Back to Journals » International Journal of Nanomedicine » Volume 17
Protection of Hearing Loss in Ototoxic Mouse Model Through SPIONs and Dexamethasone-Loaded PLGA Nanoparticle Delivery by Magnetic Attraction
Authors Park JE, Kim WC, Kim SK , Ahn Y, Ha SM, Kim G, Choi S, Yun WS, Kong TH , Lee SH, Park DJ, Choi JS, Key J , Seo YJ
Received 11 July 2022
Accepted for publication 8 November 2022
Published 13 December 2022 Volume 2022:17 Pages 6317—6334
DOI https://doi.org/10.2147/IJN.S380810
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lei Yang
Jeong-Eun Park,1,2,* Woo Cheol Kim,3,* Sung Kyun Kim,4,5,* Yeji Ahn,1,2 Sun Mok Ha,1,2 Gahee Kim,3 Seonmin Choi,3 Wan Su Yun,3 Tae Hoon Kong,1,2 Su Hoon Lee,1,2 Dong Jun Park,1,2 Jin Sil Choi,1,2 Jaehong Key,3 Young Joon Seo1,2
1Department of Otorhinolaryngology, Yonsei University Wonju College of Medicine, Wonju, 26426, South Korea; 2Research Institute of Hearing Enhancement, Yonsei University Wonju College of Medicine, Wonju, 26426, South Korea; 3Department of Biomedical Engineering, Yonsei University, Wonju, South Korea; 4Department of Otorhinolaryngology Head and Neck Surgery, Hallym University College of Medicine, Dongtan Sacred Heart Hospital, Hwaseong, South Korea; 5Laboratory of Brain & Cognitive Science for Convergence Medicine, Hallym University College of Medicine, Anyang, South Korea
*These authors contributed equally to this work
Correspondence: Young Joon Seo, Department of Otorhinolaryngology, Yonsei University Wonju College of Medicine, 20 Ilsan-ro, Wonju, 26426, South Korea, Tel +82 33 741 0644, Email [email protected] Jaehong Key, Department of Biomedical Engineering, Yonsei University, 1 Yonseidae-gil, Wonju, Gangwon-do, 26493, South Korea, Tel +82 33 760 2857, Email [email protected]
Background: Ototoxicity currently has no available treatment other than medication withdrawal as soon as toxicity is suspected. The human inner ear organs have little potential for regeneration; thus, ototoxicity-induced hair cell injury is deemed permanent. Dexamethasone (Dexa) is a synthetic steroid analog that has significant potential for otoprotection in the treatment of various inner ear diseases; however, its low absorption into the inner ear prevents significant recovery of function. Nanoparticles facilitate targeted drug delivery, stabilize drug release, and increase half-life of the drug.
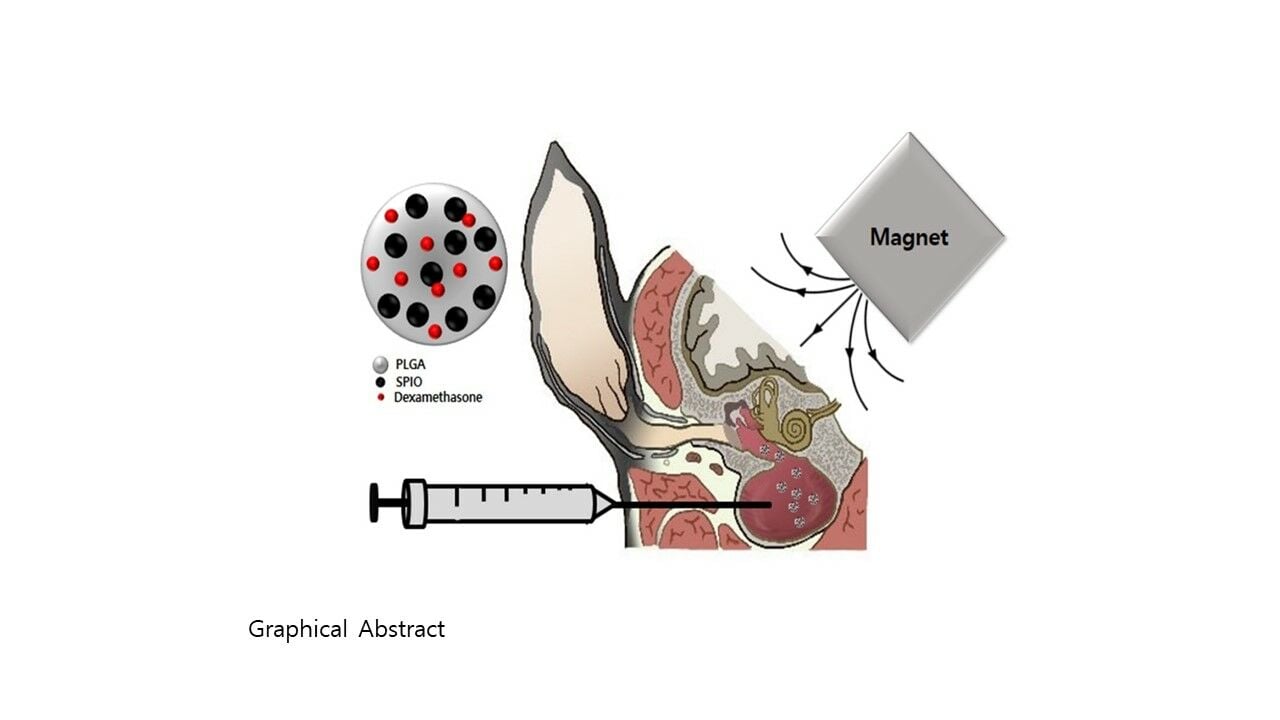
Methods: This study aimed to develop poly(lactic-co-glycolic acid) (PLGA) nanoparticles loaded superparamagnetic iron oxide nanoparticles (SPIONs) and Dexa (PSD-NPs) to control localized drug delivery by magnetic attraction in the treatment of ototoxicity-induced hearing loss. PSD-NPs and without SPIONs (PD-NPs) were prepared using a nanoprecipitation method.
Results: Using an inner ear simulating system, we confirmed that PSD-NPs has an otoprotective effect in organotypic culture that is enhanced by magnetic attraction. PSD-NPs delivered via intrabullar injection in a magnetic field penetrated the inner ear and prevented hearing loss progression to a greater degree than equivalent doses of Dexa or PSD-NPs alone (day 28: ototoxic: 80.0 ± 0.0 dB; Dexa 100: 60.0 ± 15.5 dB; PSD 100: 50.0 ± 8.2 dB; PSD 100 with magnet: 22.5 ± 5.0 dB; P < 0.05). The protective effects were confirmed in various in vivo and in vitro models of ototoxicity.
Conclusion: Our findings suggest that SPIONs with Dexa and magnetic field application prevent the progression of ototoxicity-induced hearing loss through anti-apoptotic mechanisms in the inner ear.
Keywords: superparamagnetic iron oxide nanoparticles, ototoxicity, hearing loss, magnetic attraction, dexamethasone
Graphical Abstract:
Introduction
Ototoxicity is defined as drug- or chemical-induced damage to the inner ear organs responsible for hearing and balance. Approximately 10% individuals receiving aminoglycoside antibiotics experience ototoxicity, and up to 33% damage has been reported in adult patients, with a 3% chance of the ototoxicity being permanent.1 Apart from medication withdrawal as soon as toxicity is suspected, there are currently no treatments available for ototoxicity. The human inner ear organs have little potential for regeneration; thus, ototoxicity-induced hair cell injury is deemed permanent.2 The desired goal of pharmacology is to discover a safe therapy that protects the ear from ototoxicity without affecting the therapeutic actions of the causative drug. Dexamethasone (Dexa) is a synthetic steroid analog that has significant potential for otoprotection in the treatment of various inner ear diseases, including sudden idiopathic sensorineural hearing loss, Ménière’s disease, and Bell’s palsy.3 Corticosteroids decrease reactive oxygen species (ROS) formation in the inner ear; however, their low absorption into the inner ear prevents significant recovery of function. The systemic routes of administration are challenging in inner ear therapy because of the blood-labyrinth barrier (BLB) and the significant risk of side effects, such as nausea, vomiting, upset stomach, edema, headache, dizziness, depression, anxiety, and elevated blood pressure. The BLB effectively limits the diffusion of molecules from the bloodstream into the cochlea, with the rates of entry on average being 4–6% of the total plasma concentration.4
Nanoparticle technology-based drug delivery systems are emerging as a novel treatment option for inner ear diseases. Nanoparticles (NPs) coated with polylactic acid and polyethylene glycol have been developed as a breakthrough means for sustained and controlled delivery of drugs to the cochlea.2 NPs have a size of 100 nm or less and are modified based on their size, surface potential, and physicochemical properties for use in different fields. In medicine, NPs can effectively deliver drugs over a large surface area compared with their volume, allowing the administration of small amounts of drugs to the human body and minimizing potential harm. NPs made of biodegradable polymers can be easily modified for the targeted delivery of drugs, improved biocompatibility, and controlled release of drugs in a single dose, making them effective in delivering high molecular weight drugs to impermeable areas, such as the brain vasculature,3 and inner ear.5
Iron oxide NPs are used in magnetic resonance imaging, biosensors, and cell separation. In contrast to large magnets, iron oxide NPs below 20 nm in size possess superparamagnetic characteristics; therefore, they are completely magnetized in the presence of an external magnetic field.6 However, the magnetism is not sustained in the absence of an external magnetic field (coercivity); hence, the aggregation of particles does not occur, resulting in intracellular internalization.7 Superparamagnetic iron oxide nanoparticles (SPIONs) are being developed for drug delivery.8 SPIONs are capable of precise movement using an external magnetic field and can pass through the blood-brain barrier (BBB) of mouse and in vitro human models without weakening or damaging the cells.9 Poly(lactic-co-glycolic acid (PLGA)) based nanoparticle loaded SPIONs were well internalized in the mesenchymal stromal cells (MSCs) and predominantly present in endosomal vesicles with less cytotoxicity.10 MSCs with endogenous SPIONs have increased expression of CXCR4, which can cause chemoattraction.11 It has been previously confirmed that MSCs labeled with SPIONs in an external magnetic field attachment homed into the cochlea of the mouse following intratympanic injection.12
Previous study revealed that drug delivery had limitations due to the permeation of the drug through RWM, and the drug loss in Eustachian tube by mucosa flow, therefore, concentration of the drug in the cochlea could not be increased.13 Kim et al investigated this issue by administering poly(lactic-co-glycolic acid) nanoparticles in thermosensitive gels to the middle ear.14 The continuous release of dexamethasone trapped in the PLGA nanoparticles ensured that the concentration in the cochlea remained high for an extended period.15 The biocompatibility and bioavailability of Dexa were improved by loading the drug into PLGA-based nanoparticles approved by the Food and Drug Administration (FDA) and the European Medicine Agency.16 SPION and magnetic attraction can also be used to modify drug penetration and persistence through the round window membrane (RWM), basement membrane (BM), and BLB. This study aimed to explore the possible use of SPIONs and Dexa loaded PLGA nanoparticles and magnetic field application as a therapy for ototoxic hearing loss diseases of the inner ear and various other hearing diseases.
Materials and Methods
Reagent and Materials
Kanamycin sulfate was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Dexamethasone sodium phosphate was purchased from Sigma-Aldrich (St. Louis, MO,USA). Furosemide (Lasix) was purchased from Handok Pharmaceuticals Co. (Seoul, South Korea).
PLGA (50:50, MW 38,000–54,000) polymers, Dexamethasone (≥ 98%, HPLC, powder), SPIONs solution (5 nm average particle size, 5 mg/mL in toluene) (Sigma, St. Louis, MO, USA), dichloromethane (DCM), and acetone were purchased from Sigma-Aldrich (St. Louis, MO,USA).
Synthesis of NPs
PSD-NPs were prepared using a nanoprecipitation method as follows: PLGA was dissolved in DCM (1 mg/100 µL) and into this solution, 40 µL SPIONs solution and 40 µL of Dexa (4 mg/mL) acetone solution were added dropwise in 3 mL deionized (DI) water. After that, this solution mixture was vortexed for 5 min and sonicated for 3 min (Supplementary Table S1). The solution mixture was stirred for 6 h. The PSD-NPs were obtained by filtration using centrifugation. In addition, PD-NPs were prepared without SPIONs in the same protocol as PSD-NPs.
Characterization of PSD-NPs and PD-NPs
The size and zeta potential of NPs were measured using a zetasizer (Zetasizer-ZS90, Malvern Instruments Ltd, Malvern, UK) with plastic semi-micro cuvettes (2712120 Ratiolab GmbH, Dreieich, Germany) and zeta cell DTS1070 (Malvern Panalytical, Malvern, Worcestershire, UK). PSD-NPs and PD-NPs were dispersed in DI water (1 mg / mL) before the measurement. Particle morphology was characterized via transmission electron microscopy (TEM; JEOL-F200, JEOL Ltd., Tokyo, Japan) and scanning electron microscopy (SEM; JEOL-6701F, JEOL Ltd., Tokyo, Japan). Iron quantification was determined using the methods described by Hedayati et al.17 To measure the quantification of Dexa in PSD-NPs and PD-NPs, the particles are dissolved using dimethyl sulfoxide for 24 hours and were determined at a wavelength of 270 nm using a multi-mode reader (Synergy HTX, BioTek Instruments, Winooski, VT, USA).
The stability of NPs was evaluated in DI water, phosphate-buffered saline (PBS, pH 7.4; Gibco, Grand Island, NY, USA), and perilymph at 37 °C for up to 12 h using the zetasizer. PSD-NPs and PD-NPs (20 μg) were suspended in 1 mL of DI water, PBS and perilymph and monitored.
The profile of Dexa release from NPs was assessed using a dialysis unit (Thermo Fisher Scientific) at 36°C in PBS. Dexa was measured at a wavelength of 270 nm at 1, 2, 4, 8, 12, 24, 48, 60, 80 hours for the release test. The manufacturing of perilymph was based on Philine Wangemann’s perilymph components.18
Organotypic Explant Cultures and ex vivo Otoprotective Effects of PSD-NPs
The preparation of the whole organs of Corti was conducted as previously described.19 To summarize, after collecting cochlear tissue of C57BL/6 mice at postnatal day 2, the SV, modiolus, and tectorial membrane were removed, and the organ of corti was well fixed on a plastic cover slip. In a 24-well culture plate, add 1mL of explant media (Dulbecco’s Modified Eagle Medium [DMEM]/F12 with 10% fetal bovine serum, 1% N2 supplement, and 10 µg/mL ampicillin), and incubate overnight at 37°C in a humidified 5% CO2 incubator. To evaluate otoprotective effects, dexamethasone or PSD-NPs dissolved in explant media were pretreated for 24 hours the following day, depending on the conditions. After that, 0.7 mM kanamycin was used for 24 hours to induce ototoxicity. It was stained and fixed with 4% formalin after the medium was removed.
Immunostaining
Whole organs of Corti were fixed with 4% paraformaldehyde solution for 15 min. After fixation, each whole organ of Corti was subjected to permeabilization with Triton X-100 before sequential incubation with rabbit anti-mouse myosin VIIa antibody (Abcam, Cambridge, UK), Alexa Fluor™ 647 phalloidin (Abcam), and mounting medium with 4′,6-diamidino-2-phenylindole (Fluoroshield DAPI; Sigma-Aldrich). Confocal images of stained inner ear hair cells and their cilia were acquired with a Zeiss laser scanning confocal microscope (LSM800, Carl Zeiss, Jena, Germany) and analyzed using ZEN blue 2.0 software (Carl Zeiss).
Apoptosis Assay
The organotypic cultures of cochlear tissue were stained using antibodies against cleaved caspase-3 (Cell Signaling Technology, Danvers, MA, USA). The organ of Corti tissues were immunostained with rabbit anti-mouse cleaved caspase-3 antibody (1:100) and goat anti-rabbit IgG H&L (Alexa Fluor® 488) antibody (1:500). The fluorescence images were obtained using confocal microscopy and were analyzed using the confocal microscope software (Zeiss Zen 3.4).
Establishment of the Polydimethylsiloxane (PDMS) Three-Dimensional (3D) Model
The PDMS Silicone Elastomer Kit (Dow Corning, Chungcheong-do, South Korea) was used to produce the PDMS 3D model. After mixing the silicone elastomer base and silicone elastomer curing agent in a 10:1 ratio, the mixture was poured into a mold. After storing it at 60°C for 2 h, the mold was taken out. The prepared PDMS 3D model was then placed in a confocal dish, and a 10 mm magnet was placed below it. After filling the lowest chamber of the PDMS 3D model with DMEM media, a 400 µm thick megaderm (L&C Bio, Seoul, South Korea) covered this section of the 3D model. Perilymph was then added on top and covered with a 200 µm thick megaderm layer. PBS containing PSD-NPs or PD-NPs (equivalent to 100 µg Dexa) was added to the top compartment of the PDMS 3D model. After 4, 8, 12, 24, 48, and 72 h, DMEM media were collected and centrifuged at 12,225 x g for 10 min. The precipitated NPs were dissolved in dimethyl sulfoxide, and the absorbance was measured at a wavelength of 270 nm to calculate the penetration rate of the NPs in this PDMS 3D model.
In vitro Study Using the PDMS 3D Model
The prepared rat cochlear tissue was placed in the center of a confocal dish. The PDMS 3D model was then covered and 1 mL DMEM, 400 µm thick megaderm, perilymph 200 µL, and 200 µm thick megaderm were then placed into the model in order. PBS containing PSD-NPs or PD-NPs (corresponding to 100 µg of Dexa) was then added to the top chamber of the PDMS 3D model.
After incubating the cochlear tissue in the PDMS 3D model for 24 h at 37°C, kana 0.7 was added to the medium and incubated for 24 h. For tissue staining, the medium was aspirated, and the tissue was washed twice with PBS. The sample was fixed in 4% formalin for 15 min and washed thrice with PBS. Triton X-100 at a concentration of 0.1% was used to permeate the sample for 10 min, which was subsequently washed thrice with PBS. Subsequently, 250 µL of phalloidin-iFluor 647 reagent (1:1000 dilution in PBS) was added, and the sample was incubated for 1 h at room temperature using a shaker and then washed thrice with PBS.
Animals
Five-week-old male C57BL/6 mice with 18–22 g body weight were purchased from DBL Co. (Eumsung, South Korea) to study the changes in the hearing thresholds after drug-induced ototoxicity. The mice were allowed free access to water and a regular diet and kept at room temperature under a standard 12 h light/dark cycle for 1 week of acclimatization before the experiments. The mice were anesthetized by intraperitoneal injection of 100 mg/kg ketamine (Yuhan Corporation, Seoul) and 10 mg/kg xylazine (Rompun; Bayer Korea, Ansan, South Korea) and sacrificed by cervical dislocation. The animal experiments were conducted in the animal laboratory of Yonsei University Wonju College of Medicine Wonju, Korea in accordance with the guidelines of the Institutional Animal Care and Use Committee (YWC-180703-1, YWC-201123-2).
Mouse Model of Ototoxicity
Before administration of the ototoxic drugs, the auditory-evoked brainstem responses (ABRs) of all mice were measured using click sounds. Each animal was briefly anesthetized with a mixture of 100 mg/kg ketamine and 10 mg/kg xylazine before being placed on a heating pad inside a soundproof acoustic chamber. The test stimuli were clicks generated using BioSigRP software (Tucker-Davis Technologies, Inc., Alachua, FL, USA). The stimulus intensity was gradually reduced by decrements of 10 dB from 90 dB to 10 dB until the visually identifiable ABR waveform disappeared. The lowest sound level with visualization of a waveform was defined as the threshold. In all experiments, the left ear of a mouse with intact hearing was selected and used. The right ear served as a control within the animal and was not surgically operated on. The fur from the post-auricular skin was removed caudally from the auriculocephalic crease to the shoulder girdle. The post-auricular skin was incised, and the auditory bulla was exposed. A hole was made in the bulla bone using a microdrill bit (0.12 mm in diameter). Using a 32 gauge needle-size Hamilton syringe, 5 μL of saline, Dexa, or PSD-NPs were injected into the intrabullar (IB) cavity. The hole was filled with bone wax, and the wound was closed with Steri-Strips (3M, St Paul, MN, USA). In the group with magnetic field exposure, the mice were placed in the left lateral position, and a 530 mT NdFeB magnet was placed along the vertex area for 40 min. Twenty-four hours after the bullostomy, 130 mg/kg of furosemide was injected subcutaneously, and 550 mg/kg of kanamycin was injected 30 min later. The ABR test was performed every week (days 7, 14, 21, and 28) to evaluate the protective effect of injected NPs and magnetic attraction, and the thresholds of both the left and right ears were compared.
Evaluation of the Otoprotective Effect of PSD-NPs by Staining the Cilia of the Inner Ear Hair Cells
After the ABR measurement, the cochlea from adult mice were fixed in 4% paraformaldehyde solution overnight and then decalcified in 0.5 M ethylene-diamine-tetraacetic acid (EDTA) for 2 days. All outer bones were removed from the decalcified cochlea under the microscope, and the stria vascularis (SV) and modiolus were separated from the organ of Corti (OC) where the hair cells were located. The tissues were stained using a Prussian blue staining kit (Solarbio, Beijing, China).
Histological Assessment
Cochlear dissection and tissue preparation for cryosection were performed as previously described with some modifications.20 The cochlea were immersed directly in 4% formaldehyde (Biosesang Seongnam, South Korea) for 24 h at room temperature, decalcified by immersion in 0.5 M EDTA for 48 h, dehydrated in 30% sucrose (Sigma-Aldrich, Gillingham, UK) for 24 h, embedded in optimal cutting temperature compound (Leica, Bensheim, Germany), and sectioned at 4 to 5 μm thickness in a cryostat (Leica CM1850 Cryostat; Leica, Wetzlar, Germany). The slices were mounted on slides and stained (eosin for cochlear structures and Prussian blue for PSD-NPs). The slides were microscopically examined for the localization of the PSD-NPs in the cochlea.
Statistical Analyses
The t-test for paired samples was used to compare the cell viability after treatment of the explant system with kanamycin. P < 0.05, P < 0.01, and P < 0.001 were considered statistically significant. All data obtained in the in vivo experiments were analyzed using GraphPad Prism (Version 8.01, GraphPad Software, San Diego, CA, USA). The Mann–Whitney U-test was used to compare the thresholds between the groups at 28 days after the induction of ototoxicity. This nonparametric statistical hypothesis test was used because of the relatively small group of animals in which normal distribution cannot be assumed. Data are presented as the mean ± standard error of the mean. P < 0.05 was considered to indicate a statistically significant difference.
Results
Characterization of PSD-NPs and PD-NPs
The conventional nanoprecipitation method was used for the formulation of PSD-NPs (Figure 1A and B). The morphology of the fabricated NPs was observed using TEM and SEM (Figure 1C–F). The average size of PSD-NPs was measured as 250.21 nm, and the average size of PD-NPs was measured as 130.13 nm. It is shown that the PSD-NPs were additionally loaded SPIONs to increase their size. In the case of zeta potentials, the average charge of PSD-NPs was −45.24 mV, and the average charge of PD-NPs was −60.1 mV (Figure 1G).
The stability of NPs was evaluated in DI water, PBS, and perilymph for 1, 2, 4, and 12 h using zetasizer (Figure 1H). The size of PSD-NPs and PD-NPs did not show a significant difference in DI water and PBS, respectively. However, in the perilymph, the size of the PSD-NPs decrease from 401 nm to 301 nm and the PD-NPs size decreased from 194 nm to 162 nm after 12 h.
Figure 1I shows the Dexa release profiles of PSD-NPs and PD-NPs in PBS over time. Approximately 95% of Dexa release from both NPs was observed after 80 h. The release profile data demonstrated a higher drug release from NPs in 20 h. The quantification of Dexa was measured as an average of 220.16 µg/100 µL for PSD-NPs and 90.28 µg/100 µL for PD-NPs (Table 1). The quantification of SPIONs within the PSD-NPs was measured as an average of 22.87 µg/100 µL.
 |
Table 1 Dexamethasone and Iron (Fe) Quantifications of PSD- and PD-NPs |
Protection from Kanamycin-Induced Ototoxicity and Attenuation of Apoptosis of Auditory Hair Cells by PSD-NPs in a Cochlear Explant
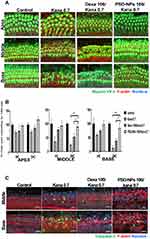
Explants were pretreated with 100 µg of Dexa solution (Dexa 100) or 100 µg of Dexa in PSD-NPs (PSD-NPs 100) for 16 h. Ototoxicity was induced via treatment with kana 0.7. Immunostaining was performed after 24 h of incubation. The numbers of normal hair cells in a length of 100 μm were counted using a confocal microscope. Three distinct regions were designated in the explant based on their positions relative to the apex, middle, and basal turn of the cochlea. Figure 2A shows representative images of cochlea that were pretreated with saline, kana 0.7, Dexa 100/kana 0.7 or PSD-NPs 100/kana 0.7 and subsequently exposed to an ototoxic insult. As shown in Figure 2B, OHCs and IHCs in the apex turn were largely unaffected by kanamycin and remained viable in all explants pretreated with saline, kana 0.7, Dexa 100/kana 0.7 or PSD-NPs 100/kana 0.7. In contrast, kanamycin application resulted in a significant reduction in OHC viability in the middle and basal turns (24.3 ± 9.5% and 13.3 ± 8.0%, respectively), indicating ototoxicity. However, the ototoxic effects were attenuated by pretreatment with Dexa 100 or PSD-NPs 100 in OHCs and IHCs. The viability of OHCs in Dexa 100-pretreated groups was 52.4 ± 12.2% in the middle turn and 24.3 ± 13% in the basal turn. The viability of OHCs in PSD-NPs 100-pretreated groups was 88.8 ± 12.6% in the middle turn and 84.2 ± 17.7% in the basal turn. These results show that PSD-NPs might protect auditory hair cells more from kanamycin-induced ototoxicity compared with Dexa alone.
Immunohistochemical staining was performed against the active form of caspase-3, a pro-apoptotic enzyme, to confirm the anti-apoptotic ability of PSD-NPs. As shown in Figure 2C, the protein expression levels of cleaved caspase-3 increased following treatment with kana 0.7. However, this protein expression was suppressed by pretreatment with Dexa 100 or PSD-NPs 100. The protective effect of Dexa observed in this study was consistent with the findings of a previous study.21 Therefore, our results indicate that PSD-NPs can reduce kanamycin-induced damage to auditory hair cells via anti-apoptotic activity, similar to Dexa.
Higher Penetration Rate of PSD-NPs in the Presence of an External Magnetic Field Than the Penetration Rate of PD-NPs
Hematoxylin and eosin staining confirmed that megaderm has excellent biocompatibility and physical properties suitable for simulating the extracellular matrix structures of the RWM and BM (Figure 3A).22 A stable 3D structure was then made. The PDMS 3D model was manufactured with a diameter of 35 mm and a height of 10 mm. Each layer had a height of 2 mm (Figure 3B and C). The 200 µm thick megaderm that simulated the RWM was cut to a diameter of 12 mm and fixed, and the 400 µm thick megaderm that simulated the BM was cut to a diameter of 8 mm and fixed (Figure 3D).
Figure 3E shows that 4.2 ± 1.2%, 10.2 ± 2.8%, 19.3 ± 2.2%, and 42.6 ± 3.6% of PSD-NPs with magnetic field application penetrated the PDMS 3D model within 4, 8, 12, and 24 h, respectively. In comparison, PD-NPs had a penetration rate of only 3.6 ± 0.2% in the PDMS 3D model after 24 h. Similarly, PSD-NPs had a penetration rate of 1.3 ± 0.1% after 24 h.
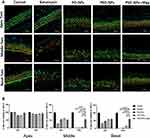
To investigate the changes in cochlear morphology, the organ of Corti was examined in all three turns of the cochlea (Figure 4A). As shown in Figure 4B, kanamycin-induced ototoxicity in OHCs and IHCs in the middle and basal turns was not attenuated by pretreatment with PD-NPs or PSD-NPs. However, there was a marked increase in the viability of OHCs from the pretreatment of PSD-NPs with magnet groups in the middle and basal turns at 55.6 ± 6.9% and 61.0 ± 8.4%, respectively (Figure 4B).
PSD-NPs showed a significant difference in the penetration rate in the PDMS 3D model when an external magnetic field was applied compared with PSD-NPs or PD-NPs alone, suggesting that an external magnetic field plays a crucial role in the successful delivery of PSD-NPs to the inner ear in future in vivo experiments.
Enhanced Protection of Hearing Loss from Kanamycin-Induced Ototoxicity Using PSD-NPs and Magnetic Attraction
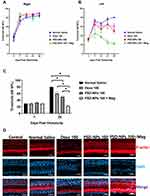
Given the results obtained ex vivo, the protective effects of PSD-NPs were assessed in vivo by employing intrabullar (IB) injection to deliver drugs to the left ear of mice (Figure 5). To evaluate the synergistic effect of magnetic force and PSD-NPs in preventing kanamycin-induced ototoxic injury, 4 of the 8 mice injected with PSD-NPs were exposed to a magnet (530 mT) for 40 min. Hearing function was then assessed by measuring the ABRs. The mice were treated with a one-shot kanamycin-furosemide regimen as previously described.11 In the normal saline treated model, the threshold was increased to 73.3 ± 3.3 dB on the 7th day, which is close to profound hearing loss. The increased threshold recovered mildly from the 14th to the 21st day; however, it increased to 80 dB on the 28th day. Similarly, the Dexa 100 and PSD-NPs 100 groups showed an increased threshold of 71.6 ± 1.7 dB and 65.0 ± 6.5 dB on the 7th day, respectively. The thresholds were reduced on the 28th day for Dexa 100 and PSD-NPs 100 (60.0 ± 6.3 dB and 50.0 ± 4.1 dB, respectively). In contrast, exposure to a magnet after injection of PSD-NPs 100 caused a modest threshold increase on the 7th day (45.0 ± 10.4 dB), with better improvement by the 28th day (22.5 ± 2.5 dB), compared with the other groups (Figure 6B).
It was confirmed previously that pretreatment with Dexa or PSD-NPs can prevent hearing loss caused by kanamycin induced ototoxicity. In vivo, the PSD-NPs 100 group had a 10 dB lower threshold than the Dexa 100 group on the 28th day; however, this difference was not statistically significant (P > 0.05). In the case of the group exposed to a magnetic field, the threshold was 37.5 dB lower than that of the Dexa 100 group and 27.5 dB lower than that of the PSD-NPs 100 group on the 28th day, and these differences were statistically significant (P = 0.01 and P = 0.025, respectively, compared with that of the PSD-NPs 100+Mag group), suggesting that the administration of PSD-NPs 100 with magnet application is more effective than Dexa 100 or PSD 100 alone in protecting from kanamycin induced ototoxicity. In contrast, progressive hearing loss was observed in the right ear where no surgical intervention was performed (Figure 6A), suggesting the successful creation of the one-shot kanamycin-furosemide induced ototoxic model and that the otoprotective effect of IB injection was limited to the left ear where the interventions were performed.
As shown in Figure 6D, kanamycin induced profound loss of OHCs in the experimental groups compared with the control group. The administration of PSD-NPs 100 with a magnet before kanamycin treatment largely preserved hair cells from kanamycin induced damage, whereas the administration of Dexa 100 or PSD-NPs 100 alone did not convey the same protective effect.
Confirmation of PSD-NP Delivery into the Cochlea with Magnetic Field Application After One Month
Two possibilities emerged that may explain the otoprotective effect of PSD-NPs. First, the PSD-NPs directly pass through the RWM and enter the cochlea to exert their effect. Second, the Dexa diffused from PSD-NPs present in the bulla can provoke this effect. Prussian blue staining of cochlear tissue was performed to find direct evidence of IB-injected PSD-NPs passing through the RWM due to the magnetic force and has transferred internally to the cochlea. Magnetically delivered PSD-NPs can be observed among the hair cells in the cochlea (Figure 7A).
Histological cross-sections of the cochlea of animals injected with PSD-NPs alone and PSD-NPs100 with magnetic field exposure revealed the presence of iron particles in the regions of the modiolus and stria vascularis of the cochlea (Figure 7B). Most clusters of iron were observed in the blood vessels and vestibular branch of the vestibulocochlear nerve. Fewer iron clusters were observed in the organ of Corti.
Discussion
In this study, an aminoglycoside-induced ototoxicity mouse model was introduced by using kanamycin and furosemide in a one-shot protocol to produce severe hearing loss.11 Using this model, the possibility of otoprotection using NPs and Dexa was assessed. The results showed a difference of 67.5 ± 5.0 dB (60% hearing recovery) in the group injected with PSD-NPs 100 and subsequently exposed to a magnetic field compared with the control group. They also showed that hearing loss prevention (27.5 dB) by PSD-NPs 100 with magnetic field application was greater than by PSD-NPs 100 without exposure to a magnetic field. This delivery strategy of Dexa into the inner ear using SPION nano-drug carriers with a magnetic field might be a novel approach that has significant implications in hearing therapy. A sufficient amount of Dexa was administered to the core SPION nanostructure to protect hair cells, which exhibits magnetic attraction in the presence of a static magnetic field. The otoprotective effects of this new method of SPION-based dexamethasone delivery with magnetic field application introduced in our study were three times higher than those exhibited by conventional dexamethasone treatment via intratympanic injection. These findings can be used for further studies such as clinical trials in ototoxicity.
The exact mechanisms of aminoglycoside induced ototoxicity remain unclear. ROS production is the primary pathway that is known to cause cellular damage resulting in apoptosis.21 Preventing damage is more important than promoting regeneration and healing of damaged hair cells; however, there are no available drugs that act as preventive therapy for ototoxicity-related sensorineural hearing loss. Several studies have shown the possibility of otoprotective drug therapy reducing the negative impact of ototoxic drugs on hearing. Currently, the number of such agents approaching or already undergoing clinical trials is encouraging. Several antioxidants (such as vitamins, magnesium, ebselen, N-acetylcysteine, coenzyme Q10, alpha-lipoic acid, sodium thiosulphate, ginkgo biloba, and steroids) have been evaluated for their protective effects against cisplatin ototoxicity.23 Ebselen (SPI-1005), an organoselenium antioxidant, is currently undergoing two clinical trials to evaluate its ability to protect from ototoxicity: a Phase II study targeting ototoxicity caused by platinum chemotherapy and a phase I/II study for the prevention and treatment of aminoglycoside induced ototoxicity.24 Most antioxidants have been shown to protect the cochlea from noise induced and ototoxic damage through the prevention of ROS formation in in vivo experiments; to repair the damage inflicted by free radicals, particularly to cell membrane lipids; and to block the triggers of intrinsic or extrinsic pathways of apoptosis. Dexa is a common synthetic steroid analog that has anti-ROS activity and the ability to increase cochlear ROS defenses and is used for the treatment of various inner ear diseases, including sudden idiopathic sensorineural hearing loss, Ménière’s disease, and Bell’s palsy. Systemic glucocorticoids are also used as empiric treatment for hearing loss with an unclear etiology. Marshak et al have shown in their study of 26 patients that the injection of 0.7 mL of dexamethasone phosphate in a 10 mg/mL solution to the middle ear prevents drug-induced ototoxicity and has a statistically significant protective effect on hearing loss.25 In this study, an aminoglycoside-induced ototoxicity mouse model was introduced using kanamycin and furosemide in a one-shot protocol that produces severe hearing loss.11 Using this model, the possibility of otoprotection using NPs and Dexa was assessed. The results showed a difference of 67.5 ± 5.0 dB (60% hearing recovery) in the group injected with PSD-NPs 100 and subsequent exposure to a magnetic field compared with the control group. They also showed that hearing loss prevention (27.5 dB) in PSD-NPs 100 with magnetic field application was greater than in PSD-NPs 100 without exposure to a magnetic field. This delivery strategy of Dexa into the inner ear using SPION nano-drug carriers with a magnetic field might be a novel approach that has significant implications in hearing therapy. A sufficient amount of Dexa was administered to protect hair cells in the core SPION nanostructure, which exhibits magnetic attraction in the presence of a static magnetic field. The otoprotective effects of PSD-NPs using magnetic field application were three times higher than Dexa treatment via intratympanic injection. These findings can be used for further studies, such as clinical trials in ototoxicity.
Intratympanic administration of drugs is a safe, inexpensive, and acceptable drug delivery route in treating several types of hearing loss. Currently, steroid therapies for inner ear disease, such as sudden idiopathic sensorineural hearing loss, rely on systemic administration of the drug. The BLB, analogous to the BBB, makes it challenging for systemic steroid administration to achieve an optimal local drug concentration in the inner ear. Moreover, side effects involving other organ systems, such as the elevation of blood sugar levels, liver obstruction, and infection, are more likely with systemic use. Thus, local delivery to the target organs might reduce the systemic side effects of steroids delivered in high doses. Cochleostomy is the best local drug delivery approach for high efficacy direct delivery of drugs. However, this method is invasive and has a significant risk of damaging hair cells during the procedure. The RWM is a semipermeable membrane with three layers that regulate the diffusion of molecules into the cochlea. Dexa can be administered via direct uptake or active endocytosis through the RWM. The magnetic attraction induces PSD-NPs to penetrate the RWM more easily to the phantom of the inner ear. In this study, the RWM was simulated with megaderm from human cadaver skin. NPs that gained easier access to the cochlea through magnetic attraction were deposited around the hair cells through the local systemic circulation. This was shown through increased staining in the stria vascularis, the microcirculation structure (Figure 6). Barrefelt et al have shown that SPIONs injected intravenously can be detected in the liver as early as 10 min post injection, with the maximum signal being detected between 24 h and 1 week post injection using magnetic resonance imaging.26 Furthermore, it was shown in their study that cytoplasmic iron accumulation shifted from clustered microbubbles in the pulmonary microvasculature to macrophages, resulting in a shift from the lungs to the spleen and liver. Briley-Saebo et al found that the half-life of iron oxide NPs coated with oxidized starch was 29 days.27 This is in contrast to the results of this study where sectioned cochlea at 4 weeks after IB injection still showed many detectable SPIONs in the scala media of the cochlea, suggesting that the early use of magnetic force upon SPION administration might help retain the NPs and prevent them from being washed out.
In vitro permeability penetration to the RWM in previous studies was performed by extracting RWMs from live animals.28,29 Little is known about the absorption and transmembrane delivery of molecules by the RWM currently. A recently published model using guinea pig RWM suggests that superparamagnetic PLGA-magnetite-Dexa-acetate NPs in a magnetic field allow better diffusion of Dexa-acetate to the inner ear.30 However, the passage of NPs was modeled with barriers that only simulate the RWM and BM in this study, using artificially produced perilymph. Previous studies have used a microneedle to penetrate the RWM or RWM niche for direct delivery of the NPs into the cochlea.31–34 However, this method is invasive and has clinical limitations due to the greater risk of causing further hearing loss than with IB injection. Furthermore, the magnetic attraction applied to NPs loaded in the bulla allows a safe and non-invasive form of drug delivery into the cochlea. It was also found that PSD-NPs injected into the bulla could protect auditory hair cells to a similar extent as Dexa alone, whereas magnetic field application to PSD-NPs appeared to largely increase this protective effect. PSD-NPs or Dexa present in the bulla drains easily through the eustachian tube, reducing the amount of Dexa reaching the cochlear hair cells over time. In contrast, magnetic attraction causes more PSD-NPs to pass through the RWM and aggregate around it, resulting in decreased drug loss and increased delivery of Dexa to the inner ear hair cells. These results demonstrate that non-invasive magnetic attraction can be an excellent approach to achieving otoprotection by maximizing drug delivery in target organs, which means increased amounts of NPs reaching the inner ear hair cells of the cochlea, as observed in this study. It is well established that Dexa alleviates auditory hair cell damage induced by aminoglycosides.31 Nuclear factor-kappa B translocation into the nucleus appears to play an important role in this protection, and it is known that glucocorticoid and glucocorticoid receptors act upstream of the cell signaling pathway that leads to its nuclear translocation.27 Dexa is directly delivered to the auditory cells in ex vivo models, unlike in in vivo models, where barriers are present. Owing to this problem, efficient drug delivery to the target area is of great importance to obtain the desired pharmacotherapeutic effects. In this study, the in vivo administration of PSD-NPs with magnetic field application led to encouraging results. Proper magnetism, angles and distances, and the production of more advanced types of particles are required to achieve better results. However, this effect is limited to pretreatment modalities. Binding to specific antibodies for more efficient drug delivery into the cochlear auditory hair cells or drugs that can induce differentiation of auditory stem cells present in the cochlea can circumvent this problem; hence, further studies are needed.33
The exact mechanism of aminoglycoside-induced ototoxicity remains unclear; however, one signaling pathway activated by aminoglycosides via ROS is the c-Jun N-terminal kinase pathway.10 This stimulates the production of pro-apoptotic caspases that lead to programmed cell death. In this study, the ototoxic drug activated pro-apoptotic caspases in the ex vivo explanted model. Previous studies have shown that Dexa protects from gentamicin induced hair cell loss in neonatal cochlea cultures. A proposed protective mechanism involves the inhibition of the mitochondrial apoptosis pathway by the regulation of Bax expression.21 Dinh et al showed that Dexa prevents tumor necrosis factor-α-induced ototoxicity in the organ of Corti explants.34 In this study, the mechanism of PSD-NPs induced protection from ototoxicity caused by kanamycin and furosemide is surmised to be through anti-apoptotic pathways. The PLGA-based nanocarrier used is approved by the FDA for human use. This carrier has greater loading capacity and superior stability that allows the delivery of slow-release hydrophobic drugs, such as Dexa. Kenyon et al reported that the encapsulation and protection of anti-inflammatory agents, such as corticosteroids in NP formulations, can improve the efficacy of drugs in the lung, by preventing their degradation and inactivation by the body.35 The controlled release of Dexa by PLGA-NP formulations was successfully prepared for potential local treatment of oral precancerous lesions using an emulsification/solvent evaporation method.29 Rather than focusing on the drug physiology, a complete preclinical study was attempted in which NPs conjugated with Dexa were locally applied to the ear as a treatment to prove the efficacy of the drug in comparison with the existing forms of Dexa in clinical use. Environments similar to human physiological states were modeled, and demonstration of the efficacy of SPIONs under a magnetic field in ex vivo inner ear to the phantom of the inner ear and in vivo inner ear models was focused upon. Furthermore, the simple, fast, and economical NP-based drugs have the benefit of using nontoxic solvents for local injection into the inner ear.
Some limitations in this study are apparent. First, no pharmacokinetic measurements of the NP-based Dexa compound in the mouse were performed to determine optimal dosing. However, we believe that the anti-inflammatory effect of the PSD-NPs observed at the 100 μg/mL dosing PD did not demonstrate efficacy. A dose of 5 mg/mL Dexa is used clinically via intratympanic injection in patients with sudden sensorineural hearing loss. Second, we did not confirm the deposition of the synthesized compounds in the inner ear at different times (days 0–28). However, Prussian blue dye-labeled nanomicelles were imaged in cochlear sections at 28 days after their administration. We are convinced that the SPION concentration in the cochlea before 28 days would be higher. Third, different timings of magnetic field applications were not assessed. The critical time for NP penetration into the cochlea is thought to be within the initial hour. Thus, these experiments should be considered an early-stage development of the therapeutic strategy. Further research on controlled magnetic fields is needed and planned.
Conclusion
In summary, this study found that mice pretreated with PSD-NPs encapsulated in PLGA-based SPIONs and subsequent magnetic field application had a lesser degree of hearing loss from kanamycin-furosemide induced ototoxicity than those pretreated with equivalent doses of Dexa alone. The otoprotective effect of the drug was shown in a series of preclinical ototoxicity models from ex vivo explanted sensory epithelium to a phantom of the inner ear and in vivo models. It is hypothesized that controlled Dexa release by local treatment of PLGA-NP formulations with a magnetic field reduces apoptosis in the ototoxicity models used in this study. The function and longevity of NPs diffused into the inner ear through magnetic field application require additional investigation to further improve their therapeutic action.
Abbreviations
ABR, auditory brainstem response; BBB, blood brain barrier; BLB, blood labyrinth barrier; BM, basilar membrane; CXCR4, C-X-C chemokine receptor type 4; DAPI, 4’,6’-diamidino-2-phenylindole; DCM, dichloromethane; Dexa, Dexamethasone; DI water, deionized water; DMEM, dulbecco’s modified eagle medium; EDTA, ethylene-diamine-tetraacetic acid; FDA, food and drug administration; IB, intrabulla; IHCs, inner hair cells; Kana, kanamycin; Mag, magnetic stromal cells; NPs, nanoparticles; OC, organ of corti; OHCs, outer hair cells; PBS, phosphate-buffered saline; PDMS, polydimethylsiloxane; PD-NPs, Polymer nanoparticles with dexamethasone; PLGA, poly(lactic-co-glycolic acid); PSD, poly(lactic-co-glycolic acid)(PLGA)-coated polymeric SPIONs with Dexamethasone; RM, reissner’s membrane; ROS, reactive oxygen species; RWM, round window membrane; SEM, scanning electron microscopy; SPION, superparamagnetic iron oxide nanoparticles; SV, stria vascularis; TM, tectorial membrane; TEM, Transmission electron microscopy.
Acknowledgments
This work was supported by National Research Foundation of Korea (NRF) grants (Nos. NRF-2020R1A2c1009789 and NRF-2018R1D1A1B07050175) and the Hallym University Research Fund. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (grants nos. 2022RIS-005, and 2022R1F1A1069516).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Selimoglu E. Aminoglycoside-induced ototoxicity. Curr Pharm Des. 2007;13(1):119–126. doi:10.2174/138161207779313731
2. Sun C, Wang X, Chen D, Lin X, Yu D, Wu H. Dexamethasone loaded nanoparticles exert protective effects against Cisplatin-induced hearing loss by systemic administration. Neurosci Lett. 2016;619:142–148. doi:10.1016/j.neulet.2016.03.012
3. Chertok B, Moffat BA, David AE, et al. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008;29(4):487–496. doi:10.1016/j.biomaterials.2007.08.050
4. Piu F, Bishop KM. Local drug delivery for the treatment of neurotology disorders. Front Cell Neurosci. 2019;13:238. doi:10.3389/fncel.2019.00238
5. Leterme G, Guigou C, Oudot A, et al. Superparamagnetic nanoparticle delivery to the cochlea through round window by external magnetic field: feasibility and toxicity. Surg Innov. 2019;26(6):646–655. doi:10.1177/1553350619867217
6. Enriquez-Navas PM, Garcia-Martin ML. Application of inorganic nanoparticles for diagnosis based on MRI. In: de la Fuente JM, Grazu V, editors. Nanobiotechnology. Elsevier; 2012:233–245.
7. Wahajuddin AS. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine. 2012;7:3445–3471. doi:10.2147/IJN.S30320
8. Kayal S, Ramanujan RV. Doxorubicin loaded PVA coated iron oxide nanoparticles for targeted drug delivery. Mat Sci Eng C Mater. 2010;30(3):484–490. doi:10.1016/j.msec.2010.01.006
9. Jiang W, Xie H, Ghoorah D, et al. Conjugation of functionalized SPIONs with transferrin for targeting and imaging brain glial tumors in rat model. PLoS One. 2012;7(5):e37376. doi:10.1371/journal.pone.0037376
10. Lee SH, Park DJ, Yun WS, et al. Endocytic trafficking of polymeric clustered superparamagnetic iron oxide nanoparticles in mesenchymal stem cells. J Control Release. 2020;326:408–418. doi:10.1016/j.jconrel.2020.07.032
11. Ju HM, Lee SH, Choi JS, Seo YJ. A simple model for inducing optimal increase of SDF-1 with aminoglycoside ototoxicity. Biomed Res Int. 2017;2017:4630241. doi:10.1155/2017/4630241
12. Ahn YJ, Yun WS, Choi JS, et al. Biodistribution of poly clustered superparamagnetic iron oxide nanoparticle labeled mesenchymal stem cells in aminoglycoside induced ototoxic mouse model. Biomed Eng Lett. 2021;11(1):39–53. doi:10.1007/s13534-020-00181-6
13. Salt AN, Plontke SK. Pharmacokinetic principles in the inner ear: influence of drug properties on intratympanic applications. Hear Res. 2018;368:28–40. doi:10.1016/j.heares.2018.03.002
14. Kim DH, Nguyen TN, Han YM, et al. Local drug delivery using poly(lactic-co-glycolic acid) nanoparticles in thermosensitive gels for inner ear disease treatment. Drug Deliv. 2021;28(1):2268–2277. doi:10.1080/10717544.2021.1992041
15. Cai H, Wen X, Wen L, et al. Enhanced local bioavailability of single or compound drugs delivery to the inner ear through application of PLGA nanoparticles via round window administration. Int J Nanomedicine. 2014;9:5591–5601. doi:10.2147/IJN.S72555
16. Sharma S, Parmar A, Kori S, Sandhir R. PLGA-based nanoparticles: a new paradigm in biomedical applications. Trends Anal Chem. 2016;80:30–40. doi:10.1016/j.trac.2015.06.014
17. Hedayati M, Abubaker-Sharif B, Khattab M, et al. An optimised spectrophotometric assay for convenient and accurate quantitation of intracellular iron from iron oxide nanoparticles. Int J Hyperthermia. 2018;34(4):373–381. doi:10.1080/02656736.2017.1354403
18. Wangemann P. Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol. 2006;576(Pt 1):11–21. doi:10.1113/jphysiol.2006.112888
19. Park JE, Lee SH, Park DJ, Seo YJ, Kim SK. In vitro time-lapse live-cell imaging to explore cell migration toward the organ of corti. J Vis Exp. 2020;166:e61947.
20. Ju HM, Lee SH, Kong TH, Kwon SH, Choi JS, Seo YJ. Usefulness of intravital multiphoton microscopy in visualizing study of mouse cochlea and volume changes in the scala media. Front Neurol. 2017;8:332. doi:10.3389/fneur.2017.00332
21. Lee JH, Oh SH, Kim TH, Go YY, Song JJ. Anti-apoptotic effect of dexamethasone in an ototoxicity model. Biomater Res. 2017;21:4. doi:10.1186/s40824-017-0090-x
22. Aarnisalo AA, Aarnisalo P, Pietola L, Wahlfors J, Jero J. Efficacy of gene transfer through the round window membrane: an in vitro model. ORL J Otorhinolaryngol Relat Spec. 2006;68(4):220–227. doi:10.1159/000092123
23. Hammill TL, Campbell KC. Protection for medication-induced hearing loss: the state of the science. Int J Audiol. 2018;57(Suppl 4):S67–s75. doi:10.1080/14992027.2018.1455114
24. Kil J, Harruff EE, Longenecker RJ. Development of ebselen for the treatment of sensorineural hearing loss and tinnitus. Hear Res. 2022;413:108209. doi:10.1016/j.heares.2021.108209
25. Marshak T, Steiner M, Kaminer M, Levy L, Shupak A. Prevention of cisplatin-induced hearing loss by intratympanic dexamethasone: a randomized controlled study. Otolaryngol Head Neck Surg. 2014;150(6):983–990. doi:10.1177/0194599814524894
26. Barrefelt Å, Saghafian M, Kuiper R, et al. Biodistribution, kinetics, and biological fate of SPION microbubbles in the rat. Int J Nanomedicine. 2013;8:3241–3254. doi:10.2147/IJN.S49948
27. Briley-Saebo KC, Johansson LO, Hustvedt SO, et al. Clearance of iron oxide particles in rat liver: effect of hydrated particle size and coating material on liver metabolism. Invest Radiol. 2006;41(7):560–571. doi:10.1097/01.rli.0000221321.90261.09
28. Wazen JM, Stevens JP, Watanabe H, Kysar JW, Lalwani AK. Silver/silver chloride microneedles can detect penetration through the round window membrane. J Biomed Mater Res B Appl Biomater. 2017;105(2):307–311. doi:10.1002/jbm.b.33557
29. Rençber S, Aydın Köse F, Karavana SY. Dexamethasone loaded PLGA nanoparticles for potential local treatment of oral precancerous lesions. Pharm Dev Technol. 2020;25(2):149–158. doi:10.1080/10837450.2019.1673407
30. Du X, Chen K, Kuriyavar S, et al. Magnetic targeted delivery of dexamethasone acetate across the round window membrane in Guinea pigs. Otol Neurotol. 2013;34(1):41–47. doi:10.1097/MAO.0b013e318277a40e
31. Wu J, Ye J, Kong W, Zhang S, Zheng Y. Programmed cell death pathways in hearing loss: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2020;53(11):e12915. doi:10.1111/cpr.12915
32. Jiang H, Sha SH, Schacht J. NF-kappaB pathway protects cochlear hair cells from aminoglycoside-induced ototoxicity. J Neurosci Res. 2005;79(5):644–651. doi:10.1002/jnr.20392
33. Mittal R, Pena SA, Zhu A, et al. Nanoparticle-based drug delivery in the inner ear: current challenges, limitations and opportunities. Artif Cells Nanomed Biotechnol. 2019;47(1):1312–1320. doi:10.1080/21691401.2019.1573182
34. Dinh CT, Haake S, Chen S, et al. Dexamethasone protects organ of corti explants against tumor necrosis factor-alpha-induced loss of auditory hair cells and alters the expression levels of apoptosis-related genes. Neuroscience. 2008;157(2):405–413. doi:10.1016/j.neuroscience.2008.09.012
35. Kenyon NJ, Bratt JM, Lee J, et al. Self-assembling nanoparticles containing dexamethasone as a novel therapy in allergic airways inflammation. PLoS One. 2013;8(10):e77730. doi:10.1371/journal.pone.0077730
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.