Back to Journals » Journal of Inflammation Research » Volume 15
Protective Effect of Crocin on Immune Checkpoint Inhibitors-Related Myocarditis Through Inhibiting NLRP3 Mediated Pyroptosis in Cardiomyocytes via NF-κB Pathway
Authors Zhang H, Lin J, Shen Y, Pan J, Wang C , Cheng L
Received 12 November 2021
Accepted for publication 9 February 2022
Published 5 March 2022 Volume 2022:15 Pages 1653—1666
DOI https://doi.org/10.2147/JIR.S348464
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Hui Zhang,1,* Jinyi Lin,2,* Yihui Shen,1 Jianan Pan,3 Chunhui Wang,4 Leilei Cheng1
1Department of Echocardiography, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, Shanghai Institute of Medical Imaging, Shanghai, People’s Republic of China; 2Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, Shanghai, People’s Republic of China; 3Department of Cardiology, Shanghai Ninth People’s Hospital, Shanghai JiaoTong University School of Medicine, Shanghai, People’s Republic of China; 4Department of Pharmacy, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Leilei Cheng, Department of Echocardiography, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, Shanghai Institute of Medical Imaging, Shanghai, 200032, People’s Republic of China, Fax +86-21-51217561, Email [email protected]
Purpose: Immune checkpoint inhibitors (ICIs)-related myocarditis is now one of the most critical immune-related adverse effects (irAEs) in tumor immunotherapy, which has raised great concern in cardio-oncology. The pathogenesis involved in cardiac injury remains elusive. Crocin, the main component of saffron, has shown distinct functions in cardioprotective and anti-inflammation properties. We therefore aimed to investigate the potential effect of crocin on the protection of ICIs-related myocarditis and its underlying molecular mechanism.
Methods: We immunized the BALB/c mice with murine cardiac troponin I (cTnI) peptide and additionally gave anti-mouse programmed death 1 (PD-1) to induce the mouse model of ICIs-related myocarditis. Mice were treated with crocin at different dosages. In vitro, HL-1 cells were pre-incubated with crocin at different concentrations and then stimulated with lipopolysaccharide (LPS). Myocardial contractile functions, myocardial inflammation and fibrosis, and myocardial injury were assessed. The expressions of pyroptosis-related proteins and nuclear factor-κB (NF-κB) pathway were evaluated.
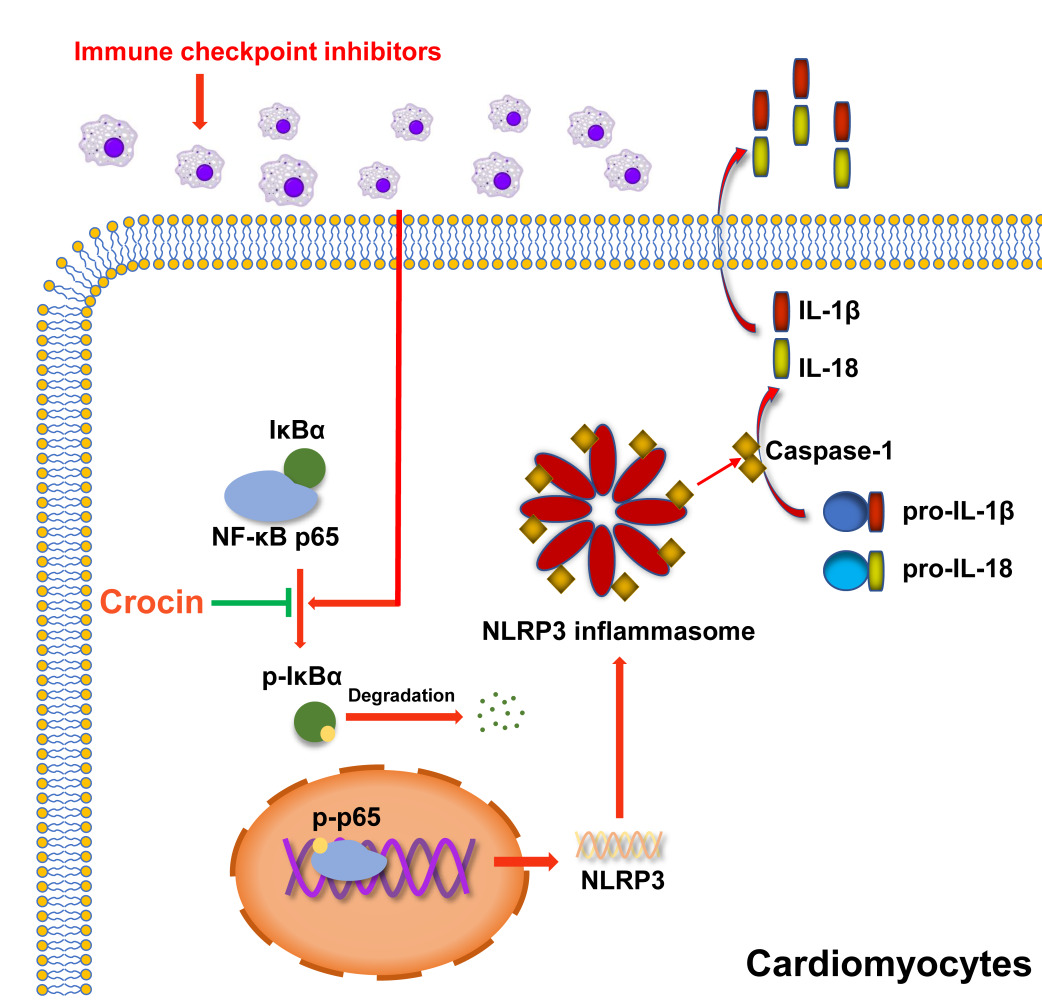
Results: Crocin treatment could partially reverse the ICIs-related myocarditis in terms of improving heart function, ameliorating inflammation and fibrosis in the myocardium, and alleviating myocardial injury. Mechanistically, ICIs administration significantly activated pyrin domain-containing protein 3 (NLRP3) inflammasome in cardiomyocytes. Crocin treatments significantly downregulated the expression of NLRP3, cleaved gasdermin D (GSDMD), cleaved caspase1, interleukin-1β (IL-1β), and IL-18. Besides, crocin inhibited the activation of NF-κB pathway, which performed as reducing the phosphorylation of p-NF-kappa-B inhibitor-α (p-IκBα), degradation of IκBα, phosphorylation of p65 and p65 DNA binding activity both in vivo and in vitro.
Conclusion: By reversing the pyroptosis in cardiomyocytes, crocin treatment in a mouse model exerted great potential to aid in the prevention of ICIs-related myocarditis from a novel target.
Keywords: cardio-oncology, immune checkpoint inhibitors, autoimmune myocarditis, programmed death 1, immune-related adverse effects
Graphical Abstract:
Introduction
In the past decade, the rise and development of immune checkpoint inhibitors (ICIs) have brought great clinical advances in cancer immunotherapy.1,2 ICIs target different immune checkpoints including the programmed death 1 (PD-1) such as Nivolumab and Pembrolizumab, programmed death ligand 1 (PD-L1) like Atezolizumab, Avelumab, and Durvalumab, and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) inhibitors like Tremelimumab and Ipilimumab.3 Since immune checkpoints also play a critical role in autoimmune tolerance, the activation of the immune system by ICIs may cause a wide range of autoimmune responses, which is called immune-related adverse effects (irAEs), including colitis, hepatitis, pneumonitis, thyroiditis, myositis, hypophysitis, and dermatitis.4–6 These irAEs are largely reversible and can typically be controlled with the administration of glucocorticoid therapy.5–7 However, severe cardiovascular effects, especially myocarditis and fatal heart failure caused by ICIs, have largely been underestimated in the past few years, which has raised great concern in cardio-oncology.8
In 2018, a multicenter clinical study showed that the incidence of ICIs-related myocarditis was 1.14%.9 In addition, the onset of ICIs-related myocarditis could be as early as 2 weeks after ICIs initiated,10 the median time from symptom onset to death was only 32 days, and the incidence of fatality even reaches 46%.11 The combination ICIs therapy such as a CTLA-4 inhibitor combined with a PD-1 inhibitor significantly increased the incidence and fatality of ICIs-related myocarditis compared to the ICIs monotherapy.11 Currently, the treatment strategies for ICIs-related myocarditis are mainly for preventing further cardiotoxicity, immunosuppression to alleviate inflammatory changes, and supportive therapy.12 However, no targeted drugs for the prevention and treatment of ICIs-related myocarditis have been developed and used.13
The pyrin domain-containing protein 3 (NLRP3) inflammasome as a sensor for pathogen-derived danger signals in the innate immune system mediates caspase-1 activation, which cleaves gasdermin D (GSDMD) and promotes the secretion of proinflammatory cytokines interleukin-1β (IL-1β)/IL-18, leading to the lytic, pro-inflammatory form of cell death, pyroptosis.14–17 Recently, many studies have shown that pyroptosis was involved in several non-inflammatory diseases, such as cryopyrin-associated periodic syndromes (CAPS), Alzheimer’s disease, diabetes, gout, autoinflammatory diseases, and atherosclerosis,18,19 which raised the possibility that anti-inflammasomes therapy may broaden its applications in diverse disease. In addition, NLRP3 has been shown to be activated in response to PD-1 blockade of CD8+ T cells in tumor cells and primary cardiomyocytes.20,21 Although the mechanism of ICIs-related myocarditis was still unclear, it might be a promising therapeutic target to inhibit pyroptosis and help alleviate the ICIs-related myocarditis.
Crocin is a major apo-carotenoid of saffron,22 which has been shown great potential in anti-oxidant, anti-hypertensive, anti-depressant, cardioprotective, and nephron-protective properties, as well as the marked anti-inflammation properties.23–26 Crocin was indicated not only to combat reactive oxygen species (ROS) production and inhibit pro-inflammatory cytokines secretion but also to suppress inflammation via regulating mainly nuclear factor-κB (NF-κB) pathway or phosphoinositide 3-kinase (PI3K)/Akt pathway in many animal models.23,27 However, the role of crocin in the protection of ICIs-related myocarditis was still unknown.
In this study, we investigated the protective effects and mechanisms of crocin against ICIs-related myocarditis in a mouse model. Moreover, we highlighted the effects of crocin in inhibiting the NLRP3 mediated pyroptosis via NF-κB pathway in vivo and in vitro. Our research provided a targeted therapeutic option for the treatment of ICIs-related myocarditis.
Materials and Methods
Materials
Crocin was a gift from Reyoung Pharmaceutical Co., Ltd. (Shanghai, China). InVivoMab Anti-mouse PD-1 was purchased from Bioxcell Co., Ltd. (West Lebanon, USA). Murine cardiac troponin I (TnI) peptide HARVDKVDEERYDVEAKVTKNITEIADLTQKIYDLRGKFKRPTLRRVRIS was synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) according to the literature before.28
Animals
Male 6-week-old BALB/c mice weighing 20–25 g were purchased from Vital River Laboratories (Shanghai, China) and housed in a standard rising condition of controlled humidity and a 12-h day/night cycle at 22°C. Animal studies were informed in compliance with the “Guide for the Care and Use of Laboratory Animals published by the US NIH (NIH publication No. 85-23, revised 2011)” and approved by the Animal Experiment Ethics Committee of Zhongshan Hospital of Fudan University (2019-109).
ICIs-Related Myocarditis Model and Animal Treatment
Except for the control group, all mice were immunized subcutaneously on day 0 and day 7, respectively, with a solution of 250ug murine cardiac TnI peptide (Sangon Biotech, China) diluted in complete Freund’s adjuvant (Sigma, USA) as described. From day 7 onwards, mice were given an intraperitoneal injection of anti-mouse PD-1 every 2 days at a dose of 5 mg/kg for 5 times to induce ICIs-related myocarditis.
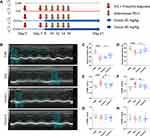
BALB/c mice were randomly assigned to four groups (n = 10 in each group): (1) control group (CON, vehicle-treated group); (2) ICIs-related myocarditis group (ICIs, model group); (3) ICIs-related myocarditis + low-crocin (Crocin-L, crocin i.p. 20mg/kg/d); (4) ICIs-related myocarditis + high-crocin (Crocin-H, crocin i.p. 50mg/kg/d). Crocin was given from day 8 for 14 consecutive days. On day 21, echocardiography was implemented, and heart tissues were obtained from mice euthanized using deep isoflurane (5%) anesthesia, rinsed in ice-cold phosphate buffer saline, and snap-frozen in liquid nitrogen. The experimental design paradigm is shown in Figure 1A
Echocardiography
Transthoracic echocardiography was performed using a 30-MHz high-frequency scan probe (Vevo 2100, VisualSonics, Canada) on day 21 in all animals. Mice were anesthetized and maintained in 2–3% isoflurane and 2 L/min 100% oxygen during the procedure. Left ventricular ejection fraction (LVEF), fractional shortening (FS), left ventricular anterior wall (LVAW) and left ventricular posterior wall (LVPW) were measured as previously described. All the measurements were performed by an investigator who was blinded to the experimental groups and the data was evaluated using Vevo Analysis software.
Histological Analysis
The heart tissue was fixed with 4% paraformaldehyde and embedded in paraffin. The paraffin-embedded hearts were then sectioned and stained with hematoxylin–eosin (HE), Masson trichrome staining, and Sirius Red staining reagent according to the manufacturer’s instructions (Servicebio Biotech, China). The images were observed and captured with a light microscope (LM, BX51, Olympus, Japan) and processed with Image J software.
Serum Markers of Myocardial Injury
The components of serum markers including the creatine kinase (CK), creatine kinase-MB (CK-MB) and lactate dehydrogenase-1 (LDH-1) were determined using commercial assay kits (Nanjing Jiancheng BioTech, China) according to the manufacturer’s instructions. The serum levels of cTnI and cTnT were quantified using commercial assays kits (Elabscience Biotechnology, China) according to the manufacturer’s instructions.
Enzyme-Linked Immunosorbent Assay (ELISA)
IL-1β and IL-18 levels in mouse serum and cell culture supernates were quantified using ELISA kits (R&D Systems, USA) according to the manufacturer’s instructions.
Cell Treatment
HL-1 mouse cardiomyocytes were purchased from Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, USA) with 10% fetal bovine serum (FBS) (Gibco, USA) and 1% penicillin–streptomycin solution (P/S) (Hyclone, USA) at 37°C in a humidified incubator containing 95% air and 5% CO2. Cells were seeded on a 6-well plate or a 96-well plate 24 hours before experiments. For crocin treatment, cells were pre-incubated with crocin at different concentrations (0, 5, 10, 20, 50μM) for 30 minutes and then stimulated with lipopolysaccharide (LPS) (Sigma, USA) at 1μg/mL for 24 hours to evaluate the protective effect of crocin in the NLRP3 mediated pyroptosis and its mechanism.
Cell Viability
HL-1 cells were seeded at a concentration of 5000 cells/well in a 96-well plate. Different treatments were implemented as described above. The cell viabilities of each group were detected using the Cell Counting Kit-8 (CCK-8, Beyotime Biotechnology, China) for measuring the absorbance values at a wavelength of 450 nm 1 hour after adding the working solution.
Western Immunoblot
Weighed heart tissue and HL-1 cells were homogenized in an ice-cold radioimmunoprecipitation assay (RIPA) lysis buffer with cocktail protease inhibitor (Beyotime Biotechnology, China) and then centrifuged at 12,000 rpm for 15 minutes at 4°C. The supernatant protein concentration was measured by the bicinchoninic acid method (Beyotime Biotechnology, China). Then, 20μg protein was size-fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto immobilon polyvinylidene difluoride membranes (Bio-Rad, USA). Membranes were cut into different parts according to the molecular weight of each protein and blocked with 5% nonfat milk (BD Biosciences, USA) for 2 hours. Each membrane was incubated with primary antibody at 4°C overnight: anti-NLRP3 (1:1000, Abcam, UK), anti-pro Caspase-1 + p10 + p12 (p10 and p12 are two kinds of cleaved Caspase-1) (1:1000, Abcam, UK), anti-Gasdermin D (1:1000, Cell Signaling Technology, USA), anti-cleaved Gasdermin D (1:1000, Cell Signaling Technology, USA), anti-NF-κB p65 (1:1000, Cell Signaling Technology, USA), anti-p-p65 (1:1000, Cell Signaling Technology, USA), anti-p-IκBα (1:1000, Cell Signaling Technology, USA), anti-IκBα (1:1000, Cell Signaling Technology, USA), anti-IL-1β (1:1000, Cell Signaling Technology, USA), anti-IL-18 (1:1000, ABclonal, China), anti-GAPDH (1:5000, Proteintech, USA). The membranes were thoroughly washed for the second day and incubated for 1 hour in the dark with secondary antibodies, DylightTM800 4XPEG-conjugated goat anti-rabbit IgG (H + L) or goat anti-mouse IgG (H + L) (1:10,000, Cell Signaling Technology, USA). The proteins were visualized and analyzed using the Odyssey Infrared Imaging System.
Immunofluorescent Staining
Slides of heart sections were deparaffinized, rehydrated, and boiled in Tris-EDTA solution (Beyotime Biotechnology, China) at 95°C for 20 min for antigen retrieval. In addition, the HL-1 cells were plated on slides, given different treatments as before, and fixed with 4% paraformaldehyde. Then, all slides were blocked with 5% goat serum and incubated with primary antibody at 4°C overnight: anti-NLRP3 (1:50, Abcam, UK), anti-cardiac Troponin T (cTnT, 1:200, Abcam, UK), anti-NF-κB p65 (1:100, Cell Signaling Technology, USA). The slides were rewarmed at 37°C for 1 hour, washed thoroughly and incubated with anti-rabbit IgG (1:1000, Alexa Fluro®594 Conjugate, Cell Signaling Technology) and anti-mouse IgG (1:1000, Alexa Fluro®488 Conjugate, Cell Signaling Technology) at 37°C for 1 hour. Nuclei were counterstained with DAPI. Finally, the stained sections were observed with a fluorescence microscope (Nikon, Japan, objective lens magnification of ×40; eyepiece magnification of ×10), and the fluorescence intensity of NLRP3 or nuclei NF-κB p65 was processed with Image J software.
Transcription Activity of NF-κB P65
Nuclear extracts from hearts and HL-1 cells were obtained by a nuclear extraction kit (Beyotime Biotechnology, China) according to the manufacturer’s protocol. The DNA-binding activity of NF-κB p65 in nuclear extracts was analyzed by using TransAM™ NF-κB p65 Colorimetric kits (Active Motif, USA) in duplicate and detected at a wavelength of 450 nm by a microplate spectrofluorometer (Bio-Tek, USA) as described.
Statistical Analysis
Continuous variables were presented as mean ± SD with at least three independent experiments. The two-tailed t-test was used to analyze the differences between the 2 groups. And, for comparisons among multiple groups, a one-way ANOVA followed by a Bonferroni post hoc test was used. P values <0.05 were considered to be statistically significant. Statistical analyses were performed using SPSS 22.0 software package.
Results
Effect of Crocin on ICIs-Related Myocarditis in Myocardial Contractile Function
The therapeutic potential of crocin in ICIs-related myocarditis was investigated in a mouse model as described above. On day 21, the left ventricular function and the thickness of the anterior and posterior walls of the left ventricle were assessed by echocardiography (Figure 1B). The results showed that the left ventricular ejection fraction and fraction shortening were significantly reduced in the ICIs group, compared to the control group. Administration of crocin in the high-dose group rather than crocin in the low-dose group significantly improved the left ventricular function (Figure 1C and D). The thickness of the anterior walls of the left ventricle but not the posterior walls was significantly higher in the ICIs group than in the control group, which was reduced in both the Crocin-L group and Crocin-H group (Figure 1E–H).
Effect of Crocin on ICIs-Related Myocarditis in Myocardial Inflammation and Fibrosis
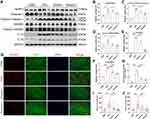
HE staining showed the inflammatory manifestations caused by ICIs (Figure 2A). In the ICIs group, the myocardial tissues were induced to a variable extent of inflammatory infiltration, ranging from 25% to 40%, compared to the control group, which received complete Freund’s adjuvant injections without TnI peptide and anti-mouse PD-1. The inflammatory infiltration was significantly reduced in both Crocin-L group and Crocin-H group (Figure 2D). Consistently, myocardial fibrosis and collagen deposition detected by Masson trichrome and Sirius Red staining were shown in the ICIs group, whereas the fibrotic scar formation was significantly reduced by crocin in a dose-dependent manner (Figure 2B, C, E and F).
Effect of Crocin on ICIs-Related Myocarditis in Myocardial Injury
Further, the serum levels of creatine kinase (CK), creatine kinase-MB (CK-MB), and lactate dehydrogenase-1 (LDH-1) were detected to evaluate the myocardial injury. As are shown in Figure 2G–I, the induction of ICIs-related myocarditis resulted in a substantial rise in CK, CK-MB, and LDH-1. Administration with crocin of 20 mg/kg/d partially reversed the level of CK-MB and LDH-1, while crocin in 50 mg/kg/d significantly reduced the level of CK, CK-MB, and LDH-1. Besides, serum cTnT and cTnI were also quantified to evaluate the cardiac injury. Consistently, crocin treatments significantly reversed high levels of cTnT and cTnI caused by ICIs (Figure 2J and K).
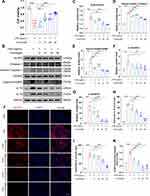
Effect of Crocin on ICIs-Related Myocarditis in NLRP3 Mediated Pyroptosis
In order to verify the role of NLRP3 mediated pyroptosis in ICIs-related myocarditis and identify the potential effect of crocin in reversing the pyroptosis, the heart tissue of the mice was extracted. Western blot showed that ICIs significantly upregulated the expression of NLRP3, cleaved Caspase 1, cleaved GSDMD, IL-1β and IL-18, which were significantly reversed in both crocin treated groups (Figure 3A–F). Meanwhile, the double immunofluorescent staining showed that red-stained NLRP3 positive signals were more intense in the ICIs group, which were located in the green-stained cTnT positive cardiomyocytes. This phenomenon was significantly ameliorated in the Crocin-L group and Crocin-H group (Figure 3G and H).
Moreover, the serum levels of IL-1β and IL-18 were significantly elevated in ICIs groups, which were processed as a result of caspase 1 activation. Treatment with crocin at both dosages significantly inhibited the productions of these cytokines consistently (Figure 3I and J).
Effect of Crocin on LPS-Induced Pyroptosis in vitro
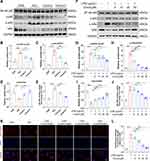
To further investigate the role of crocin in alleviating the pyroptosis in cardiomyocytes, HL-1 cell lines were used, and LPS was used to induce pyroptosis in vitro.29,30 The cell viabilities were detected by the CCK-8 kit. The results showed that LPS induced a significant decrease in cell viability, which was reversed by the pretreatment of crocin at the dose of 10μM, 20μM, and 50μM (Figure 4A). Western Blot showed that LPS induced a significant increase in the expression of NLRP3, cleaved Caspase 1, cleaved GSDMD, IL-1β and IL-18, compared to the control group. Pretreatment of crocin significantly reversed the effect by a dose-dependent reduction in NLRP3, cleaved Caspase 1, cleaved GSDMD, IL-1β and IL-18 expression (Figure 4B–G). Consistently, the levels of IL-1β and IL-18 in cell culture supernates were elevated by the stimulation of LPS, which were inhibited by the crocin pretreatments (Figure 4H and I). Meanwhile, the immunofluorescent staining showed similar results that LPS induced stronger red-stained NLRP3 signals, which were significantly alleviated by crocin pretreatments (Figure 4J and K).
Effect of Crocin on NF-κB Pathway in vivo and in vitro
The activation of NF-κB pathway has been reported participating in the process of pyroptosis. Therefore, the role of crocin in regulating the NF-κB pathway in ICIs-related cardiomyocytes’ pyroptosis was accessed. As are shown in Figure 5A–E, the NF-κB pathway was activated in the ICIs groups, demonstrated by an increased level of p-p65 and p-IκBα, a decreased level of IκBα expression, as well as the upregulation of DNA binding activity of p65. The treatment of crocin at both dosages significantly inhibited the activation of NF-κB pathway. In agreement with the findings in vivo, the pretreatment of crocin significantly inhibited LPS-induced activation of NF-κB pathway in HL-1 cell lines, as characterized by reducing the phosphorylation of p65 and IκBα, the degradation of IκBα, and the level of p65 DNA binding activity (Figure 5F–J). Moreover, LPS stimulation significantly enhanced NF-κB p65 nuclear transfer performed as the red stained p65 positive in the nuclear, which was significantly alleviated by the pretreatments of crocin (Figure 5K and L).
Discussion
The present study evaluated the phenotype and underlying mechanism of ICIs-related myocarditis in a mouse model. Crocin supplementation may alleviate ICIs-related myocarditis in terms of improving heart function, ameliorating inflammation, and inhibiting pyroptosis via NF-κB pathway. In addition, we verified the protective effect of crocin against NLRP3 mediated pyroptosis in vitro. Collectively, we supposed that crocin would be a potential therapeutic agent in preventing ICIs-related myocarditis by inhibiting the pyroptosis in cardiomyocytes.
In view of the fact that ICIs are widely used in the treatment of tumors, the occurrence of irAEs has gradually attracted attention. The American Society of Clinical Oncology (ASCO) developed the guideline for the management of irAEs, which separated myocarditis into 4 grades of severity, with grade 1 being the mildest. Concerned about the high mortality of ICIs-related myocarditis, it is recommended to hold the ICIs even for grade 1 toxicity and high-dose corticosteroids should be administered rapidly for all grades.31,32 As the indications of ICIs continue to expand, further research is needed for the identification of ICIs-related myocarditis, especially in its mechanism and treatments.
The mechanism of ICIs-related myocarditis was mostly reported to be related to autoimmune disorders caused by excessive T cell activation.33–35 Prior studies demonstrated that fatal myocarditis mediated by T cells and myeloid cells was shown in PD-1 deficient MRL mice, which had an autoimmune tendency, while CTLA-4 deficient mice also showed similar early lethal lymphocytes proliferative disease.34,36 Cardiac troponin I was considered to be the specific cardiac antigens, leading to the development of autoimmune cardiotoxicity.28 Therefore, we immunized mice with cTnI, and additionally gave anti-mouse PD-1 to simulate the clinical ICIs-related myocarditis. Here we found the impaired myocardial contractile function, the inflammatory manifestations, the myocardial fibrosis, and the increased level of myocardial enzyme profile in the model group, which provided a new mouse model for mechanism exploration and drug screening in ICIs-related myocarditis.
A recent study showed that anti-PD1 therapy disrupted immune homeostasis and induced dysregulated metabolism, exhibiting profound detrimental effects on cardiac integrity.37 In addition, some scholars believed that common antigens in tissues could be recognized by T cells, and the increased levels of interferon-γ (IFN-γ), granzyme B, and tumor necrosis factor-α (TNF-α) produced by activated T cells could cause heart damage.8 However, the direct association between ICIs-induced immune reactions and cardiac injury is still unknown. The previous study showed that PD-L1-dependent activation of the NLRP3 inflammasome in tumor tissues directly linked to CD8+ T cell activity by the recruitment of granulocytic myeloid-derived suppressor cells (PMN-MDSCs) to the tumor bed in response to the anti-PD1 therapy.20 Besides, the AC16 human cardiomyocytes co-cultured with human peripheral blood mononuclear cells (hPBMCs) treated with ipilimumab manifested the overexpression of NLRP3/MyD88 and cytokines, which was likely a result of the lymphocytic infiltration of lymphocytes.21 Similarly, the present study indicated that NLRP3 inflammasome was significantly activated in cardiomyocytes of the entire heart tissue by the ICIs treatment, not necessarily in the inflammation area. Therefore, we hypothesized that the excessive activation of T cells response to ICIs, upregulating the autoimmune activity of the cardiomyocytes, which leads to myocardial pyroptosis widely.
Immunosuppressive therapy is preferred as a treatment option for ICIs-related myocarditis, but it can counteract the anti-cancer effects as well.13 Other treatments such as the blockade of TNF-α and the blockade of Janus kinase (JAK) signals have also been reported to limit changes in cardiac immunity.37,38 Our study found that crocin, as a derivative from traditional Chinese herbs, was able to alleviate the phenotype of ICIs-related myocarditis in mice via inhibiting the NLRP3 mediated pyroptosis in cardiomyocytes. Crocin is now widely used as a cardioprotective agent. It has been suggested to attenuate isoprenaline-induced myocardial fibrosis by targeting toll-like receptor 4 (TLR4)/NF-κB signaling.27 In addition, crocin alleviated the LPS-induced toxicity in H9c2 cells via reducing the levels of inflammatory factors.39 In line with the previous findings, our results showed the protective effect of crocin in inhibiting the activation of the NLRP3 inflammasome both in vivo and in vitro.
Mechanically, the traditional NF-κB pathway was widely reported as the first signal of the activation of NLRP3 inflammasome.40–42 IκBα, a member of the NF-κB inhibitor family, was degraded by ubiquitin-dependent proteasomes, allowing p65 phosphorylation and transferred to the nucleus,43 which upregulated the expression of NLRP3. In addition, NF-κB pathway exerted multiple therapeutic targets since its regulatory network ranged from cancers to inflammatory and immune disorders.44–46 We found that crocin effectively reversed the activation of the NF-κB pathway both in vivo and in vitro, suggesting that NF-κB signaling was involved in the regulation of crocin in pyroptosis. Further, how crocin inhibited the activation of the NF-κB pathway might appear more complicated. Physiologically, NF-κB pathway was required for adaptive changes in gene expression and tissue homeostasis, which was responsive to oxidative stress, DNA damage, immune activation and growth regulatory signals.43 Interestingly, crocin was reported to physically bind to various cellular proteins, such as structural proteins, membrane transporters, and enzymes, which are involved in adenosine triphosphate (ATP) synthesis, redox homeostasis, and signal transduction.47 Substantial evidence showed that crocin reduced ROS generation and provided a potential explanation.48,49 A recent study suggested that toll-like receptor (TLR) 4 signaling might be a target for crocin to inhibit the activation of the NF-κB pathway, which still requires more validation.27 Considering this, our findings indicated the potential effect of crocin on inhibiting NLRP3 mediated pyroptosis via regulating the NF-κB pathway in ICIs-related myocarditis.
Limitation
However, there were still some limitations in the current study. First, the mechanism of how the excessive T cells induced the activation of NLRP3 inflammasome needs further verification. Secondly, the co-culture of ICIs-treated PBMCs and cardiomyocytes was lacking, although we used LPS to stimulate the pyroptosis of cardiomyocytes. Finally, we initially evaluated the potential use of crocin in ameliorating the ICIs-related myocarditis, and its role in cancer and other irAEs still needs further exploration.
Conclusion
In summary, our study provided evidence that NLRP3 inflammasome was strongly activated in cardiomyocytes, which played a role in the disrupted immune homeostasis of the heart in ICIs therapy. Crocin treatment could partially ameliorate ICIs-related myocarditis and contributed to the suppression of pyroptosis by regulating NF-κB pathway. Our findings may provide a novel therapeutic agent to treat ICIs-induced cardiac dysfunction.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 82170359, 81771840), Clinical Research Fund of Zhongshan Hospital (2020ZSLC21), and Smart medical treatment project of Zhongshan Hospital (2020ZHZS16).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–249. doi:10.1146/annurev-pathol-042020-042741
2. Singh S, Hassan D, Aldawsari HM, Molugulu N, Shukla R, Kesharwani P. Immune checkpoint inhibitors: a promising anticancer therapy. Drug Discov Today. 2020;25(1):223–229. doi:10.1016/j.drudis.2019.11.003
3. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi:10.1126/science.aar4060
4. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors. JAMA Oncol. 2018;4(12):1721–1728. doi:10.1001/jamaoncol.2018.3923
5. Johnson DB, Chandra S, Sosman JA. Immune checkpoint inhibitor toxicity in 2018. JAMA. 2018;320(16):1702–1703. doi:10.1001/jama.2018.13995
6. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi:10.1056/NEJMra1703481
7. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2(10):1346–1353. doi:10.1001/jamaoncol.2016.1051
8. Varricchi G, Galdiero MR, Marone G, et al. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open. 2017;2(4):e000247. doi:10.1136/esmoopen-2017-000247
9. Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–1764. doi:10.1016/j.jacc.2018.02.037
10. Chen DY, Huang WK, Chien-Chia Wu V, et al. Cardiovascular toxicity of immune checkpoint inhibitors in cancer patients: a review when cardiology meets immuno-oncology. J Formos Med Assoc 2020;119(10):1461–1475. doi:10.1016/j.jfma.2019.07.025
11. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–1755. doi:10.1056/NEJMoa1609214
12. Hu JR, Florido R, Lipson EJ, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. 2019;115(5):854–868. doi:10.1093/cvr/cvz026
13. Salem JE, Allenbach Y, Vozy A, et al. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med. 2019;380(24):2377–2379. doi:10.1056/NEJMc1901677
14. Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–1022. doi:10.1016/j.cell.2014.04.007
15. Liu X, Zhang Z, Ruan J, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153–158. doi:10.1038/nature18629
16. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. doi:10.1016/S1097-2765(02)00599-3
17. Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi:10.1038/nature15514
18. Guo H, Callaway JB, Ting JPY. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–687. doi:10.1038/nm.3893
19. Menu P, Vince JE. The NLRP3 inflammasome in health and disease: the good, the bad and the ugly. Clin Exp Immunol. 2011;166(1):1–15. doi:10.1111/j.1365-2249.2011.04440.x
20. Theivanthiran B, Evans KS, DeVito NC, et al. A tumor-intrinsic PD-L1/NLRP3 inflammasome signaling pathway drives resistance to anti-PD-1 immunotherapy. J Clin Invest. 2020;130(5):2570–2586. doi:10.1172/JCI133055
21. Quagliariello V, De Laurentiis M, Cocco S, et al. NLRP3 as putative marker of ipilimumab-induced cardiotoxicity in the presence of hyperglycemia in estrogen-responsive and triple-negative breast cancer cells. Int J Mol Sci. 2020;21(20):7802. doi:10.3390/ijms21207802
22. Bukhari SI, Manzoor M, Dhar MK. A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomed Pharmacother. 2018;98:733–745. doi:10.1016/j.biopha.2017.12.090
23. Hashemzaei M, Mamoulakis C, Tsarouhas K, et al. Crocin: a fighter against inflammation and pain. Food Chem Toxicol. 2020;143:111521.
24. Hashemzaei M, Rezaee R, Nabatzehi M, et al. Anti-hypertensive effect of crocin and hesperidin combination in high-fat diet treated rats. Exp Ther Med. 2020;19(6):3840–3844. doi:10.3892/etm.2020.8650
25. Korani S, Korani M, Sathyapalan T, Sahebkar A. Therapeutic effects of Crocin in autoimmune diseases: a review. Bio Factors Oxf Engl. 2019;45(6):835–843. doi:10.1002/biof.1557
26. Hatziagapiou K, Kakouri E, Lambrou GI, Bethanis K, Tarantilis PA. Antioxidant properties of Crocus sativus L. and its constituents and relevance to neurodegenerative diseases; focus on Alzheimer’s and Parkinson’s disease. Curr Neuropharmacol. 2019;17(4):377–402. doi:10.2174/1570159X16666180321095705
27. Jin W, Zhang Y, Xue Y, et al. Crocin attenuates isoprenaline-induced myocardial fibrosis by targeting TLR4/NF-κB signaling: connecting oxidative stress, inflammation, and apoptosis. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(1):13–23. doi:10.1007/s00210-019-01704-4
28. Bockstahler M, Fischer A, Goetzke CC, et al. Heart-specific immune responses in an animal model of autoimmune-related myocarditis mitigated by an immunoproteasome inhibitor and genetic ablation. Circulation. 2020;141(23):1885–1902. doi:10.1161/CIRCULATIONAHA.119.043171
29. Qiu Y, Ma Y, Jiang M, et al. Melatonin alleviates LPS-induced pyroptotic cell death in human stem cell-derived cardiomyocytes by activating autophagy. Stem Cells Int. 2021;2021:8120403. doi:10.1155/2021/8120403
30. Qiu Z, He Y, Ming H, Lei S, Leng Y, Xia ZY. Lipopolysaccharide (LPS) aggravates high glucose- and hypoxia/reoxygenation-induced injury through activating ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2 cardiomyocytes. J Diabetes Res. 2019;2019:8151836. doi:10.1155/2019/8151836
31. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–1768. doi:10.1200/JCO.2017.77.6385
32. Palaskas N, Lopez‐Mattei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. 2020;9(2):e013757.
33. Ji C, Roy MD, Golas J, et al. Myocarditis in cynomolgus monkeys following treatment with immune checkpoint inhibitors. Clin Cancer Res. 2019;25(15):4735–4748. doi:10.1158/1078-0432.CCR-18-4083
34. Wang J, Okazaki IM, Yoshida T, et al. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol. 2010;22(6):443–452. doi:10.1093/intimm/dxq026
35. Grabie N, Gotsman I, DaCosta R, et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation. 2007;116(18):2062–2071. doi:10.1161/CIRCULATIONAHA.107.709360
36. Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in ctla-4. Science. 1995;270(5238):985–988. doi:10.1126/science.270.5238.985
37. Michel L, Helfrich I, Hendgen-Cotta UB, et al. Targeting early stages of cardiotoxicity from anti-PD1 immune checkpoint inhibitor therapy. Eur Heart J. 2021;43(4):ehab430.
38. Liu X, Zhang X, Ye L, Yuan H. Protective mechanisms of berberine against experimental autoimmune myocarditis in a rat model. Biomed Pharmacother. 2016;79:222–230. doi:10.1016/j.biopha.2016.02.015
39. Baradaran Rahim V, Khammar MT, Rakhshandeh H, Samzadeh-Kermani A, Hosseini A, Askari VR. Crocin protects cardiomyocytes against LPS-induced inflammation. Pharmacol Rep. 2019;71(6):1228–1234. doi:10.1016/j.pharep.2019.07.007
40. Zhao W, Ma L, Cai C, Gong X. Caffeine Inhibits NLRP3 inflammasome activation by suppressing MAPK/NF-κB and A2aR signaling in LPS-induced THP-1 macrophages. Int J Biol Sci. 2019;15(8):1571–1581. doi:10.7150/ijbs.34211
41. Boaru SG, Borkham-Kamphorst E, Van de Leur E, Lehnen E, Liedtke C, Weiskirchen R. NLRP3 inflammasome expression is driven by NF-κB in cultured hepatocytes. Biochem Biophys Res Commun. 2015;458(3):700–706. doi:10.1016/j.bbrc.2015.02.029
42. Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12(8):695–708. doi:10.1038/ni.2065
43. Mitchell S, Vargas J, Hoffmann A. Signaling via the NFκB system. Wiley Interdiscip Rev Syst Biol Med. 2016;8(3):227–241. doi:10.1002/wsbm.1331
44. Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8(1):33–40. doi:10.1038/nrd2781
45. Cheong R, Hoffmann A, Levchenko A. Understanding NF-kappaB signaling via mathematical modeling. Mol Syst Biol. 2008;4:192. doi:10.1038/msb.2008.30
46. Kearns JD, Hoffmann A. Integrating computational and biochemical studies to explore mechanisms in NF-κB Signaling. J Biol Chem. 2009;284(9):5439–5443. doi:10.1074/jbc.R800008200
47. Hosseinzadeh H, Mehri S, Heshmati A, Ramezani M, Sahebkar A, Abnous K. Proteomic screening of molecular targets of crocin. DARU J Pharm Sci. 2014;22(1):5. doi:10.1186/2008-2231-22-5
48. Ochiai T, Ohno S, Soeda S, Tanaka H, Shoyama Y, Shimeno H. Crocin prevents the death of rat pheochromyctoma (PC-12) cells by its antioxidant effects stronger than those of alpha-tocopherol. Neurosci Lett. 2004;362(1):61–64. doi:10.1016/j.neulet.2004.02.067
49. Liang Y, Zheng B, Li J, et al. Crocin ameliorates arsenic trioxide‑induced cardiotoxicity via Keap1-Nrf2/HO-1 pathway: reducing oxidative stress, inflammation, and apoptosis. Biomed Pharmacother. 2020;131:110713. doi:10.1016/j.biopha.2020.110713
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.