oxidized per min per mg protein.
Statistical Analysis
Normality was investigated and one-way ANOVA was used for evaluating the differences between groups. Tukey's post hoc test was used for evaluation of the significant difference among groups. To investigate the behavioral responses, repeated measures ANOVA test was applied.
Results
SEM Image
The image for SEM is shown in Figure 2. Particles are small and adjoining. The smaller sized curcumin nano-phytosome the higher interfacial surface area for drug absorption.
 |
Figure 2 SEM image for nano-phytosome of curcumin. |
Antioxidant Properties
The data for activities of antioxidant enzymes are shown in Figure 3(A–D). Regarding all the enzymes, antioxidant activity in the cara+sal group was significantly lower compared to the control group (P<0.05). However, those which received curcumin nano-phytosome had higher activity compared to the cara+sal group (P<0.05). Using indomethacin could not augment the nano-phytosome efficiency. Significant difference between indomethacin and curcumin groups was not observed for GRx (P>0.05). The CAT and SOD activities were significantly higher in the indomethacin group compared to the curcumin group (P<0.05). In sum, the nano-phytosome group showed higher antioxidant activity compared to the curcumin group (P<0.05).
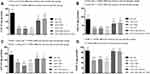 |
Figure 3 The serum concentrations of antioxidant enzymes in the mice that received carrageenan and treated with curcumin and its nano-phytosome. (A) CAT, (B) GRx, (C) GPX and (D) SOD. |
Behavioral Responses
Behavioral responses for hot plate and tail-pinch tests are respectively shown in Figures 4 and 5. In both tests and at all times, the latency was significantly lower in the cara+sal group compared to the sal+sal group (P<0.05). Oral administration of all the agents increased latency time compared to the cara+sal group (P<0.05). Curcumin group showed lower latency time compared to the nano-phytosome groups (P<0.05). Indomethacin decreased latency time compared to those which received indomethacin and nano-phytosome (P<0.05).
 |
Figure 4 The behavioral responses in the hot plate test in mice which received carrageenan and were treated with curcumin and its nano-phytosome. |
 |
Figure 5 The behavioral responses for tail-pinch in the mice that received carrageenan and were treated with curcumin and its nano-phytosome. |
Discussion
In this study, we investigated the effects of curcumin and its nano-phytosome in the mice with carrageenan-induced inflammation. The results showed that nano-phytosome of curcumin improved antioxidant and behavioral responses in the mice with carrageenan-induced inflammation. There is a link between inflammation and antioxidant properties, as mice which received carrageenan showed lower antioxidant activity. Oxidative stress is induced due to an imbalance between oxidant and antioxidant systems. Glutathione has a protective role against toxic effects. Antioxidant enzymes eliminate free radicals and modulate in repairing radicals that are produced by biological damage. SOD and CAT are essential enzymes that eliminate free radicals.20 Decreased activity of the antioxidant enzymes is expected during some disorders.21 SOD has key roles in the conversion of superoxide radicals into H2O2 and molecular oxygen.20 CAT protects tissues against reactive radicals produced during catalyzing the hydrogen peroxide.22 Decreased activity level of antioxidant enzymes is due to increased consumption during oxidative stress and inflammation. Comparing negative and positive groups shows that antioxidant enzymes are significantly consumed by oxidative stress after administration of carrageenan. However, mice in the curcumin nano-phytosome group showed higher levels of antioxidant enzymes. Antioxidant activity of curcumin is due to H-atom donation in the phenolic group.23,24 Antioxidant activity of curcumin can be attributed to its ability in eliminating free radicals in peroxidation processes and also preventing the cell membrane against oxidative damage.9,10 Using nano-phytosome can upgrade the antioxidant properties. Nano-phytosomes protect curcumin and increase its absorption. Seemingly, curcumin nano-phytosome increases activities of antioxidant enzymes and/or decreases oxidative stress. Curcumin and its nano-phytosome showed antinociceptive activity by increasing the latency time of responses. Opioid receptors are involved in such activities. Spinal, supraspinal, and peripheral analgesia are related to the activation of opioid receptors.25 Curcumin and especially its nano-phytosome can have analgesic activity. Curcumin and its nano-phytosome show analgesic activity by activating opioid receptors in the central nervous system. However, such statements require further investigation by conducting mechanistic studies.
Conclusion
In conclusion, the administration of carrageenan decreased activities of antioxidant enzymes and latency time in behavioral responses. Using nano-phytosome curcumin could maintain the levels of antioxidant enzymes and increase the behavioral latency time. It can be recommended to apply the curcumin nano-phytosome in patients suffering from inflammation.
Author Contributions
All authors contributed in analysing the data, drafting and revising the paper, gave final approval of the version for publication and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mueller M, Hobiger S, Jungbauer A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem J. 2010;122:987–996. doi:10.1016/j.foodchem.2010.03.041
2. Borthakur A, Bhattacharyya S, Anbazhagan AN, Kumar A, Dudeja PK, Tobacman JK. Prolongation of carrageenan-induced inflammation in human colonic epithelial cells by activation of an NFκB-BCL10 loop. Biochim Biophys Acta. 2012;1822:1300–1307. doi:10.1016/j.bbadis.2012.05.001
3. West J, Miller KN. California’s Living Marine Resources: A Status Report. Agarophytes and carrageenophytes.California Department of Fish and Game; 2001: 286–287.
4. Hajjar DP, Gotto AM. Biological relevance of inflammation and oxidative stress in the pathogenesis of arterial diseases. Am J Pathol. 2013;182:1474–1481. doi:10.1016/j.ajpath.2013.01.010
5. Leonarduzzi G, Gamba P, Gargiulo S, Biasi F, Poli G. Inflammation- related gene expression by lipid oxidation-derived products in the progression of atherosclerosis. Free Radic Biol Med. 2012;52:19–34. doi:10.1016/j.freeradbiomed.2011.09.031
6. Boroumand N, Samarghandian S, Hashemy SI. Immunomodulatory, anti-inflammatory, and antioxidant effects of curcumin. J Herbmed Pharmacol. 2018;7(4):211–219. doi:10.15171/jhp.2018.33
7. Fadus MC, Lau C, Bikhchandani J, Lynch HT. Curcumin: an age-old anti-inflammatory and anti-neoplastic agent. J Tradit Complement Med. 2017;7(3):339–346. doi:10.1016/j.jtcme.2016.08.002
8. Soltani A, Salmaninejad A, Jalili-Nik M, et al. 5’-Adenosine monophosphate-activated protein kinase: a potential target for disease prevention by curcumin. J Cell Physiol. 2018. doi:10.1002/jcp.27192
9. Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–125. doi:10.1007/978-0-387-46401-5-3
10. Wright JS. Predicting the antioxidant activity of curcumin and curcuminoids. J Mol Struct. 2002;591(1–3):207–217. doi:10.1016/S0166-1280(02)00242-7
11. Bombardelli E, Curri SB, Della RL, Del NP, Tubaro A, Gariboldi P. Complexes between phospholipids and vegetal derivatives of biological interest. Fitoterapia. 1989;60:1–9.
12. Shivanand P, Kinjal P. Phytosomes: technical revolution in phytomedicine. International J PharmTech Res, 2010;2(1), 627–631.
13. Xia S, Xu S, Zhang X. Optimization in the preparation of coenzyme Q10 nanoliposomes. J Agric Food Chem. 2006;54:6358−6366. doi:10.1021/jf060405o
14. Almasirad A, Mousavi Z, Tajik M, Assarzadeh MJ, Shafiee A. Synthesis, analgesic and anti-inflammatory activities of new methyl-imidazolyl-1, 3, 4-oxadiazoles and 1, 2, 4-triazoles. Daru J Pharm Sci. 2014;22(1):22. doi:10.1186/2008-2231-22-22
15. Bakhtiarian A, Araghi S, Khanavi M. Inhibition of Carrageenan-induced edema by Stachys fruticulosa extract. Toxicol Lett. 2012;211(S):207–208. doi:10.1016/j.toxlet.2012.03.744
16. Emik U, Unal Y, Arslan M, Demirel CB. The effects of memantine on recovery, cognitive functions, and pain after propofol anesthesia. Rev Bras Anestesiol. 2016;66(5):485–491. doi:10.1016/j.bjane.2015.03.002
17. Genet S, Kale RK, Baquer NZ. Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: effect of vanadate and fenugreek (Trigonella foenum graecum). Mol Cell Biochem. 2002;236:7–12. [PMID: 12190123]. doi:10.1023/A:1016103131408
18. Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra W. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(588–590). [PMID: 4686466]. doi:10.1126/science.179.4073.588
19. Pinto RE, Bartley W. The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem J. 1969;112:109–115. doi:10.1042/bj1120109
20. Sellamuthu PS, Arulselvan P, Kamalraj S, Fakurazi S, Kandasamy M. Protective nature of mangiferin on oxidative stress and antioxidant status in tissues of streptozotocin-induced diabetic rats. ISRN Pharmacol. 2013;2013:1–10. doi:10.1155/2013/750109
21. Srinivasan S, Pari L. Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chemico-Biol Interact. 2012;195:43–51. doi:10.1016/j.cbi.2011.10.003
22. Omotayo EO, Gurtu S, Sulaiman SA, Wahab MSA, Sirajudeen KNS, Salleh MSM. Hypoglycemic and antioxidant effects of honey supplementation in streptozotocin-induced diabetic rats. Int J Vitamin Nutr Res. 2010;80:74–82. doi:10.1024/0300-9831/a000008
23. Ak T, Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174(1):27–37. doi:10.1016/j.cbi.2008.05.003
24. Shahmoradi MK, Askaripour M, Rajabi S, Dzigandzli G. Beneficial effects of curcumin on rats with polycystic ovary syndrome: evaluation of the gene expression of GLUT4, Erα and insulin resistance. GMJ Med. 2018;2(1):80–87. doi:10.29088/GMJM.2018.80
25. Furst S. Transmitters involved in antinociception in the spinal cord. Brain Res Bull. 1999;48:129–141. doi:10.1016/S0361-9230(98)00159-2
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
