Back to Journals » Journal of Inflammation Research » Volume 16
Prophylactic Treatment of Intestinal Ischemia-Reperfusion Injury Reduces Mucosal Damage and Improves Intestinal Absorption
Authors Garcia-Alonso I , Velasco-Oraa X , Cearra I , Iturrizaga Correcher S, Mar Medina C , Alonso-Varona A, García Ruiz de Gordejuela A , Ruiz-Montesinos I , Herrero de la Parte B
Received 6 July 2023
Accepted for publication 2 September 2023
Published 20 September 2023 Volume 2023:16 Pages 4141—4152
DOI https://doi.org/10.2147/JIR.S426396
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Ignacio Garcia-Alonso,1,2,* Xabier Velasco-Oraa,3,* Iñigo Cearra,1,4,5 Sira Iturrizaga Correcher,6 Carmen Mar Medina,6 Ana Alonso-Varona,7 Amador García Ruiz de Gordejuela,1,8 Inmaculada Ruiz-Montesinos,1,8 Borja Herrero de la Parte1,2,*
1Department of Surgery and Radiology and Physical Medicine, Faculty of Medicine and Nursing, University of the Basque Country UPV/EHU, Leioa, 48940, Spain; 2Interventional Radiology Research Group, Biocruces Bizkaia Health Research Institute, Barakaldo, 48903, Spain; 3Department of Anaesthesia, Hospital Clínic of Barcelona, Barcelona, 08036, Spain; 4Department of Orthopedics, Basurto University Hospital, Osakidetza Basque Health Service, Bilbao, 48013, Spain; 5Regenerative Therapies, Osteoarticular and Tendon Pathology Research Group, Biocruces Bizkaia Health Research Institute, Barakaldo, 48903, Spain; 6Department of Clinical Analyses, Galdakao-Usansolo University Hospital, Galdakao, 48960, Spain; 7Department of Cell Biology and Histology, Faculty of Medicine and Nursing, University of the Basque Country UPV/EHU, Leioa, 48940, Spain; 8Department of Gastrointestinal Surgery, Donostia University Hospital, Osakidetza Basque Health Service, Donostia, 20014, Spain
*These authors contributed equally to this work
Correspondence: Borja Herrero de la Parte, Department of Surgery and Radiology and Physical Medicine, University of The Basque Country, Barrio Sarriena s/n, Leioa, 48940, Spain, Tel +34 946015590, Email [email protected]
Purpose: Intestinal ischemia-reperfusion injury (i–IRI) involves a blood flow interruption in an intestinal segment followed by blood flow restoration. When blood flow is restored, oxidative and inflammatory molecules are distributed throughout the bloodstream, triggering both local and systemic damage. Our goal was to evaluate the potential of three antioxidant and/or anti–inflammatory compounds (curcumin, dexmedetomidine and α-tocopherol) to prevent or reverse local and systemic damage induced by i–IRI.
Methods: i-IRI was induced by placing a microvascular clip in the superior mesenteric artery of female WAG/RijHsd rats; the clip was removed after 1h and reperfusion was allowed for 4h. Curcumin (200 mg/kg, orally), α-tocopherol (20 mg/kg, i.p.), and dexmedetomidine (5 or 20 μg/kg, s.c.; DEX5 and DEX20, respectively) were administered. Blood and terminal ileum specimens were collected for biochemical and histological determination. Furthermore, D-xylose absorption test was performed to evaluate intestinal absorption; after completing the 1-hour ischemia and 4-hour reperfusion period, 1 mL of aqueous D-xylose solution (0.615 mg/mL) was administered orally, and one hour later, plasma D-xylose levels were quantified.
Results: The histological injury degree (HID) measured by the Chiu scale was significantly reduced when the treatments were applied (non-treated rats, 2.6 ± 0.75; curcumin, 1.54 ± 0.8; DEX5, 1.47 ± 0.7; DEX20 1.14 ± 0.5; and α-tocopherol, 1.01 ± 0.6); intestinal absorptive capacity also improved in all cases healthy rats (2.06 ± 0.07 μg/mL; non-treated, 1.18 ± 0.07 μg/mL; curcumin 1.76 ± 0.3 μg/mL; DEX5, 2.29 ± 0.2 μg/mL; DEX20, 2.25 ± 0.26 μg/mL; and α-tocopherol 1.66 ± 0.21 μg/mL). However, it failed to reduce liver enzyme levels. Finally, only dexmedetomidine significantly reduced urea and creatinine levels compared to non-treated animals.
Conclusion: All drugs were effective in reducing HID, although α-tocopherol was effective to a greater extent. Only dexmedetomidine reverted intestinal absorption to normal values of healthy animals.
Keywords: intestinal ischemia-reperfusion, i-IRI, female rat, α-tocopherol, curcumin, dexmedetomidine, intestinal mucosal damage, absorptive function, antioxidant therapy
Introduction
Acute mesenteric ischemia (AMI) is a medical condition defined as an abrupt decrease or interruption in intestinal blood flow, resulting in an inadequate supply of oxygen and nutrients leading to intestinal infarction.1 Under inadequate oxygen supply to the enterocyte, xanthine dehydrogenase is converted into xanthine oxidase, an enzyme which has a high formation capacity of reactive oxygen species (ROS). During reperfusion, ROS are released into the bloodstream and distributed throughout the organism, not only resulting in local injury, but also systemic damage, leading to intestinal ischemia reperfusion injury (i–IRI).2 i–IRI translates into systemic damage to other organs, especially those with a high proportion of endothelial cells, such as liver, kidney, or lung. Restoration of blood flow in the ischemic intestinal segment further exacerbates the high mortality associated with intestinal ischemia; the systemic inflammatory response and the generalized distribution of ROS alter the structure and function of multiple organs, ultimately leading to endotoxemia, systemic inflammatory response syndrome (SIRS), and even multiple organ dysfunction syndrome (MODS).3–5
According to its origin, IMA can be roughly differentiated between occlusive mesenteric ischemia (which includes arterial embolism, arterial thrombosis and mesenteric venous thrombosis) and non-occlusive mesenteric ischemia (NOMI).6,7 In spite of its low incidence, the estimated incidence of AMI ranges from 7.3 to 12 cases per 100,000 people8,9 and 5 out of every 10,000 hospital admissions,10 its mean mortality rate averages 60%, while some studies report mortality rates as high as 93%.10–12 Depending on the precipitating causes, namely hypotension during hemodialysis, hypoperfusion, cardiopulmonary bypass surgery, sepsis, low cardiac output,1,13 NOMI accounts for approximately 4% to 60% of AMI cases14,15 and its mortality is even higher, surpassing mortality rates of 80% or even reaching 100% in some case series.14,16
In 2017 and 2022, the World Society for Emergency Surgery published the guidelines for AMI management.1,17 These documents indicate that if NOMI is suspected, clinical efforts should focus on treatment of the underlying cause and rapid restoration of mesenteric flow; in addition, any infarcted intestinal sections should be resected.18 Therefore, its focus lies only on prevention of ischemic injury, reduction of time to reperfusion as short as possible, and multiorgan failure, but does not address the treatment of reperfusion syndrome after restoration of blood flow.
Only experimental studies have explored preventive treatment options for various ischemia reperfusion syndromes (IRI), both i–IRI as well as liver, lower limb, kidney or even retina.19–22 In this context, plant-derived compounds have shown great potential in the clinical setting, not only in IRI therapies, but also for oncology or rheumatic disorders.23,24 For example, curcumin and α-tocopherol, have demonstrated their benefits in cardiovascular diseases, diabetes or cancer, as well as their role as anti–inflammatory and antioxidant agents.25,26
As indicated, patients suffering i–IRI are considered to be critical and treated in Intensive Care Units. In these units, the use of sedative agents such as dexmedetomidine, a highly selective α-2 agonist, is a frequent practice.27 Dexmedetomidine, besides having a protective effect against delirium and agitation, appears to have no clinically relevant cardiac and/or respiratory effects in patients requiring intensive care.28 There is little evidence about the underlying mechanism of the protective effect of dexmedetomidine against oxidative stress, but it has been shown that it activates the nuclear factor erythroid-2-related factor 2/heme oxygenase-1 (NrF-2/HO-1) signaling pathways, protecting against oxidative stress-induced DNA damage and apoptosis.29 Because of its promptness in peaking plasma concentrations (15 minutes) and its prolonged effect (up to 4 h), is a promising agent for treating i–IRI.30 In fact, it was successfully used to prevent damage to the lungs31 and liver32 after i–IRI, and even to the intestine.33
In this piece of work, we present an experimental study conducted in female rats aimed to assess the intestinal mucosal damage, as well as the impact on the intestinal absorption rate and the systemic effect of i–IRI.
Materials and Methods
Female WAG/RijHsd rats, 3–4 months old, husbanded in the animal facilities of the University of the Basque Country, were used. Animals were kept with food and water ad libitum and under a constant temperature of 24°C and a 12:12 h light–dark cycle. Procedures were carried out in accordance with Spanish (Real Decreto 53/2013) and European Union legislation (Directive 2010/63/EU) and were approved by the Institutional Review Board of the University of the Basque Country (ref. number M20/2021/012).
Experimental Groups, Drugs and Reagents
The study has been carried out with groups of 12 rats each: (1) normal/healthy animals (control group, not subjected to ischemia), (2) ischemia w/o treatment (i–IRI group), and treated with (3) curcumin (CUR group), (4) α-tocopherol (ATF group), and dexmedetomidine at (5) low dose (DEX5 group) or at (6) normal dose (DEX20 group). Half of the animals assigned to each experimental group (6 animals) were randomly selected for histological and biochemical analyses, and the other half (6 animals) for absorptive studies.
Because of its low solubility, curcumin (C1386-5G, Merk, Darmstadt, Germany) was suspended in sesame oil at 30°C to a final concentration of 80 mg/mL. Curcumin (200 mg/kg)34 was given by oral gavage 24 and 2 hours prior to ischemia induction, by a 18 G polypropylene cannula (Instech, Plymouth Meeting, Pennsylvania, USA). The curcumin suspension was freshly made at the time of each administration.
Two doses of dexmedetomidine (Dexdor® 100 µg/mL, Orion Pharma S.L., Madrid, Spain) were also tested: low dose (5 µg/kg; DEX5 group) or normal dose (20 µg/kg; DEX20 group).33,35
To be administered, the stock vial was diluted in saline to a final concentration of 10 µg/mL (DEX5) or 40 µg/mL (DEX20) for subcutaneous injection 30 minutes prior to the onset of ischemia.
Finally, α-tocopherol vials (Ephynal® 100 mg/2mL, Bayer Hispania S.L., Sant Joan Despí, Spain) was kindly provided by the Hospital Universitario de Basurto. It was suspended in saline (4 mg/mL) for intraperitoneal (i.p.) injection (20 mg/kg) and given 2 hours prior to ischemia.36
For absorptive studies, we used the D-xylose test, first described by Eberts et al.37 D-xylose (Acros Organics, Geel, Belgium) was solved in water and administered by oral gavage through a flexible 18 G polypropylene cannula.
Intestinal Ischemia-Reperfusion Model
The i–IRI model used is widely accepted and has been used by several research groups.31–33,38 Briefly, under 1.5% (v/v) isoflurane anesthesia the superior mesenteric artery (SMA) was identified and cross-clamped with a Yasargil microclamp.
Proper placement of the microclamp was ensured by complete dissection of the connective tissue around the SMA. Atraumatic microdissection forceps and surgical head glasses with 2.5x magnification were used for this purpose; a small piece of nitrile glove was also placed behind the artery to prevent the microclamp tip from clamping tissue posterior to the SMA.
Once the Yasargil clamp was properly placed and the absence of pulse in the arteries of the small intestine was verified, the laparotomy was closed, analgesia was administered (2 mg/kg meloxicam, i.p.), and the animal was placed in a warm cage for 1 hour. Then, the animals were re-anesthetized following the same protocol and the laparotomy was reopened; the clamp removed and the laparotomy was closed with interrupted stitches. The animals were placed again in a warm cage allowing intestinal reperfusion for 4 hours.
Sample Collection, Processing and Analysis
Serum and intestinal tissue samples were extracted for the different studies. Under isoflurane anesthesia, 5 mL of blood was retrieved from the abdominal aorta; thereafter, the terminal ileum was excised and fixed in formalin; subsequently, the animal was sacrificed.
Histological damage in the intestine was assessed on hematoxylin/eosin stained 5 µm thick transversal slices of the ileum (spaced 1 cm from each other) by a skilled scientist. Each slice was divided into four quadrants, and the histological injury degree (HID) score was established in each quadrant, using Chiu’s Scale (Table 1);39 a total of four slices were evaluated for each animal. The HID of each animal was calculated as the mean of all of them, and also a comparison analysis of the mesenteric and antimesenteric HID was performed. Samples were blinded to the observer.
 |
Table 1 Chiu Scale for Intestinal Damage Assessment |
Serum samples were analyzed in a Cobas® 80 00 modular clinical analyzer, equipped with a Cobas c702 module (Hoffmann-La Roche, Basel, Basel-Stadt, Switzerland), and kits for enzymes’ quantification (all from Roche Diagnostics GMBH, Rotkreuz, Zug, Switzerland). To analyze the potential systemic damage induced by i–IRI, we studied enzymes that show abnormalities in the liver: alanine transaminase (ALT) and aspartate transaminase (AST); kidney: creatinine and urea; and other tissues: creatine kinase (CK) and lactate dehydrogenase (LDH).
Finally, for absorptive studies, once the total period of ischemia-reperfusion was concluded, 1 mL of aqueous solution of D-Xylose (0.615 mg/mL) was given. One hour after the administration of the D-Xylose solution, under isoflurane anesthesia, 5 mL of blood was retrieved as previously described. Serum samples were reacted with chromogen (a mixture of phloroglucinol, hydrochloric acid and acetic acid) in boiling water for 4 minutes; the absorbance was then quantified with Shimadzu UV-1700 PharmaSpec spectrophotometer (Shimadzu, Kyoto, Japan) at a wavelength of 554 nm.37,40
Statistical Analysis
All analyses were performed with Prism 9.4.1 (GraphPad Software, San Diego, CA, USA). The data obtained were represented as the mean and standard deviation, once their fit to a normal distribution had been checked using the Kolmogorov−Smirnov test. To compare three or more groups, analysis of the variance (ANOVA) was performed. Following confirmation of the existence of statistically significant differences, Tukey’s multiple comparison test was used to determine the differences between groups. A 95% confidence level was accepted as significant.
Results
Following SMA clamping, no adverse effects were observed in the animals. The whole procedure was well tolerated, with no bleeding, nor did it cause the death of any animal during the experimental process.
Histological Injury Degree
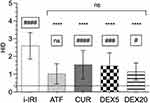
Figure 1 shows representative histological slices: control, i–IRI, ATF, CUR, DEX5 and DEX20 (Figure 1A–F, respectively). The score of the whole bowel section of i–IRI animals, including the 4 quadrants analyzed (both mesenteric and antimesenteric border), reached a mean value of 2.6 ± 0.75 in Chiu’s scale.
Both CUR- and ATF-treated animals showed significantly less mucosal damage (1.54 ± 0.8 and 1.01 ± 0.57, respectively; p < 0.0001); treatment with dexmedetomidine prevented mucosal damage to a similar extent as in the CUR group. The dexmedetomidine dose no making any difference; the mean HID index was 1.47 ± 0.7 (DEX5 group), and 1.14 ± 0.49 (DEX20 group) (Figure 2).
Considering the mesenteric and the antimesenteric border (Figure 3A and B, respectively), in the i–IRI group the HID in the mesenteric border was almost half that in the antimesenteric border (1.84 ± 0.5 vs 3.4 ± 0.5). ATF and DEX20 achieved the greatest reduction in HID compared to the control group (0.40 ± 0.38, vs 0.81 ± 0.43, vs 0.25 ± 0.2, respectively). No therapeutic effect was noticed in the antimesenteric border.
Biochemical Determination
To explore the consequences of i–IRI on other organs/tissues we performed some serum analysis (Table 2). Compared to control, intestinal ischemia-reperfusion has not modified serum ALT levels (4.17 ± 0.16 IU/L vs 4.33 ± 1.63 IU/L); neither curcumin nor α-tocopherol treatment modified this enzyme. Only the groups receiving dexmedetomidine, either DEX5 (28.0 ± 3.5 IU/L) or DEX20 (25.9 ± 11.1 IU/L), presented a significant increase in ALT serum values (p < 0.0001). AST significantly increased in animals subjected to i–IRI relative to control (111 ± 26 vs 5.1 ± 0.26 IU/L, p < 0.0001). Neither treatment significantly blocked this elevation.
Following i–IRI, a significant increase in urea was found (83.6 ± 19.6 mg/dL, p < 0.0001). All of the treatments reduced this increase to some degree. However, only the lowest dose of dexmedetomidine (DEX5) was able to reduce urea levels to same levels as in the healthy animals (49.2 ± 7.7 mg/dL; p > 0.05); DEX20 also showed a significant reduction but was not able to return urea to baseline concentrations. Focusing on creatinine, the i–IRI group doubled the serum concentration of this parameter with respect to the control (0.55 ± 0.15 vs 0.26 ± 0.10 mg/dL, p < 0.001). Curcumin showed no effect at all, and the reduction observed in animals receiving α-tocopherol was not statistically significant. Meanwhile, dexmedetomidine-treated animals reached a significant reduction in serum creatinine concentration, both DEX5 (0.32 ± 0.03 mg/dL, p < 0.01) or DEX20 (0.28 ± 0.07 mg/dL, p < 0.001).
Lastly, no treatment exerted any influence on CK and LDH serum elevations.
Absorptive Studies
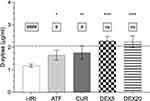
Absorptive intestinal function (Figure 4) was significantly reduced in those animals subjected to i–IRI (1.18 ± 0.07 µg/mL). Alpha-tocopherol and curcumin increased serum D-xylose concentration by 40% and 50% (1.66 ± 0.21 and 1.76 ± 0.30 µg/mL, respectively; p < 0.05). Dexmedetomidine restored basal serum D-Xylose values; there was no difference between the DEX5 and DEX20 groups (2.29 ± 0.2 vs 2.25 ± 0.26 µg/mL).
Discussion
As mentioned, several factors are involved in the onset of damage, both local and systemic, following i–IRI. Among them, the activation of neutrophils and macrophages as a consequence of endothelial injury of the affected organs, the ROS release into the bloodstream and the cytokines production, which enhances the inflammatory response and leads to the dissemination of the tissues affected by ischemia; all these events eventually lead to a MODS.15
The model we have used to induce i–IRI has been widely used. However, the experimental conditions have not been widely agreed on, and there has been considerable variability in terms of the periods of ischemia and reperfusion which range from 30 minutes to 2 hours and 45 minutes to 72 hours, respectively.38,41–47
We designed a study with 1 hour of ischemia trying to translate human usual clinical practice into a rodent model. In this clinical scenario, it usually takes 6 to 8 hours to achieve a complete reperfusion. Taking into account the faster life span of rodents (6.4 times higher metabolism, 4.7 times higher heart rate and 6.3 times faster respiratory rate48), this time may reflect properly an usual clinical practice.
On the other hand, shorter times may not allow us to find significant findings as a consequence of the treatment in the parameters analyzed. Something similar would lead us to discard times of 120 minutes, which induce such an exacerbated injury that it makes it difficult to determine the benefits of the substances under study.38 Regarding reperfusion time, we established 4 hours to allow enough time for pathological lesions derived from immunological activation due to the i–IRI to develop.49
Early studies in i–IRI mainly involved antioxidant substances due to their well-known role in reducing oxidative stress damage.50 However, nowadays it is known that the key factor in the damage caused by IRI is the activation of the innate immune system and the systemic establishment of an uncontrolled proinflammatory state. In this way, the molecules modified by ROS are responsible for this uncontrolled state as they are recognized as foreign elements by the immune system, which triggers their attack and elimination.51
Our experiment analyzed the effect of curcumin, an antioxidant compound, and dexmedetomidine, a substance with anti–inflammatory activity; in addition, it includes α-tocopherol as a drug with previously proven efficacy.52 The three drugs have been shown to significantly decrease the HID, with no statistically significant differences between them; however, α-tocopherol and 20 µg/kg dexmedetomidine were found to achieve the greatest reduction in HID, around 60%. Conversely, the drop observed when curcumin or 5 µg/kg dexmedetomidine were administered was around 40%. Bilbao et al showed that α-tocopherol treatment reduced mucosal damage by 20% when a 2-hour ischemia was induced, followed by 1-hour reperfusion.36 Gutierrez-Sanchez et al also applied the same temporal schedule (2h + 1h) and found that treatment with 10 mg/kg nitroindazole reduced the intestinal injury by 38%; however, when they used a 1-hour ischemia period, the HID decrease reached almost 70%.38
Günel et al, following a single intramuscular injection of 10 mg/kg α-tocopherol 15 minutes before reperfusion, reported a slight decrease in intestinal damage (23%) in rabbits after 1h of ischemia and 1h of reperfusion.53 This lower effect, compared to our findings, may be due to several factors: the route of administration (intramuscular vs intraperitoneal), the dose (10 mg/kg vs 20 mg/kg) and the administration timing (in the last 15 minutes of ischemia vs 2 hours before ischemia).
Focusing on curcumin therapy, the differences between our results and those previously published may also lie in the dosage and the administration time employed. Other authors were able to reduce histological damage by 60–70%, despite the fact that they used greatly differing experimental conditions.54,55 While Karatepe treated for 15 days prior to ischemia with 40 mg/kg curcumin, Yucel pretreated for 3 days prior to ischemia with 100 mg/kg; in addition, they also used different reperfusion times (3h vs 1h, respectively). In contrast, both used oral administration and a 1-hour ischemia period by clamping the SMA. Önder et al administered the same dose as we did (200 mg/kg), but 15 minutes before ischemia. They quantified the damage after one hour of reperfusion, but failed to find statistically significant differences.34 In contrast, with the same dose, we obtained a statistically significant reduction. This finding could be partly explained by the low absorption of curcumin after oral administration,56 as in our study we started pretreatment 24h and 4h before ischemia, and not only 1h before. Furthermore, by allowing only 1 hour of reperfusion, it is possible that the therapeutic effect of curcumin has not reached its maximum peak of action. Bo and Feng supported the fact that the oral route of administration requires more time between the administration and the ischemia-reperfusion event.57
Finally, the published literature shows that dexmedetomidine acts in a dose-dependent manner on preventing intestinal damage due to i–IRI, which is consistent with our findings. Zhang et al administered various doses of dexmedetomidine (10, 20 or 50 µg/kg) 30 minutes before ischemia. Two hours after reperfusion, they observed a reduction in the HID, which was greater the higher the dexmedetomidine dose; however, the differences did not reach statistical significance.58 Another study also reported that lower doses of dexmedetomidine (5 µg/kg) also reduced intestinal damage after clamping the SMA for 1 h followed by 2 h reperfusion;33,45 which is also consistent with our findings.
Examining the HID figures when the mesenteric and antimesenteric borders were separately studied, we can observe a completely different injury pattern between both of them. The antimesenteric border doubled the damage observed in the mesenteric border of the i–IRI group. These findings are consistent with those reported in 2007 by Higa et al, who also reported more than twice as much damage to the antimesenteric border concerning villi, necrosis and hemorrhagic infarction.59 When we applied α-tocopherol, curcumin or both doses of dexmedetomidine, this differential damage was also observed. Overall, only the mesenteric border was able to preserve a normal histologic structure in the intestinal mucosa. It is likely that these findings are due to the worse blood supply to the antimesenteric border, as the intestinal vasculature runs from the mesenteric border, making this region more susceptible to ischemic injury. These results are also consistent with previously published studies. Kazmers et al analyzed small intestinal sections by scanning electron microscopy in glucagon-treated rats after 85 minutes of SMA clamping and 20 minutes of reperfusion.60
Concerning the absorptive study, our results show that i–IRI reduces the absorptive capacity of the intestine, in agreement with Morini.61 In an ex vivo model, Schoots et al also found that intestinal absorption was affected. They reported an 11% and 44% decrease in the absorption of glucose and glutamine, respectively; however, the barrier function for large molecules (between 376 D and 40 kD) was preserved.62 In our study, all three drugs were able to increase blood D-xylose concentrations. Among them, dexmedetomidine was the compound that most successfully increased D-xylose concentrations, reaching concentrations similar to those of healthy animals; however, no differences were observed between low or normal doses. These results may be related to the harmful changes in the intestinal epithelium resulting from i–IRI. The destruction of the villi leads to a decrease in the absorptive capacity of the small intestine,63 that may justify this reduction in blood D-xylose levels. However, it should be noted that the specific reason for this reduction is not entirely clear. It should be noted that α-tocopherol though being the compound more effective reducing the damage at the histological level, it has not been the one inducing the higher increase in the concentrations of D-xylose in the blood (since this corresponds to dexmedetomidine). It is conceivable that since dexmedetomidine, being an α-2 adrenergic, improves intestinal motility, this effect may in turn result in an improvement in absorptive capacity.64
Focusing now on systemic affectation, as measured by various enzymatic markers, we see that i–IRI induces intense changes in many of them. Firstly, liver impairment was especially noteworthy, as evidenced by the elevation of AST, ALT and LDH levels after i–IRI. Hepatic involvement in i–IRI is well documented in the literature.29 Beside the general mechanism of endothelium damage induced by activated lymphocytes in liver, kidneys or lungs, because of the anatomical relationship between the intestine and the liver, all the toxic substances accumulated during ischemia are drained directly to the liver through the portal vein adding more stress to the organ. Despite the positive effects on the intestine (both mucosal damage and absorptive function), none of the treatments employed was able to reduce the elevation of these enzymes.
On the other hand, elevated creatinine and urea serum levels also showed kidney dysfunction after i–IRI. Lai et al have already proved that i–IRI affect distant organs, such as the kidney.65 They observed, after 90 minutes of SMA clamping and 6 hours of reperfusion, elevation of kidney damage markers (blood urea nitrogen and creatinine) and the existence of microscopic renal lesions. In our study, treatment with dexmedetomidine, at any dose, was able to normalize urea and creatinine values after i–IRI. However, it is important to note that this may not be purely attributable to its effect against inflammation, oxidative stress and apoptosis,33 since dexmedetomidine also increases renal vasodilation and increases glomerular filtration, while also reducing glomerular congestion, renal tubular epithelial cell inflammation and luminal stenosis, which could also contribute to reducing renal damage during reperfusion.
To conclude, we would like to emphasize a differential aspect of our work, the sex of the animals used. There are few studies in which the study subjects are not male.66 The hormonal environment plays a key role in the establishment or development of the inflammatory response. Among other activities, estrogens act both by protecting endothelial integrity and by regulating the redox state.67,68 Unfortunately, because we have not been able to find published studies with the same experimental design as the one we used it is not feasible to compare whether the initial damage after i–IRI, without any treatment, varies in magnitude according to gender.
Few studies use both males and females and evaluate the differential efficacy of the treatment according to gender. Szabó et al analyzed the differences at the microcirculatory and epithelial levels in male and female mice subjected to 30-min intestinal ischemia and 90-min reperfusion.69 They concluded that, in i–IRI, the leukocyte response and the alterations at the level of the intestinal microcirculation develop more rapidly and are initially more pronounced in males, but that female hormones are unable to prevent the final manifestations of i–IRI injury. Under a similar approach, Wu et al proved that NS-398, a selective cyclooxygenase-2 inhibitor, after 30 minutes of ischemia and ranging from 15 to 120 minutes of reperfusion, attenuated i–IRI–induced injury, total peroxidase levels and prostaglandin E2 production in males, but not in females.70 Therefore, relying on these studies, the gender should be considered to design differential approaches to the therapeutic management of i–IRI.
An important limitation of this study is the absence of evaluation of the blood pressure. Changes in blood pressure are frequent during i-IRI, in particular during the reperfusion phase.
Kozhura et al revealed that during the ischemia period there was no significant variation in blood pressure; however, a sudden decrease in blood pressure was noticed early in the reperfusion period and continued to decrease until the end of the experiments.71 They hypothesized that reperfusion could dysregulate the systemic blood pressure support system and trigger its gradual decline in the reperfusion period. Another related constraint is related to the oral administration of curcumin, or any other drug, in patients suffering from intestinal ischemia. The damage to the intestinal mucosa impairs the absorptive capacity of the intestine, as we determined in our experiments, as well as other studies.72 In our experimental setting, this is not an issue, since oral administration was performed prior to ischemic damage; however, It may be difficult to translate into real clinical practice. Finally, we would also like to emphasize the strengths of this study, such as the realization of the intestinal absorption tests, as well as the use of the Chiu scale, established in 1970 and widely used in i-IRI studies Papers using both tests are scarce, which allows us to demonstrate, without interstudy variation bias, the relationship between mucosal damage and absorptive deficit.
Conclusion
In our experimental model of i-IRI, prophylactic treatment with anti-inflammatory or antioxidant substances has allowed both a decrease in localized injury to the intestinal mucosa and a substantial improvement in intestinal absorption. Finally, in terms of systemic effects, none of the treatments achieved a relevant reduction in hepatic damage, and only dexmedetomidine succeeded in reducing renal damage, as measured by urea and creatinine levels.
Funding
This research received funding from the University of The Basque Country UPV/EHU (grant reference GIU21/054).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bala M, Kashuk J, Moore EE, et al. Acute mesenteric ischemia-guidelines of the world society of emergency surgery. World J Emerg Surg. 2017;12(38). doi:10.1186/s13017-017-0150-5
2. Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2013;20(7):1126–1167. doi:10.1089/ars.2012.5149
3. Tadros T, Traber DL, Heggers JP, Herndon DN. Effects of Interleukin-1α administration on intestinal ischemia and reperfusion injury, mucosal permeability, and bacterial translocation in burn and sepsis. Ann Surg. 2003;237(1):101–109. doi:10.1097/00000658-200301000-00014
4. Turnage RH, Guice KS, Oldham KT. Endotoxemia and remote organ injury following intestinal reperfusion. J Surg Res. 1994;56(6):571–578. doi:10.1006/jsre.1994.1091
5. Shi Y, Zhang X, Wan Z, et al. Mesenchymal stem cells against intestinal ischemia–reperfusion injury: a systematic review and meta-analysis of preclinical studies. Stem Cell Res Ther. 2022;13(1):216. doi:10.1186/s13287-022-02896-y
6. Oldenburg WA, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164(10):1054–1062. doi:10.1001/archinte.164.10.1054
7. Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Plast Surg. 2004;91(1):17–27. doi:10.1002/bjs.4459
8. Kase K, Reintam Blaser A, Tamme K, et al. Epidemiology of acute mesenteric ischemia: a population-based investigation. World J Surg. 2023;47(1):173–181. doi:10.1007/s00268-022-06805-5
9. Kärkkäinen JM, Lehtimäki TT, Manninen H, Paajanen H. Acute mesenteric ischemia is a more common cause than expected of acute abdomen in the elderly. J Gastrointest Surg. 2015;19(8):1407–1414. doi:10.1007/s11605-015-2830-3
10. Tamme K, Blaser AR, Laisaar KT, et al. Incidence and outcomes of acute mesenteric ischaemia: a systematic review and meta-analysis. BMJ Open. 2022;12(10):e062846. doi:10.1136/bmjopen-2022-062846
11. Brandt LJ, Boley SJ. AGA technical review on intestinal ischemia. Gastroenterology. 2000;118(5):954–968. doi:10.1016/S0016-5085(00)70183-1
12. Acosta S. Epidemiology of mesenteric vascular disease: clinical implications. Semin Vasc Surg. 2010;23(1):4–8. doi:10.1053/j.semvascsurg.2009.12.001
13. Quiroga B, Verde E, Abad S, et al. Detection of patients at high risk for non-occlusive mesenteric ischemia in hemodialysis. J Surg Res. 2013;180(1):51–55. doi:10.1016/j.jss.2012.10.008
14. Guillaume A, Pili-Floury S, Chocron S, et al. Acute mesenteric ischemia among postcardiac surgery patients presenting with multiple organ failure. Shock. 2017;47(3):296–302. doi:10.1097/SHK.0000000000000720
15. Bourcier S, Klug J, Nguyen LS. Non-occlusive mesenteric ischemia: diagnostic challenges and perspectives in the era of artificial intelligence. World J Gastroenterol. 2021;27(26):4088. doi:10.3748/wjg.v27.i26.4088
16. Paul M, Bougouin W, Legriel S, et al. Frequency, risk factors, and outcomes of non-occlusive mesenteric ischaemia after cardiac arrest. Resuscitation. 2020;157:211–218. doi:10.1016/j.resuscitation.2020.09.028
17. Bala M, Catena F, Kashuk J, et al. Acute mesenteric ischemia: updated guidelines of the world society of emergency surgery. World J Emerg Surg. 2022;17(1):54. doi:10.1186/s13017-022-00443-x
18. Savlania A, Tripathi RK. Acute mesenteric ischemia: current multidisciplinary approach. J Card Surg. 2017;58(2):339–350. doi:10.23736/S0021-9509.16.09751-2
19. Cearra I, Herrero de la Parte B, Ruiz Montesinos I, Alonso-Varona A, Moreno-Franco DI, García-Alonso I. Effects of folinic acid administration on lower limb ischemia/reperfusion injury in rats. Antioxidants. 2021;10(12):1887. doi:10.3390/antiox10121887
20. Akhtar MZ, Henderson T, Sutherland A, Vogel T, Friend PJ. Novel approaches to preventing ischemia-reperfusion injury during liver transplantation. Transplant Proc. 2013;45(6):2083–2092. doi:10.1016/j.transproceed.2013.04.004
21. Knight SF, Kundu K, Joseph G, et al. Folate receptor–targeted antioxidant therapy ameliorates renal ischemia–reperfusion injury. J Am Soc Nephrol. 2012;23(5):793–800. doi:10.1681/ASN.2011070711
22. Nucci C, Tartaglione R, Cerulli A, et al. Retinal damage caused by high intraocular pressure–induced transient ischemia is prevented by coenzyme Q10 in rat. Neuroinfl Neuronal Death Rep. 2007;82:397–406. doi:10.1016/S0074-7742(07)82022-8
23. Alfonzetti T, Yasmin-Karim S, Ngwa W, Avery S. Phytoradiotherapy: an integrative approach to cancer treatment by combining radiotherapy with phytomedicines. Front Oncol. 2021;10:624663. doi:10.3389/fonc.2020.624663
24. Dragos D, Gilca M, Gaman L, et al. Phytomedicine in Joint Disorders. Nutrients. 2017;9(1):70. doi:10.3390/nu9010070
25. Jayaprakasha GK, Jaganmohan Rao L, Sakariah KK. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006;98(4):720–724. doi:10.1016/j.foodchem.2005.06.037
26. Müller L, Theile K, Böhm V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol Nutr Food Res. 2010;54(5):731–742. doi:10.1002/mnfr.200900399
27. Keating GM. Dexmedetomidine: a review of its use for sedation in the intensive care setting. Drugs. 2015;75(10):1119–1130. doi:10.1007/s40265-015-0419-5
28. Venn RM, Hell J, Michael Grounds R. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care. 2000;4(5):302. doi:10.1186/cc712
29. Zhao Y, Kong GY, Pei WM, Zhou B, Zhang QQ, Pan BB. Dexmedetomidine alleviates hepatic injury via the inhibition of oxidative stress and activation of the Nrf2/HO-1 signaling pathway. Eur Cytokine Netw. 2019;30(3):88–97. doi:10.1684/ecn.2019.0431
30. Uusalo P, Al-Ramahi D, Tilli I, Aantaa RA, Scheinin M, Saari TI. Subcutaneously administered dexmedetomidine is efficiently absorbed and is associated with attenuated cardiovascular effects in healthy volunteers. Eur J Clin Pharmacol. 2018;74(8):1047–1054. doi:10.1007/s00228-018-2461-1
31. Chen Y, Bian W, Xu B. Pretreatment with dexmedetomidine alleviates lung injury in a rat model of intestinal ischemia reperfusion. Mol Med Rep. 2020;21(3):1233–1241. doi:10.3892/mmr.2020.10942
32. Fan X, Du J, Wang MH, et al. Irisin contributes to the hepatoprotection of dexmedetomidine during intestinal ischemia/Reperfusion. Oxid Med Cell Longev. 2019;2019:7857082. doi:10.1155/2019/7857082
33. Zhang XY, Liu ZM, Wen SH, et al. Dexmedetomidine administration before, but not after, ischemia attenuates intestinal injury induced by intestinal ischemia-reperfusion in rats. Anesthesiology. 2012;116(5):1035–1046. doi:10.1097/ALN.0b013e3182503964
34. Önder A, Kapan M, Gümüş M, et al. The protective effects of curcumin on intestine and remote organs against mesenteric ischemia/reperfusion injury. Turk J Gastroenterol. 2012;23(2):141–147. doi:10.4318/tjg.2012.0446
35. Liu XM, Chen QH, Hu Q, et al. Dexmedetomidine protects intestinal ischemia-reperfusion injury via inhibiting p38 MAPK cascades. Exp Mol Pathol. 2020;115:104444. doi:10.1016/j.yexmp.2020.104444
36. Bilbao JE, Garcia-Alonso I, Portugal V, Barceló P, Ortiz Lacorzana J, Méndez J. Therapeutic usefulness of antioxidant drugs in experimental intestinal reperfusion syndrome. Rev Esp Enferm Dig. 1991;80(4):237–241.
37. Eberts TJ, Sample RH, Glick MR, Ellis GH. A simplified, colorimetric micromethod for xylose in serum or urine, with phloroglucinol. Clin Chem. 1979;25(8):1440–1443. doi:10.1093/clinchem/25.8.1440
38. Gutiérrez-Sánchez G, García-Alonso I, Gutiérrez Sáenz de Santa María J, Alonso-Varona A, Herrero de la Parte B. Antioxidant-based therapy reduces early-stage intestinal ischemia-reperfusion injury in rats. Antioxidants. 2021;10(6):853. doi:10.3390/antiox10060853
39. Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101(4):478–483. doi:10.1001/archsurg.1970.01340280030009
40. Antunes DMF, Da Costa JP, Campos SMN, et al. The serum D-xylose test as a useful tool to identify malabsorption in rats with antigen specific gut inflammatory reaction. Int J Exp Pathol. 2009;90(2):141–147. doi:10.1111/j.1365-2613.2008.00627.x
41. Dominowski L, Kirsch M. Synergistic Effect of β-alanine and aprotinin on mesenteric ischemia. J Surg Res. 2021;263:78–88. doi:10.1016/j.jss.2021.01.026
42. Almoiliqy M, Wen J, Xu B, et al. Cinnamaldehyde protects against rat intestinal ischemia/reperfusion injuries by synergistic inhibition of NF-κB and p53. Acta Pharmacol Sin. 2020;41(9):1208–1222. doi:10.1038/s41401-020-0359-9
43. Toropova YG, Pechnikova NA, Zelinskaya IA, et al. Nicotinamide riboside has protective effects in a rat model of mesenteric ischaemia-reperfusion. Int J Exp Pathol. 2018;99(6):304–311. doi:10.1111/iep.12302
44. Ucar BI, Erikci A, Kosemehmetoglu K, et al. Effects of endothelin receptor blockade and COX inhibition on intestinal I/R injury in a rat model: experimental research. Int J Surg. 2020;83:89–97. doi:10.1016/j.ijsu.2020.08.061
45. Sampaio de Holanda G, Dos Santos Valença S, Maran Carra A, et al. Sulforaphane and albumin attenuate experimental intestinal ischemia-reperfusion injury. J Surg Res. 2021;262:212–223. doi:10.1016/j.jss.2021.01.014
46. Ikiz Ö, Kahramansoy N, Erkol H, Koçoğlu E, Fırat T. Effects of lycopene in intestinal ischemia reperfusion injury via intestinal immunoglobulin A. J Surg Res. 2021;267:63–70. doi:10.1016/j.jss.2021.04.039
47. Wu MB, Ma B, Zhang TX, Zhao K, Cui SM, He SC. Propofol improves intestinal ischemia-reperfusion injury in rats through NF-κB pathway. Eur Rev Med Pharmacol Sci. 2020;24(11):6463–6469. doi:10.26355/eurrev_202006_21545
48. Agoston DV. How to translate time? The temporal aspect of human and rodent biology. Front Neurol. 2017;8:92. doi:10.3389/fneur.2017.00092
49. Warrington R, Watson W, Kim HL, Antonetti FR. An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol. 2011;7(1):S1. doi:10.1186/1710-1492-7-S1-S1
50. Vajdovich P. Free radicals and antioxidants in inflammatory processes and ischemia-reperfusion injury. Vet Clin North Am Small Anim. 2008;38(1):31–123. doi:10.1016/j.cvsm.2007.11.008
51. Lee SM, Hutchinson M, Saint DA. The role of Toll-like receptor 4 (TLR4) in cardiac ischaemic-reperfusion injury, cardioprotection and preconditioning. Clin Exp Pharmacol Physiol. 2016;43(9):864–871. doi:10.1111/1440-1681.12602
52. Yağmurdur MC, Özdemir A, Özenç A, Kilinç K. The effects of alpha-tocopherol and verapamil on mucosal functions after gut ischemia/reperfusion. Turk J Gastroenterol. 2003;14(1):26–32.
53. Günel E, Çaǧlayan F, Çaǧlayan O, Dilsiz A, Duman S, Aktan M. Treatment of intestinal reperfusion injury using antioxidative agents. J Pediatr Surg. 1998;33(10):1536–1539. doi:10.1016/S0022-3468(98)90492-4
54. Yucel AF, Kanter M, Pergel A, Erboga M, Guzel A. The role of curcumin on intestinal oxidative stress, cell proliferation and apoptosis after ischemia/reperfusion injury in rats. J Mol Histol. 2011;42(6):579–587. doi:10.1007/s10735-011-9364-0
55. Karatepe O, Gulcicek OB, Ugurlucan M, et al. Curcumin nutrition for the prevention of mesenteric ischemia–reperfusion injury: an experimental rodent model. Transplant Proc. 2009;41(9):3611–3616. doi:10.1016/j.transproceed.2009.08.002
56. Tian C, Asghar S, Wu Y, et al. Improving intestinal absorption and oral bioavailability of curcumin via taurocholic acid-modified nanostructured lipid carriers. Int J Nanomedicine. 2017;12:7897–7911. doi:10.2147/IJN.S145988
57. Bo H, Feng X. Post-treatment curcumin reduced ischemia–reperfusion-induced pulmonary injury via the Notch2/Hes-1 pathway. J Int Med Res. 2019;48(4):1–11. doi:10.1177/0300060519892432
58. Zhang X, Zhou J, Hu Q, et al. The role of janus kinase/signal transducer and activator of transcription signalling on preventing intestinal ischemia/reperfusion injury with dexmedetomidine. J Nanosci Nanotechnol. 2019;20(5):3295–3302. doi:10.1166/jnn.2020.16416
59. Higa OH, Parra ER, Ab’Saber AM, Farhat C, Higa R, Capelozzi VL. Protective effects of ascorbic acid pretreatment in a rat model of intestinal ischemia-reperfusion injury: a histomorphometric study. Clinics. 2007;62(3):315–320. doi:10.1590/S1807-59322007000300017
60. Kazmers A, Zwolak R, Appelman HD, et al. Pharmacologic interventions in acute mesenteric ischemia: improved survival with intravenous glucagon, methylprednisolone, and prostacyclin. J Vasc Surg. 1984;1(3):472–481. doi:10.1016/0741-5214(84)90088-0
61. Morini S, Elias G, Brown M, Subbotin V, Rastellini C, Cicalese L. Chronic morpho-functional damage as a consequence of transient ischemia/reperfusion injury of the small bowel. Histol Histopathol. 2010;25:277–286. doi:10.14670/HH-25.277
62. Schoots IG, Bijlsma PB, Koffeman GI, van Gulik TM. Hypoxia/reoxygenation impairs glucose absorption and cAMP-mediated secretion more profoundly than glutamine absorption and Ca2+/PKC-mediated secretion in rat ileum in vitro. Surgery. 2006;139(2):244–253. doi:10.1016/j.surg.2005.07.032
63. Udassin R, Haskel Y, Samuni A. Nitroxide radical attenuates ischaemia/reperfusion injury to the rat small intestine. Gut. 1998;42(5):623. doi:10.1136/gut.42.5.623
64. Tufanogullari B, White PF, Peixoto MP, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesth Analg. 2008;106(6):1741–1748. doi:10.1213/ane.0b013e318172c47c
65. Lai HJ, Zhan YQ, Qiu YX, et al. HMGB1 signaling-regulated endoplasmic reticulum stress mediates intestinal ischemia/reperfusion-induced acute renal damage. Surgery. 2021;170(1):239–248. doi:10.1016/j.surg.2021.01.042
66. Wang J, Zhang W, Wu G. Intestinal ischemic reperfusion injury: recommended rats model and comprehensive review for protective strategies. Biomed Pharmacother. 2021;138:111482. doi:10.1016/j.biopha.2021.111482
67. Ba ZF, Yokoyama Y, Toth B, Rue LW, Bland KI, Chaudry IH. Gender differences in small intestinal endothelial function: inhibitory role of androgens. Am J Physiol. 2004;286(3):G452–G457. doi:10.1152/ajpgi.00357.2003
68. Doucet D, Badami C, Palange D, et al. Estrogen receptor hormone agonists limit trauma hemorrhage shock-induced gut and lung injury in rats. PLoS One. 2010;5(2):e9421. doi:10.1371/journal.pone.0009421
69. Szabó A, Vollmar B, Boros M, Menger MD. Gender differences in ischemia-reperfusion-induced microcirculatory and epithelial dysfunctions in the small intestine. Life Sci. 2006;78(26):3058–3065. doi:10.1016/j.lfs.2005.12.012
70. Wu M, Rowe JM, Fleming SD. Eicosanoid production varies by sex in mesenteric ischemia reperfusion injury. Clin Immunol. 2020;220:108596. doi:10.1016/j.clim.2020.108596
71. Kozhura VL, Basarab DA, Golubev AM, Reshetnyak VI, Moroz VV. Reperfusion injury after critical intestinal ischemia and its correction with perfluorochemical emulsion “perftoran. World J Gastroenterol. 2005;11(45):7084–7090. doi:10.3748/wjg.v11.i45.7084
72. Huber TS, Björck M, Chandra A, et al. Chronic mesenteric ischemia: clinical practice guidelines from the society for vascular surgery. J Vasc Surg. 2021;73(1):87S–115S. doi:10.1016/j.jvs.2020.10.029
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.