Back to Journals » Journal of Inflammation Research » Volume 14
Proprotein Convertase Subtilisin/Kexin Type 9 and Systemic Inflammatory Biomarker Pentraxin 3 for Risk Stratification Among STEMI Patients Undergoing Primary PCI
Authors Zhao X, Song L, Wang Y, Li J, Zhou J, Chen R , Liu C, Zhou P, Sheng Z, Chen Y, Zhao H, Yan H
Received 1 September 2021
Accepted for publication 7 October 2021
Published 14 October 2021 Volume 2021:14 Pages 5319—5335
DOI https://doi.org/10.2147/JIR.S334246
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Xiaoxiao Zhao,1,* Li Song,1,* Ying Wang,1,* Jiannan Li,1 Jinying Zhou,1 Runzhen Chen,1 Chen Liu,1 Peng Zhou,1 Zhaoxue Sheng,1 Yi Chen,1 Hanjun Zhao,1 Hongbing Yan2
1Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Peking Union Medical College & Chinese Academy of Medical Sciences, BeiJing, People’s Republic of China; 2Department of Cardiology, Fuwai Hospital Chinese Academy of Medical Sciences, ShenZhen, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongbing Yan
Fuwai Hospital, Chinese Academy of Medical Sciences, 12 Langshan Road, Shenzhen, 518000, People’s Republic of China
Tel +86-13701339287
Email [email protected]
Hanjun Zhao
Department of Cardiology, Fuwai Hospital, Chinese Academy of Medical Sciences, No. 167, Beijing, 100037, People’s Republic of China
Tel +86-15210020808
Email [email protected]
Background and Aim: The aim of prospective study was to determine the prognostic value of combined measures of plasma proprotein convertase subtilisin/kexin type 9 (PCSK9) and pentraxin 3 (PTX3) according to the culprit-plaque morphology (plaque rupture versus plaque erosion) in relation to the in patients with acute ST-elevated myocardial infarction (STEMI) who underwent primary percutaneous coronary intervention.
Methods: A total of 434 patients with STEMI aged ≥ 18 years who underwent pre-intervention OCT imaging of culprit lesions between March 2017 and March 2019 were enrolled. Finally, 235 patients who meet the inclusion criteria were enrolled and the cohort was divided into 3 groups according to PCSK9 and PTX3 levels: group A: PCSK9 < median and Pentraxin 3 (N = 72/30.6%); group B: PCSK9 ≥ median or Pentraxin 3≥ median (N = 91/38.7%); group C: PCSK9 ≥ median and Pentraxin 3≥ median (N = 72/30.6%). MACEs were defined as a composite of all-cause death, myocardial infarction (MI) recurrence, and ischemic stroke, revascularization and heart failure.
Outcomes: During a median follow-up of 2.01 years, 50 patients has occurred MACE. Two-year MACE was higher in group C (23/31.9%) than in group B (16/17.6%) and group A (11/15.3%) (p = 0.028). There was a correlation between PCSK9 and PTX3 (r = 0.302, p < 0.003). In multivariable analysis adjusted for age, gender, risk factors, and serum indexes, being in group C remained independently associated with an increased risk of MACE (hazard ratio [HR]: 2.90; p = 0.010), and group B tended to have higher MACE (HR: 1.76; p = 0.172) compared with group A. Among patients with plaque erosion by OCT, group C was independently associated with an increased risk of MACE (HR: 9.04; p = 0.048) after fully adjustment. However, the significant association was absence among patients with plaque rupture.
Conclusion and Relevance: This study demonstrated the usefulness of combined measures of PCSK9 and PTX3 to enhance risk stratification in patients with STEMI especially among patients with plaque erosion. Patients with elevation of both PCSK9 and PTX3 had a markedly increased risk of MACE.
Keywords: pentraxin-3, PCSK9, plaque morphology characteristics, optical coherence tomography, major adverse cardiovascular events, ST-segment elevation myocardial infarction
Introduction
Despite the advances in contemporary therapies, cardiovascular atherosclerotic disease remains the leading cause of morbidity and mortality worldwide. Inflammation is a significant process in atherosclerosis, leading to plaque rupture and acute coronary syndrome. Pro-protein convertase subtilisin/kexin type 9 (PCSK9) directly or indirectly contribute to atherosclerosis.1 It induces the expression of secretion of inflammatory cytokines, endothelial cell apoptosis, inhibition of platelet activation and chemoattractants, thereby increasing inflammatory cell adhesion, and inflammation at the atherosclerotic vascular wall.2 Furthermore, PCSK9 levels are considered causal in the development of atherosclerotic cardiovascular disease (ASCVD).3–5 Pentraxin-3 (PTX3), produced mainly by dendritic cells, macrophages, and endothelial cells in response to primary inflammatory stimuli1, was involved in the pathogenesis of atherosclerosis and influenced clinical outcomes.6–8 Optical coherence tomography (OCT), a cross-sectional and high-resolution intravascular imaging technique, allows the acquisition of detailed in vivo images of coronary plaque morphology characteristics, including plaque rupture (PR) and plaque erosion (PE) which are responsible for the majority of acute coronary events.9–11 PCSK9 inhibitor has been reported that it could reduce plaque lipid content and inflammatory index of lipid-core.12–14 Furthermore, PTX3 was proved to be a useful inflammatory marker to reflect the vulnerability of the plaque.15 However, the clinical significance and incremental prognostic value of PCSK9 and PTX3 in the context of coronary plaque morphology characteristics are presently unknown. The aim of prospective study was to determine the prognostic value of combined measures of PCSK9 and PTX3 according to the culprit-plaque morphology (plaque rupture versus plaque erosion) in relation to the in patients with acute ST-elevated myocardial infarction (STEMI) who underwent primary percutaneous coronary intervention.
Methods
Study Population
The Optical Coherence Tomography Examination in Acute Myocardial Infarction registry (OCTAMI) consecutive enrolled 434 STEMI patients hospitalized from March 2017 to March 2019 at Peking Union Medical College Fuwai Hospital. Finally, 235 patients who meet the inclusion criteria were enrolled. In terms of the principles of Declaration of Helsinki, the protocol was conducted and was given permission by the Ethics Committee of Fuwai Hospital. Personal information of the enrolled patients were not given away by concealing them. Patients have provided written informed consent specific to the OCT study (clinical trials.gov: NCT03593928).
The exclusion criteria of conducting OCT examinations were contraindication to aspirin or ticagrelor cardiac shock, allergy to contrast media, serious liver dysfunction, severe renal and hepatic insufficiency and lesions with characteristics that raised the difficulty and risk of performing OCT including left main coronary artery diseases, chronic total occlusion and heavily calcified vessels. The definition of STEMI followed the established criteria published previously.16 There were only 17 cases of calcified nodules. Therefore, we excluded the cases of nodular calcification in the study in order to avoid the bias.
Primary Stenting and Antiplatelet Therapy
Emergent coronary angiography and procedures of primary PCI were conducted through radial access routinely. Primary PCI (including stenting/balloon dilatation/thrombus aspiration) was conducted using the standard criteria. During period of periprocedural anticoagulant therapy, bivalirudin or heparin (100 IU/kg) was routinely administered. Glycoprotein IIb/IIIa inhibitors were used at the discretion of the physicians and stents commercially available were used. According to Thrombolysis in Myocardial Infarction (TIMI) grading system, the blood flow of infarct-related artery was evaluated. The relevant devices and certain PCI techniques including stenting, balloon dilation, intra-aortic balloon pump distal protection device, excimer laser coronary angioplasty and thrombus aspiration were at the discretion by operators. Patients undergone primary PCI were moved in the coronary care unit subsequently. The dual antiplatelet therapy following primary PCI consisted of oral aspirin and a P2Y12 inhibitor for at least 12 months.
OCT Image Acquisition
The imagines of intravascular OCT were performed in accordance with the described methods previously.8 In summary, OCT images of culprit lesions were obtained by a catheter (Dragonfly™; LightLab Imaging, Inc., Westford, MA, USA) and a frequency-domain OCT system (ILUMIEN OPTIS™; St. Jude Medical/Abbott, St. Paul, MN, USA).
Quantitative Analysis of OCT Image
Three independent observers (JN.L., RZ.C., ZX. S.) who blinded to the basic clinical characteristics and angiographic presentations performed the OCT image analysis by the offline review workstation (Ilumien Optis, St Jude Medical). Since there existed disagreements and divergences of views among observers on the results of OCT, it is necessary to have further in-depth discussions of this issue and resolve them by consensus. Based on the established criteria,17–20 thin-cap fibroatheroma, lipid-rich plaque, lipid arc, macrophage infiltration, calcification, micro-vessels, white thrombi plaque rupture, plaque erosions and cholesterol crystals were defined (Figure 1).
Laboratory Tests
Venous blood samples were collected into a tube containing ethylene diamine tetraacetate (EDTA) before PCI and were centrifuged at 2000 × g for 15min at room temperature and the plasma was frozen for PTX3 and stored at −80°C until analysis. ELISA tests were performed on the instrumental platform of MultiSkan MK3 (Thermo). We have conducted a more detailed protocol and deposited it as supplement data. Measurement of circulating PCSK9 levels Circulating levels of PCSK9 were measured using enzyme-linked immunosorbent assay (DY3888; R&D Systems, Catalog) according to the manufacturer’s instructions and compared with purified human PCSK9 standards. Circulating levels of PTX3 were determined by enzyme-linked immunosorbent assay (ELISA; R&D systems, USA).
Measurements
The clinical demographics such as age, gender, history of disease (including chronic kidney disease, hypertension, diabetes mellitus and hyperlipidemia), smoking status, primary PCI procedures, and medical treatments, were obtained from the electronic records of hospital.
Endpoints and Follow-Up
The composite of myocardial infarction recurrence, all-cause mortality, cardiac death, ischemic stroke, heart failure and revascularization were defined as MACEs. Well-trained physicians conducted the follow-up via records of hospital discharge and clinical notes by cellphone and direct interviews. The protocol of follow-up has been approved from the Institutional Review Board of Chinese Academy of Medical Sciences Fuwai Hospital. Well-trained physicians in charge of the follow-up endpoints, including all cause death, all-cause death, MI recurrence, revascularization, heart failure and ischemic stroke. They identified and extracted the primary endpoints from hospital records, medical records, emergency records, laboratory reports, and clinical notes which required to be sent to coronary disease centers. More than two professional physicians who were blinded to the clinical, angiographic and laboratory data confirmed the clinical endpoints.
Statistical Analysis
The continuous variables and categorical data are presented as the median (25th and 75th percentiles) and numbers (percentages). Mann–Whitney U-test and independent sample t-test were used to analysis the difference of groups for non-normally and normally distributed data respectively among continuous data. Categorical data were compared by Pearson’s chi-squared (χ2) test or Fisher’s exact test, as appropriate. In order to assess the correlation of PCSK9 and PTX3 index with plaque morphology characteristics, multivariable cox regression with adjustments for confounding variables were conducted. Kaplan–Meier survival curves were performed to assess the incidence rate of MACEs and Log rank test was used to compare the discrepancy rates of cumulative events. Adjustments for confounding factors were made for variables including sex, gender, status of smoking, ejection fraction, hypertension, diabetes mellitus, hyperlipidemia, creatine kinase, heart rate, C-reactive protein, low-density lipoprotein and lipase activator. The areas under the receiver operating characteristic curve (ROC), sensitivity, specificity, Youden index and 95% confidence interval (CI) were calculated to evaluate the predictive ability of PCSK9 combined with PTX3 for MACEs.
Statistical significance was set at P < 0.05, and all p values were from a 2-sided test. Statistical analysis was performed using R Programming Language X64 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria), SPSS (version 20.0; IBM Corp., Armonk, NY, USA) software and MedCalc version 18.2.1 (MedCalc Software, Ostend, Belgium).
Results
Comparison of Baseline Characteristics According to PCSK9 and PTX3 Level
A total of 235 patients who met the inclusion criteria were enrolled. A flow diagram illustrating the study sample selection process is shown in Figure 2. Table 1 describes the baseline clinical and biochemical characteristics of 235 participants stratified according to achieved PCSK9 levels and PTX3 at baseline (group A: PCSK9 <median and Pentraxin 3; group B: PCSK9 ≥ median or Pentraxin 3≥ median; group C: PCSK9 ≥ median and Pentraxin 3≥ median). Among the 235 patients included in this study (mean age: IQR 58 (50, 67) years; 80.9% men), 146 (62.1%) had hypertension and 70 (29.8%) had diabetes. Eighty-seven percent of patients were hyperlipidemia, 138 (58.7%) were in the status of current smoking, and 20 (8.5%) had a history of PCI. Incidence of MACE was 21.3%, mortality was 1.7%, MI recurrence was 3.8% and ischemic stroke was 3.8%. Thirty-two patients (13.6%) underwent revascularization after discharge and 1.7% experience heart failure. There were 72 patients (30.6%) in group A, 91 patients (38.7%) in group B, and 71 patients (30.6%) in group C. Patients in group C were lower prevalence of current smoking (p = 0.003) and the incidence of MACE (p = 0.028) and re-vascularization (p = 0.012) were more frequently compared with those in the 2 other groups (Table 1).
 |
Table 1 Baseline Clinical and Procedure Characteristics of the Study Population Divided by PCSK9 and PTX3 |
In order to prove the prognostic of PCSK9 value alone, we conducted the ROC for predicting MACEs to evaluate the diagnostic value of PCSK9 after fully adjustment (including gender, age, ejective fraction, basic syntax score, residual syntax score, hypertension, hyperlipidemia, diabetes mellitus, history of myocardial infarction, history of PCI, height, weight, heart rate, systolic blood pressure, diastolic blood pressure, killip classification, the use of aspirin and statin, baseline and peak level of TnI, baseline and peak level of BNP, hs-CRP, white blood cell, hemoglobin, platelet, creatinine, fasting glucose, hemoglobin A1c, total cholesterol, triglyceride, low-lipid protein and TyG index). The area under the ROC curve of PCSK9 was 0.887 (95% CI, 0.829–0.931). The Specificity is 92.91% and the Sensitivity is 69.44%. The results indicated that inflammatory biomarkers of PCSK9 could predict MACE with prognostic value (Supplement Figure 1). Correlation between LDL-chol and PCSK9 values among patients with primary PCI was conducted in Supplement Table 1. The results showed that there are no correlations between the LDL-chol and PCSK9 values among our cohort (p = 0.806).
Relationship Between PCSK9 and PTX3 and Characteristics of Plaque
There was a correlation between PCSK9 and PTX3 (r = 0.302, p < 0.003) (Figure 3). The proportion of patients with PTX3 level ≥median was 61.02% in the subset of patients with PCSK9 ≥median and 38.46% in the subset with a PCSK9 <median. PCSK9 correlated with characteristics of plaque by OCT including calcification (p = 0.033), arc of calcification (p = 0.005), micro-calcification (p = 0.005) and cholesterol crystal (p = 0.047) (Table 2). Correlation between biomarkers reflecting myocardial damage and PCSK9 and PTX3 among enrolled patients has been shown in Table 3. Table 3 has indicated that peak level of troponin was correlated with Circulation PCSK9 value (p = 0.0146). We divided the cohort by micro-structural OCT features of culprit lesions (plaque rupture and plaque erosion). Circulation PCSK9 value was significantly associated with calcification arc (p = 0.014) and micro-calcification (p = 0.021) among patients with plaque rupture. Among patients with plaque erosion, PCSK9 was correlated with thrombus characteristics of culprit lesions. Furthermore, PTX3 correlated with parameters of healing plaque (p = 0.004) and minimal lumen area (p = 0.012) among all enrolled cohort. Among group of plaque erosion, PTX3 correlated with fibrous plaque (p = 0.009) and healing plaque (p = 0.010). However, PTX3 was significantly associated with minimal lumen area merely (p = 0.001) among patients with plaque rupture.
 |
Table 2 Correlation Between Blood Biomarkers and OCT Characteristics Among Patients with Plaque Rupture or Plaque Erosion |
 |
Table 3 Correlation Between Biomarkers Reflecting Myocardial Damage and PCSK9 and PTX3 Among Enrolled Patients |
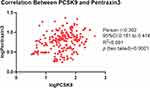 |
Figure 3 Correlation between PCSK9 and pentraxin 3. Table 4 revealed the Impact of PCSK9 and PTX3 on endpoints among all enrolled cohort by univariable and Multivariable Analyses. Table 5 revealed the Impact of PCSK9 and PTX3 on Major adverse cardiovascular events among plaque rupture and plaque erosion cohort by univariable and Multivariable Analyses. Abbreviations: Adj., adjusted; MACE, major adverse cardiovascular events; PCSK9, proprotein convertase subtilisin/kexin type 9; PTX3, pentraxin 3; REF, reference. Notes: Data presented are HRs and 95% CI. Adjust I model adjusts for sex and age; Adjust II model adjusts for adjust I plus ejection fraction, smoke, hypertension, hyperlipidemia, diabetes mellitus; Adjust III model adjusts for adjust II + creatine kinase, heart rate, low-density lipoprotein; lipase activator and C-reactive protein. *P < 0.05. |
Outcomes According to PCSK9 and PTX3 Plasma Level
During a median follow-up of 2.01 years (interquartile range: 1.96 to 2.96 years), 50 patients occurred MACE. Kaplan–Meier curves were generated for the cumulative incidence of MACEs for up to a median of 2.01 years stratified by PCSK9 and PTX3 among the PR and PE subgroups. The 2-year MACE rate was higher (p = 0.0075) in group C (23/31.9%) than in group B (16/17.6%) and group A (11/15.3%) in all enrolled cohort (Figure 4A). Notably, among patients with plaque erosion, there were significant differences among the group with PCSK9 ≥ median and Pentraxin 3≥ median (p = 0.028) (Figure 4B). However, the difference was not significant among the patients with plaque rupture (p = 0.170) (Figure 4C).
We tried to prove the prognostic function of combined PCSK9 and PTX3 values for cardiac events are as follows. Firstly, the prognostic value of PCSK9 combined with PTX3 is higher than PCSK9 values alone. We conducted the area under the receiver operating characteristic curve (ROC) for predicting MACEs to evaluate the diagnostic value of PCSK9 combined with PTX3 after fully adjustment (adjusted factors including gender, age, hypertension, hyperlipidemia, diabetes mellitus, the use of statin, hs-CRP, triglyceride, low-lipid protein). The area under the ROC curve of PCSK9 and PCSK9 combined with PTX3 after fully adjustment was 0.730 (95% CI, 0.650–0.770) and 0.732 (95% CI, 0.671–0.778). The results indicated that the combination of two inflammatory biomarkers could predict MACE with more prognostic value compared with PCSK9 or PTX3 values alone (Figure 5).
Tables 4 and 5 show the crude and adjusted multivariable relationships between MACEs stratified according to PCSK9 and PTX3 index with PR and PE among the subgroups (Tables 4 and 5, respectively). In univariable analysis, group C was associated with a 2.71-fold increase (p = 0.007) and group B with a 1.28-fold increase (p = 0.531) in MACEs compared with group A (Tables 4 and 5) among all enrolled cohort (p for trend = 0.005). After adjustments for several variables in different models, including sex, age, ejection fraction, smoke, hypertension, hyperlipidemia, diabetes mellitus, creatine kinase, heart rate, C-reactive protein, low-density lipoprotein and lipase activator, group C remained independently associated with increased risk of MACE (HR, 2.90; 95% CI, 1.29–6.25; P = 0.010), whereas there was a trend for an association between group B and increased MACE compared with group A (HR, 1.76; 95% CI, 0.78–3.94; P = 0.172) (p for trend=0.010) (Table 4). Notably, the effect of elevation of PCSK9 and PTX3 on MACEs was similar in the fully adjusted Cox regression models among patients with plaque erosion (HR: 9.04; 95% CI = 1.02 to 79.83; p = 0.048). However, the significant association was absent among patients with plaque rupture (Table 5).
 |
Table 4 Univariable and Multivariable Analyses of the Impact of PCSK9 and PTX3 on Endpoints Among All Enrolled Cohort |
 |
Table 5 Univariable and Multivariable Analyses of the Impact of PCSK9 and PTX3 on Major Adverse Cardiovascular Events Among Plaque Rupture and Plaque Erosion Cohort |
Disscussion
Main Finding
To the best of our knowledge, the present study is the first study to explore the prognostic value of combined measures of PCSK9 and PTX3 according to the culprit-plaque morphology (PR versus PE) in relation to the in patients with STEMI who underwent primary PCI via OCT. The main findings of this study can be summarized as 8 follows: The main findings of the present study were as follows: 1) measurement of PCSK9 plasma levels provided incremental prognostic information beyond that obtained by PTX3; and 2) patients with elevation of both PCSK9 and PTX3 had a markedly increased risk of MACEs compared with those without elevation of these 2 biomarkers.
PCSK9 and PTX3 and Cardiovascular Risk
The previous study has tried to make the combination of PCSK9 and PTX3 among patients with severe sepsis or septic shock. However, the effects on the patients with acute coronary syndrome are remained unknown. In the sub-analysis of the ALBIOS trial, Vecchie21 et al has investigated the correlations between levels of PCSK9 and PTX3 among patients with severe sepsis or septic shock with ranked Spearman’s coefficients and found that PCSK9 correlated positively with PTX3 at the three time-points. They found that patients with septic shock presenting with lower plasma PCSK9 levels experienced higher mortality rate.21 PTX3, an acute-phase reactant22 belonging to the CRP family (pentraxins), has been evaluated as a biomarker of disease severity and outcomes.23–25 Rannikko et al26 have observed that there is a significant positive correlation between PCSK9 and CRP levels. The expression of PCSK9 is stimulated by mediators of inflammatory27 and the elevated level of PCSK9 is correlated with remarkable systemic inflammation. The positive correlation between PCSK9 and PTX3 has been confirmed by previous study.21
PCSK9, through promoting lysosome degradation of hepatic low-density lipoprotein (LDL) receptor to decrease the clearance of plasma LDLs, is now identified as a major player in hypercholesterolaemia and atherosclerosis pathophysiology.28 Besides the hepatocyte LDL-receptor, PCSK9 can regulate LDL-receptor in other cells, including macrophages, lymphocytes, vascular smooth muscle cells and endothelial cells.29 PTX3, as a significant symbol of systemic inflammation, has been described to be associated with endothelial dysfunction in different cardiovascular disorders and correlated with increased risk of MACEs which widely established in previous studies in line with our results.30,31 Marked as an innate immunity protein, large amounts of circulating PTX3 increase during inflammation to affect the cardiovascular system.32 The present study showed that patients with elevation of circulating PCSK9 and PTX3 has been exposure higher incidence of MACE compared with those with none or only 1 of these 2 biomarkers elevated. Furthermore, the association between elevation of these blood biomarkers and MCAEs persisted after adjustment for these factors. The present study has showed that hsCRP values were higher in group B and C than in group A. The inflammatory index of hs-CRP represents a major biomarker of inflammation and associated risk in cardiovascular disease. The levels of hs-CRP identify groups of patients with higher risk of cardiovascular disease achieving better identify groups of prevention in response to PCSK9 inhibition. In the study of FOURIER, there was a stepwise risk increment by level of hs-CRP even in patients with extremely low levels of LDL-C.33 The correlation of elevated CRP levels and cardiovascular disease has been well established. Furthermore, the effect of lacking of CRP-lowering does not bring doubts the clinical benefit of PCSK9 inhibitors which have been shown to reduce cardiovascular outcomes.34,35 It would be of paramount significant to elucidate the role of PCSK9 beyond hs-CRP modulation and pathways connecting PCSK9 to vascular inflammation. There are evident to revealed that PCSK9 is expressed in atherosclerotic plaques36 and small interfering RNA-mediated knockdown of PCSK9 in human macrophages has been found to reduce inhibitor of nuclear factor kappa B-α degradation, thereby reducing the expression of pro-inflammatory genes.37 In order to regulate related inflammation together with the complement system, the functions of CRP and PTX3 which are deeply involved in the pathogenesis of cardiovascular disease linked to inflammation are to coordinate spatially and temporally targeted clearance of injured tissue components.38 It has been proved that circulating elevated CRP and PTX3 levels could provide prognostic information for a variety of clinical diagnosis of cardiovascular disease.39
In the present study, there was only a strong correlation between PCSK9 and PTX3, and these 2 biomarkers were complementary in terms of prognostication. Approximately one-half of the patients with PCSK9 level ≥median had elevation of PTX3. In contrast, approximately one-quarter of patients with PCSK9 <median had PTX3 level ≥median. These findings might be explained by the fact that PCSK9 and PTX3 are activated by different mechanisms in response to various pathophysiological pathways and/or stimuli. There is accumulating evidence indicating that PCSK9 could increase vascular inflammation locally and contribute to atherosclerotic plaque progression among hyper-cholesterolaemia cohort.40 Among several previously published epidemiological studies, the connection between PCSK9 and key inflammation markers (including white blood cells, fibrinogen, and hs-CRP) has been investigated.41 In a single-centre study of CAD patients who were not on lipid-lowering therapy in China, plasma levels of PCSK9 were positively and independently associated with white blood cell count subsets, lymphocyte count and neutrophil count.42 A cross-sectional study has verified that the circulating levels of PCSK9 were positively associated with those of fibrinogen among stable CAD and the interaction was suggested to be one of the underlying mechanisms involved in the progression of atherosclerosis.43
PCSK9 and PTX3 and Plaque Morphology Characteristics by OCT
The most striking finding in the current study was the prognostic role of the PCSK9 combined with PTX3 among patients with plaque rupture or plaque erosion. After adjustment for confounding factors, the incidence of MACEs was significantly higher among participants in the group with PCSK9 ≥ median and Pentraxin 3≥ median in the presence of PE. However, this tendency was absent in patients with PE. Rupture and erosion of the coronary culprit plaques are the primary cause of acute coronary disease.44 Previous studies comparing the influence of PE and PR in acute MI demonstrated that despite similar clinical characteristics, patients with PE tended to present high-risk clinical characteristics, more complex angiographic features and high-risk plaque features, such as larger infarct sizes,45 more multi-vessel lesions,46 large lipids core and inflammation activity.47 Nevertheless, the current study failed to prove the independent prognostic role of PR, though the incidence of MACE among group C was associated with a 2.4-fold increase among patients with plaque rupture. The discrepancy might be caused by insufficient follow-up time or small sample size. In addition, a small-size study of acute coronary syndrome cohort from Hu et al was in line with our results.48 Due to the inconsistency on cardiovascular effect of plaque rupture, it is warranted to conduct larger cohort studies to confirm the results.
Due to the lack of clinical and basic evidence, further studies are needed to confirm that PCSK9 may reflect thrombus type such as red, white or mixed thrombus or thrombus volume such as large or small number of thrombus. The possible mechanism can be explained as follows. The previous researches29,49 have demonstrated that higher level of PCSK9 could lead to the inflammation of atherosclerosis by increasing infiltration of Ly6 monocytes in the plaque which supports the notion that PCSK9 inhibitors may improve cardiovascular outcomes through lipid independent and lipid-dependent pathways. The erosions of plaque trigger atherothrombosis by exposing thrombogenic material which inside the plaque, including matrix molecules, tissue factor, phospholipids and coagulation factors.50 Atherothrombi can expand and fill the lumen rapidly which leading to infarction, ischemia and atherothrombosis.42 The plaque with fibrous cap fracture more likely to have a larger lipid core and procoagulant tissue was localized among these cores.43 Plaque erosion, another substrate for thrombus formation, shows less inflammatory cell accumulation which indicates exposed plaque constituents induce local hemorheology, activation systemic clotting activity, increased fibrinolytic function and sensitivity of the end organ to ischemia.41 Therefore, pathogenetic mechanisms are different between these two types of plaque and should be considered separately. Disturbance of the balance between fibrinolysis activity and prothrombotic plays a significant role for precipitating the thrombotic event.51 However, the precise sequence of events has not been known. Previous studies52 have recognized thrombi involved on damaged vascular surfaces. Davies et al observed that ruptured plaques display large lipid core and thin fibrous caps regions. Furthermore, more studies53,54 have revealed that culprit lesions of fatal thrombi in coronary arteries contain reduced amounts of mature and increased levels of collagen-degrading enzymes. Tissue factor which expressed by vascular smooth muscle cells in the atherosclerotic plaque could initiate the blood coagulation cascade that leads to fibrin formation.55
Mechanism
By motivating the adhesion of white blood cells and platelets to the vascular wall and stimulating plaque growth, elevated plasma levels of fibrinogen could accelerate the progression of atherosclerosis and raise the mortality.51 It is worth noting that the expression of PCSK9 was increased in vascular smooth muscle cells at the sites of inflammation with low-shear stress.38,56 From the mechanistic view, NADPH oxidase dependent reactive oxygen species, generated as by-products of the metabolism of molecular oxygen, mediated the circulating PCSK9 expression.57 PCSK9 monoclonal antibodies (mAbs), including evolocumab and alirocumab which used to decrease the level of circulating PCSK9, are proven to provide substantial contributions for patients at very high risk of cardiovascular events.58 The team of Kastelein et al59 argued that ezetimibe might not share the lipid-independent effects of statins on anti-inflammatory properties and endothelial function. Previous study has demonstrated that PTX3 is strongly associated the incidence of cardiovascular and mortality among large Chinese cohort.60 PTX3/P-selectin pathway has been identified to induce the vascular changes and they are implicated in the pathogenesis of vascular diseases such as plaque rupture, atherosclerosis, aortic aneurysms and neointimal hyperplasia.61 Furthermore, previous studies has revealed that PTX3 induced an impairment in NO signaling that enhanced smooth muscle vasoconstriction and evoked endothelial dysfunction increasing vascular resistance.62,63
Our previous study64 has found that patients with PR/high-PTX3 and PE/high-PTX3 presented a poorer prognosis than those with PE/low-PTX3. Combining the culprit-plaque morphology with PTX3 enhanced the predictive ability for MACE and contributed to better identification of high-risk patients. Furthermore, this research was conducted to verify the combination of PTX3 levels with plaque morphology to identify STEMI patients and revealed that plasma PTX3 and plaque rupture added important prognostic information. The team of Koga et al15 has demonstrated that the frequency of TCFA significantly increased according to elevations in systemic PTX3 levels indicating that PTX3 is a useful biomarker to reflect coronary plaque vulnerability. What is more, higher levels of PTX3 were the most powerful predictor of TCFA in patients with stable angina pectoris and the entire study population. The typical morphology of TCFA is definite by a large necrotic core, large plaque burden, combining with thin-fibrous cap with infiltrating macrophages, and positive remodeling.65 Nebuloni et al.66 found that PTX3 localized in the interstitium of heart tissues of patients with AMI at autopsy is produced by endothelium, macrophages, to a lesser extent, myocardiocytes. Previous studies67 have reported that PTX3 plays a crucial part in the progression of atherosclerotic lesions by the soluble pattern recognition in innate immunity. Increasing levels of PTX3 has been confirmed that it could reflect a compensatory cardioprotective response to an activated inflammatory response.68 The present result is in line with previous researches that elevated PTX3 is correlated with increased risk of MACE. The current findings may suggest higher prognostic value of PTX3 compared with PCSK9 in STEMI lesions with plaque erosion. In large cohort healthy adults from the Cardiovascular Health Study,60 the results showed a strong positive association between level of PTX3 and incident all-cause death and cardiovascular disease.
Study Limitations
The present study has some limitations which must be given consideration. First, the use of a single baseline PCSK9 and PTX3 measurement which absence of evaluating effects of longitudinal changes during the follow-up period to predict outcomes did not allow us to assess the causal risk for incident cardiovascular endpoints. Therefore, further studies are warranted to clarify the mechanisms underlying the association between PCSK9 levels and PTX3. Thirdly, it is unclear whether our findings in STEMI individuals in China can be generalized to other diseases or other ethnicities and further prospective investigations among diverse populations, larger sample sizes, and studies with long-term follow-up should be undertaken.
Conclusion
Present study indicated that the combined measurement of PCSK9/PTX3 combined with morphology characteristics might add prognostic information and contribute to improve risk stratification among patients with STEMI. Patients with elevations of both PCSK9 and PTX3 have at least a 2.9-fold increased risk of MACE among all enrolled cohort and 9.04-fold increased risk of MACE among patients with plaque erosion. Further studies with a larger number of patients are needed to confirm these results.
Sponsor Role
This study was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2016-I2M-1–009), National Natural Science Funds (number: 81970308), the Fund of “Sanming” Project of Medicine in Shenzhen (number: SZSM201911017) and Shenzhen Key Medical Discipline Construction Fund (number: SZXK001).
Data Sharing Statement
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
It is from the ethics committee of the department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Peking Union Medical College, China.
Consent for Publication
Written informed consent for publication was obtained from all participants.
Acknowledgments
The authors gratefully acknowledge all individuals who participated in this study.
Disclosure
No conflicts of interest, financial or otherwise, are declared by the authors.
References
1. Tang Y, Li SL, Hu JH, et al. Research progress on alternative non-classical mechanisms of PCSK9 in atherosclerosis in patients with and without diabetes. Cardiovasc Diabetol. 2020;19(1):33. doi:10.1186/s12933-020-01009-4
2. Ferri N, Marchianò S, Tibolla G, et al. PCSK9 knock-out mice are protected from neoin- timal formation in response to perivascular carotid collar placement. Atherosclerosis. 2016;253:214–224. doi:10.1016/j.atherosclerosis.2016.07.910
3. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722. doi:10.1056/NEJMoa1615664
4. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–2107. doi:10.1056/NEJMoa1801174
5. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5(12):941–950. doi:10.1016/S2213-8587(17)30313-3
6. Goodman AR, Cardozo T, Abagyan R, et al. Long pentraxins: an emerging group of proteins with diverse functions. Cytokine 4 Growth Factor Rev. 1996;7:191–202. doi:10.1016/1359-6101(96)00019-6
7. Rolph MS, Zimmer S, Bottazzi B, et al. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:e10–4. doi:10.1161/01.ATV.0000015595.95497.2F
8. Kimura S, Sugiyama T, Hishikari K, et al. Relationship of systemic pentraxin-3 values with coronary plaque components on optical coherence tomography and post-percutaneous coronary intervention outcomes in patients with stable angina pectoris. Atherosclerosis. 2020;292:127–135. doi:10.1016/j.atherosclerosis.2019.11.022
9. Uetani T, Amano T, Harada K, et al. Impact of insulin resistance on post-procedural myocardial injury and clinical outcomes in patients who underwent elective coro- nary interventions with drug-eluting stents. JACC Cardiovasc Interv. 2012;5(11):1159–1167. doi:10.1016/j.jcin.2012.07.008
10. Fiorentino TV, Marini MA, Succurro E, et al. Relationships of surrogate indexes of insulin resistance with insulin sensitivity assessed by euglycemic hyperinsulinemic clamp and subclinical vascular damage. BMJ Open Diabetes Res Care. 2019;7(1):e911. doi:10.1136/bmjdrc-2019-000911
11. Du T, Yuan G, Zhang M, et al. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13:146. doi:10.1186/s12933-014-0146-3
12. Yurtseven E, Ural D, Baysal K, Tokgözoğlu L. An update on the role of PCSK9 in atherosclerosis. J Atheroscler Thromb. 2020;27(9):909–918. doi:10.5551/jat.55400
13. Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on coronary plaque composition. J Am Coll Cardiol. 2018;72(17):2012–2021. doi:10.1016/j.jacc.2018.06.078
14. Sun J, Lepor NE, Cantón G, et al. Serial magnetic resonance imaging detects a rapid reduction in plaque lipid content under PCSK9 inhibition with alirocumab. Int J Cardiovasc Imaging. 2021;37(4):1415–1422. doi:10.1007/s10554-020-02115-w
15. Koga S, Ikeda S, Yoshida T, et al. Elevated levels of systemic pentraxin 3 are associated with thin-cap 9 fibroatheroma in coronary culprit lesions: assessment by optical coherence tomography 10 and intravascular ultrasound. JACC Cardiovasc Interv. 2013;6:945–954.
16. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177.
17. Stevens PE, Levin A; Kidney disease: improving global outcomes chronic kidney disease guideline development work group, M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi:10.7326/0003-4819-158-11-201306040-00007
18. Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59(12):1058–1072. doi:10.1016/j.jacc.2011.09.079
19. Tian J, Ren X, Vergallo R, et al. Distinct morphological features of ruptured culprit plaque for acute coronary events compared to those with silent rupture and thin-cap fibroatheroma: a combined optical coherence tomography and intravascular ultrasound study. J Am Coll Cardiol. 2014;63(21):2209–2216. doi:10.1016/j.jacc.2014.01.061
20. Prati F, Regar E, Mintz GS, et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31:401–415. doi:10.1093/eurheartj/ehp433
21. Vecchie A, Bonaventura A, Meessen J, et al. PCSK9 is associated with mortality in patients with septic shock: data from the ALBIOS study. J Intern Med. 2021;289(2):179–192. doi:10.1111/joim.13150
22. Casula M, Montecucco F, Bonaventura A, et al. Update on the role of Pentraxin 3 in atherosclerosis and cardiovascular diseases. Vascul Pharmacol. 2017;99:1–12. doi:10.1016/j.vph.2017.10.003
23. Liu S, Qu X, Liu F, et al. Pentraxin 3 as a prognostic biomarker in patients with systemic inflammation or infection. Mediators Inflamm. 2014;2014:421429. doi:10.1155/2014/421429
24. Muller B, Peri G, Doni A, et al. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med. 2001;29:1404–1407. doi:10.1097/00003246-200107000-00017
25. Bastrup-Birk S, Munthe-Fog L, Skjoedt MO, et al. Pentraxin-3 level at admission is a strong predictor of short-term mortality in a community-based hospital setting. J Intern Med. 2015;277:562–572. doi:10.1111/joim.12294
26. Rannikko J, Jacome Sanz D, Ortutay Z, et al. Reduced plasma PCSK9 response in patients with bacteraemia is associated with mortality. J Intern Med. 2019;286:553–561. doi:10.1111/joim.12946
27. Mauri T, Bellani G, Patroniti N, et al. Persisting high levels of plasma pentraxin 3 over the first days after severe sepsis and septic shock onset are associated with mortality. Intensive Care Med. 2010;36:621–629. doi:10.1007/s00134-010-1752-5
28. Lambert G, Charlton F, Rye K-A, et al. Molecular basis of PCSK9 function. Atherosclerosis. 2009;203:1–7. doi:10.1016/j.atherosclerosis.2008.06.010
29. Lagace TA. PCSK9 and LDLR degradation: regulatory mechanisms in circulation and in cells. Curr Opin Lipidol. 2014;25:387–393.
30. Norata GD, Garlanda C, Catapano AL. The long pentraxin PTX3: a modulator of the immunoinflammatory response in atherosclerosis and cardiovascular diseases. Trends Cardiovasc Med. 2010;20:35–40. doi:10.1016/j.tcm.2010.03.005
31. Parlak A, Aydoğan U, Iyisoy A, et al. Elevated pentraxin-3 levels are related to blood pressure levels in hypertensive patients: an observational study. Anadolu Kardiyol Derg. 2012;12:298–304.
32. Presta M, Camozzi M, Salvatori G, et al. Role of the soluble pattern recognition receptor PTX3 in vascular biology. J Cell Mol Med. 2007;11:723–738. doi:10.1111/j.1582-4934.2007.00061.x
33. Navarese EP, Kolodziejczak M, Schulze V, et al. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic r eview and meta-analysis. Ann Intern Med. 2015;163:40–51. doi:10.7326/M14-2957
34. Sabatine MS, Giugliano RP, Wiviott SD, et al; Open-Label Study of Long-TermEvaluation against LDL Choles terol (OSLER) Investigators. Efficacy and safety of evolocumab in r educing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–1509. doi:10.1056/NEJMoa1500858
35. Robinson JG, Farnier M, Krempf M, et al; ODYSSEY LONGTERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 372;2015:1489–1499. doi:10.1056/NEJMoa1501031
36. Ferri N, Tibolla G, Pirillo A, et al. Proprotein convertase subtilisin kexin type 9 (PCSK9) secreted by cultured smooth muscle cells reduces macrophages LDLR levels. Atherosclerosis. 2012;220:381–386. doi:10.1016/j.atherosclerosis.2011.11.026
37. Tang Z, Jiang L, Peng J, et al. PCSK9 si RNA suppresses the inflammatory response induced b yoxLDL through inhibition of NF-κB activation in THP-1-derivedmacrophages. Int J Mol Med. 2012;30:931–938. doi:10.3892/ijmm.2012.1072
38. Volanakis JE, Narkates AJ. Interaction of C-reactive protein with artificial phosphatidylcholine bilayers and complement. J Immunol. 1981;126:1820–1825.
39. Maekawa Y, Nagai T, Anzai A. Pentraxins: CRP and PTX3 and cardiovascular disease. Inflamm Allergy Drug Targets. 2011;10(4):229–235. doi:10.2174/187152811796117744
40. Momtazi-Borojeni AA, Sabouri-Rad S, Gotto AM, et al. PCSK9 and inflammation: a review of experimental and clinical evidence. Eur Heart J Cardiovasc Pharmacother. 2019;5(4):237–245. doi:10.1093/ehjcvp/pvz022
41. Li S, Zhang Y, Xu R-X, et al. Proprotein convertase subtilisin-kexin type 9 as a biomarker for the severity of coronary artery disease. Ann Med. 2015;47:386–393.
42. Zhang Y, Zhu C-G, Xu R-X, et al. Relation of circulating PCSK9 concentration to fibrinogen in patients with stable coronary artery disease. J Clin Lipidol. 2014;8:494–500.
43. Gencer B, Montecucco F, Nanchen D, et al. Prognostic value of PCSK9 levels in patients with acute coronary syndromes. Eur Heart J. 2016;37:546–553.
44. Fuster V, Badimon L, Badimon JJ, et al. The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med. 1992;326:242–250. doi:10.1056/NEJM199201233260406
45. Yahagi K, Kolodgie FD, Lutter C, et al. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2017;37:191–204. doi:10.1161/ATVBAHA.116.306256
46. Mather AN, Crean A, Abidin N, et al. Relationship of dysglycemia to acute myocardial infarct size and cardiovascular outcome as determined by cardiovascular magnetic resonance. J Cardiovasc Magn Resonan. 2010;12:61. doi:10.1186/1532-429X-12-61
47. Kim HO, Kim CJ, Kurihara O, et al. Angiographic features of patients with coronary plaque erosion. Int J Cardiol. 2019;288:12–16. doi:10.1016/j.ijcard.2019.03.039
48. Hu S, Zhu Y, Zhang Y, et al. Management and outcome of patients with acute coronary syndrome caused by plaque rupture versus plaque erosion: an Intravascular Optical Coherence Tomography Study. J Am Heart Assoc. 2017;6:004730.
49. Schulz R, Schluter KD, Laufs U. Molecular and cellular function of the proprotein convertase subtilisin/kexin type 9 (PCSK9). Basic Res Cardiol. 2015;110:4. doi:10.1007/s00395-015-0463-z
50. Li S, Guo Y-L, Xu R-X, et al. Association of plasma PCSK9 levels with white blood cell count and its subsets in patients with stable coronary artery disease. Atherosclerosis. 2014;234:441–445. doi:10.1016/j.atherosclerosis.2014.04.001
51. Pearson TA, Mensah GA, Hong Y, et al. CDC/AHA workshop on markers of inflammation and cardiovascular disease. Circulation. 2004;110:e543–e544.
52. Danesh J, Lewington S, Thompson SG, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant metaanalysis. JAMA. 2005;294:1799–1809.
53. Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32:274–278. doi:10.3109/07853890009011772
54. Zhang Y, Cliff W, Schoefl G, et al. Coronary C-reactive protein distribution: its relation to development of atherosclerosis. Atherosclerosis. 1999;145:375–379. doi:10.1016/S0021-9150(99)00105-7
55. Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140.
56. Ding Z, Liu S, Wang X, et al. Hemodynamic shear stress via ROS modulates PCSK9 expression in human vascular endothelial and smooth muscle cells and along the mouse aorta. Antioxid Redox Signal. 2015;22:760–771. doi:10.1089/ars.2014.6054
57. Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res. 2005;65:16–27. doi:10.1016/j.cardiores.2004.08.007
58. Reyes-Sofer G, Pavlyha M, Ngai C, et al. Effects of PCSK9 inhibition with alirocumab on lipoprotein metabolism in healthy humans. Circulation. 2017;135(4):352–362. doi:10.1161/CIRCULATIONAHA.116.025253
59. Kastelein JJP, Akdim F, Stroes ESG, et al; for the ENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 358;2008:1431–1443. doi:10.1056/NEJMoa0800742
60. Jenny NS, Arnold AM, Kuller LH, Tracy RP, Psaty BM. Associations of pentraxin 3 with cardiovascular disease and all-cause death: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2009;29:594–599.
61. Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. doi:10.1161/res.90.3.251
62. Volpe M, Iaccarino G, Vecchione C, et al. Association and cosegregation of stroke with impaired endothelium dependent vasorelaxation in stroke prone, spontaneously hypertensive rats. J Clin Invest. 1996;98:256–261. doi:10.1172/JCI118787
63. Peri G, Introna M, Corradi D, et al. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–641. doi:10.1161/01.CIR.102.6.636
64. Wang Y, Zhao X, Zhou P, et al. Plasma Pentraxin-3 combined with plaque characteristics predict cardiovascular risk in ST-segment elevated myocardial infarction: an Optical Coherence Tomography Study. J Inflamm Res. 2021;14:4409–4419. doi:10.2147/JIR.S330600
65. Vancraeynest D, Pasquet A, Roelants V, et al. Imaging the vulnerable plaque. J Am Coll Cardiol. 2011;57:1961. doi:10.1016/j.jacc.2011.02.018
66. Nebuloni M, Pasqualini F, Zerbi P, et al. PTX3 expression in the heart tissues of patients with myocardial infarction and infectious myocarditis. Cardiovasc Pathol. 2011;20:e27–35. doi:10.1016/j.carpath.2010.02.005
67. Savchenko A, Imamura M, Ohashi R, et al. Expression of pen-traxin 3 (PTX3) in human atherosclerotic lesions. J Pathol. 2008;215:48–55. doi:10.1002/path.2314
68. Norata GD, Marchesi P, Pulakazhi Venu VK, et al. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atheroscle-rosis. Circulation. 2009;120:699–708. doi:10.1161/CIRCULATIONAHA.108.806547
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.