Back to Journals » ClinicoEconomics and Outcomes Research » Volume 15
Projected Savings Associated with Lowering the Risk of Total Hip Arthroplasty Revision Due to Dislocation in Patients with Spinopelvic Pathology
Authors Ackerman SJ, Vigdorchik JM, Siljander BR, Gililland JM, Sculco PK, Polly DW
Received 28 February 2023
Accepted for publication 15 April 2023
Published 28 April 2023 Volume 2023:15 Pages 321—330
DOI https://doi.org/10.2147/CEOR.S410453
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Stacey J Ackerman,1 Jonathan M Vigdorchik,2 Breana R Siljander,2,3 Jeremy M Gililland,4 Peter K Sculco,2 David W Polly3
1Department of Biomedical Engineering, Johns Hopkins University, San Diego, CA, USA; 2Department of Orthopedic Surgery, Hospital for Special Surgery, New York, NY, USA; 3Department of Orthopedic Surgery, University of Minnesota, Minneapolis, MN, USA; 4Department of Orthopedic Surgery, University of Utah, Salt Lake City, UT, USA
Correspondence: Stacey J Ackerman, Email [email protected]
Purpose: In the United States (US), total hip arthroplasty (THA) is the most common hospital inpatient operation among Medicare beneficiaries and is ranked fourth when considering all payers. Spinopelvic pathology (SPP) is associated with an increased risk of THA revision (rTHA) due to dislocation. Several strategies have been proposed to mitigate the risk of instability in this population, including use of dual-mobility implants, anterior-based surgical approaches, and technology-assistance (digital 2D/3D pre-surgical planning, computer navigation, and robotic assistance). For primary THA (pTHA) patients with SPP who subsequently undergo rTHA due to dislocation, we aimed to estimate (1) target population size; (2) economic burden; and (3) 10-year projected savings to the US payer of lowering the risk of rTHA due to dislocation among pTHA patients with SPP.
Methods: A budget impact analysis from the US payer perspective was undertaken using published literature; American Academy of Orthopaedic Surgeons American Joint Replacement Registry 2021 Annual Report; Centers for Medicare & Medicaid Services MEDPAR 2019; and National (Nationwide) Inpatient Sample (NIS) 2019. Expenditures were inflation-adjusted to 2021 US dollars using the Medical Care component of the Consumer Price Index. Sensitivity analyses were performed.
Results: The target population size in 2021 was estimated at 5040 (range, 4830– 6309) for Medicare (fee-for-service plus Medicare Advantage) and 8003 (range, 7669– 10,018) for all-payer. Annual rTHA episode-of-care (through 90 days) expenditures for Medicare and all-payer were $185 million and $314 million, respectively. Using a 4.14% compound annual growth rate from NIS, the estimated number of applicable rTHA procedures that will be performed from 2022– 2031 was 63,419 Medicare and 100,697 all-payer. With each 10% reduction in relative risk of rTHA due to dislocation, Medicare and all-payer could save $233 million and $395 million, respectively, over a 10-year period.
Conclusion: Among pTHA patients with spinopelvic pathology, a modest reduction in the risk of rTHA due to dislocation could achieve substantial cumulative savings to payers while improving healthcare quality.
Keywords: total hip arthroplasty, revision, spinopelvic pathology, dislocation, economics
Introduction
In the United States (US), total hip arthroplasty (THA) is the most common hospital inpatient operation among Medicare beneficiaries and is ranked fourth when considering all payers.1 Spinopelvic pathology (SPP) is present in approximately 16% of patients undergoing primary THA (pTHA) and is associated with an increased risk of THA revision (rTHA) due to dislocation (estimated at 8% in pTHA patients with SPP).2–4 A hip dislocation after THA leads to a marked decrease in health-related quality of life largely due to difficulties with usual activities, self-care, and anxiety and depression.5
Dislocation is the second most frequent reason for rTHA based on the primary diagnosis reported on the insurance claim, resulting in substantial costs to the payer.6,7 Strategies to lower the incidence of rTHA due to dislocation in this higher-risk population are warranted to achieve reductions in payer expenditures while improving quality of care. Several strategies can be used to mitigate instability in this population, such as dual-mobility implants, anterior-based surgical approaches, and technology-assistance (for example, digital 2D/3D pre-surgical planning, computer navigation, and robotic assistance) (Figure 1).4,8–10
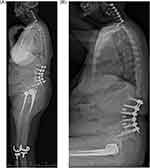 |
Figure 1 Sample case: (A) standing and (B) sitting lateral stereoradiographic images (EOS Imaging, Paris, France / ATEC Spine, Carlsbad, CA) demonstrating both a spinal deformity correction and bilateral hip arthroplasty. Notes: Examining the images in the standing and sitting positions informs both the spinal deformity and amount of spinal mobility present, thus helping to classify patients according to the Hip-Spine Classification system and guiding treatment.8 |
To our knowledge, the financial burden of rTHA due to dislocation in this elevated-risk population has not yet been explored. We aimed to estimate (1) the target population size; (2) economic burden; and (3) 10-year projected savings to the US payer of lowering the risk of rTHA due to dislocation among pTHA patients with spinopelvic pathology.
Materials and Methods
Analysis Overview
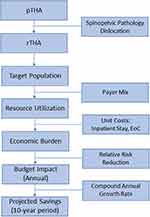
Using established research principles, a budget impact model was developed in Microsoft Excel to estimate the target population size, financial burden, and 10-year projected savings from the US payer perspective.11 Data sources included published literature; American Academy of Orthopaedic Surgeons American Joint Replacement Registry 2021 Annual Report;6 Centers for Medicare & Medicaid Services Medicare Inpatient Hospitals by Geography and Service dataset (administrative claims data from the Medicare Provider Analysis and Review [MEDPAR] 2019 file containing hospital inpatient data for 100% of Medicare beneficiaries);12 and Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project, National (Nationwide) Inpatient Sample (NIS) 2019 (a 20% sample of discharges from short-term, non-Federal community hospitals).1 Our study used solely public, published sources. It did not include any interaction or intervention with human subjects or include any access to identifiable private information. Therefore, IRB approval was not required. Expenditures were inflation-adjusted to 2021 US dollars using the Medical Care component of the Consumer Price Index.13 Sensitivity analyses were performed by generating a series of scenario analyses.11 Figure 2 depicts a graphical representation of the budget impact model analytical framework.
The target population was pTHA patients with SPP who subsequently undergo rTHA due to dislocation. SPP was defined as diagnoses of various conditions associated with a stiff spine or flatback, such as degenerative disc disease, scoliosis, history of fracture or tumor, post-laminectomy syndrome, and post spinal fusion. The target population did not exclude patients based on age, body mass index, pelvic incidence, number of fused segments (if any), or instrumentation. We assumed that patients undergo rTHA open surgery in the hospital inpatient setting (in lieu of a closed reduction in the emergency department) due to the higher risk of subsequent dislocation.14
Target Population Size
The target population size was estimated for base case and scenario analyses using MEDPAR data and other sources (Table 1).1,12,15–18 Given that diagnosis-related groups (DRGs) 466–468 include both rTHA and revision total knee arthroplasty (rTKA) discharges, the percent Medicare THA (primary plus revision) was derived using NIS.1
 |
Table 1 Input Parameter Values and Data Sources |
Among the nearly 63 million Medicare program beneficiaries in 2019, approximately 63% were enrolled in Medicare fee-for-service (FFS) and 37% in Medicare Advantage (MA).15 The budget impact analysis accounted for payer mix as well as racial disparities reported in THA use for MA beneficiaries.17 We estimated the total number of Medicare (FFS+MA) primary and revision THA and TKA discharges at 753,116 versus 756,950 reported from NIS (0.5% difference), which supports the validity of our methodological approach as the basis for estimating the target population size.1
Base Case
The American Academy of Orthopaedic Surgeons American Joint Replacement Registry 2021 Annual Report states that 18.2% of 3803 rTHA were due to dislocation/instability based on 2020 all-payer data; for comparison, Ackerman et al reported dislocation as the primary diagnosis for rTHA in 18.3% of 344 Medicare patients using data from the Medicare 5% Standard Analytical Files (SAF) (longitudinal data across practice settings for a 5% random sample of Medicare beneficiaries).6,7 Vigdorchik et al found a 67% prevalence of spinopelvic risk factors among 48 patients with post-operative hip dislocations.19 The percentage of rTHA due to dislocation among patients with SPP (12.2% [18.2% x 67%]) was applied to the total Medicare (FFS+MA) discharges for rTHA. The growth in the target population size from 2019–2021 was calculated by applying the compound annual growth rate (CAGR) derived from NIS for THA discharges (primary plus revision) from 2016–2019.1,18 The payer mix for THA (primary plus revision) was based on 2019 NIS.1
Scenario Analyses
Uncertainty in the target population size was addressed using scenario (sensitivity) analyses. For the first sensitivity analysis, Novikov et al reported that 4.3% (5/117) of rTHA had SPP, with or without fusion; and Yang et al reported that spinopelvic fixation patients were associated with a greater than 3.5-fold increase in hip dislocation risk.20,21 These values were applied to the Medicare rTHA discharges.
For the second sensitivity analysis, we referred to MEDPAR and other sources to determine the number of pTHA discharges for Medicare FFS+MA beneficiaries (Table 1). Additionally, Buckland et al found that 16.0% (174/1088) of patients undergoing pTHA had sagittal spinal deformity; and DelSole reported that, among pTHA patients with a diagnosis of sagittal spinal deformity, 8.0% (11/139) had hip dislocations.3,4 Therefore, 1.3% (16.0% x 8.0%) of pTHA patients have both SPP and hip dislocation. This figure was applied to the Medicare pTHA discharges.
Economic Burden
The economic burden of the rTHA inpatient stay was estimated using the weighted average Medicare payments for DRGs 466–468 (Table 1).12 Episode-of-care (EoC) costs were defined as the rTHA inpatient stay plus 90 days reflecting the Medicare bundled payment global period. Ackerman et al previously reported that the mean rTHA index stay expenditures were 62.5% of the mean rTHA EoC expenditures based on the Medicare 5% SAF.7 Therefore, the Medicare rTHA EoC costs were calculated by dividing the weighted average Medicare payment amount for the rTHA index stay by 62.5%. These values were inflation-adjusted to 2021 USD using the Medical Care component of the Consumer Price Index.13 Furthermore, Fang et al reported that private insurers paid 23.2% more than Medicare for total joint arthroplasty revisions, which was applied to calculate private payer rTHA index stay and EoC expenditures.22 Total annual medical expenditures were calculated by multiplying the target population size by the per patient EoC costs by payer.
Of note, investigators have reported that the average payments to hospitals were similar for stays with a primary payer of Medicare FFS and MA; as such, the Medicare FFS payment amounts were also applied to MA.23,24
Projected Savings Associated with Risk-Mitigation Strategies
Among pTHA patients with spinopelvic pathology, the number of subsequent rTHAs due to dislocation that would be performed over the next decade (from 2022–2031) was estimated using the CAGR derived from NIS 2016–2019.1,18 The annual and 10-year projected savings to Medicare and all-payer (by using more advanced technologies and techniques) were then calculated for an assumed reduction in revision risk, ranging from 0–50% (Table 1). The 10-year time period is consistent with that used by the Congressional Budget Office for projections of Medicare spending.25 The results are presented by annual budget period as undiscounted costs to reflect the budget holder’s interest in the impact expected at each time point.11 The savings per target patient was calculated as the total annual savings divided by the target population size.
Results
For pTHA patients with spinopelvic pathology who subsequently undergo rTHA due to dislocation, the target population size in 2021 was estimated at 5040 (range, 4830–6309) for Medicare (FFS+MA) and 8003 (range, 7669–10,018) for all-payer. Among Medicare beneficiaries, the rTHA inpatient stay and EoC costs per patient were $23,000 and $36,777, respectively (2021 USD). Private payer expenditures for the rTHA inpatient stay and EoC costs per patient were estimated at $28,337 and $45,309, respectively. Total annual EoC expenditures for Medicare were $185 million and for all-payer were $314 million (Table 2). With each 10% reduction in the risk of rTHA due to dislocation (by using more advanced technologies and techniques), Medicare and all-payer could save $18.5 million and $31.4 million annually, respectively (504 Medicare and 800 all-payer rTHA averted; $3688 Medicare and $3923 all-payer cost-savings on average per target patient [that is, per capita]).
 |
Table 2 Annual Expenditures for rTHA Due to Dislocation Among pTHA Patients with Spinopelvic Pathology (2021 USD) (N=8003) |
Using a 4.14% CAGR from NIS, the estimated number of applicable rTHA procedures that will be performed from 2022–2031 was 63,419 Medicare and 100,697 all-payer. Given the uncertainty in the actual relative risk reduction (RRR), we explored a wide range from 0–50% (in 5% increments), where the RRR reflects the reduction in risk of rTHA due to dislocation among pTHA patients with SPP. Figures 3 and 4 illustrate the projected offsets over a 10-year period; with each 10% reduction in risk of rTHA due to dislocation, Medicare and all-payer could save $233 million and $395 million, respectively (6342 Medicare and 10,070 all-payer rTHA averted).
Discussion
Budget impact analyses are designed to inform payer, health system, and health policy decision-making by estimating expenditures and potential cost-offsets. Budget impact analyses provide an analytical framework to view financial estimates under alternative plausible scenarios. As recommended by the International Society for Pharmacoeconomics and Outcomes Research Task Force on Budget Impact Analysis, the model was populated using real-life use and costs from registry and health insurance claims databases from the budget holder’s perspective.11
We evaluated the economic burden and budget impact of lowering the risk of rTHA due to dislocation for pTHA patients with SPP. The RRR could be conferred by multiple risk-mitigation strategies, including use of dual-mobility implants, anterior-based surgical approaches, and technology-assistance (digital 2D/3D pre-surgical planning, computer navigation, and robotic assistance). The present study estimated that with each 10% RRR in rTHA due to dislocation among pTHA patients with SPP, Medicare and all-payer could save $233 million and $395 million, respectively, over a 10-year period. Considering Medicare rTHA due to any reason, investigators have reported that with a 1% absolute reduction in rTHA 5-year cumulative revision risk (from 4.2% to 3.2%; that is, an RRR of 23.8%), Medicare could save $985 million over a 10-year period (2016 USD).7 Achieving a 1% absolute reduction (nearly 25% RRR) appears to be feasible based on an Australian patient registry that reported a 5-year cumulative percent rTHA of 2.9% by using advanced technologies and techniques.10 Our study differs from these reports in that it focuses on a subpopulation at elevated risk of rTHA due to dislocation – pTHA patients with spinopelvic pathology – and considers the 10-year projected savings to both Medicare and all-payer.
After first-time rTHA due to dislocation, recurrent dislocation and re-revision are common. Based on the Danish Hip Arthroplasty Register, Hermansen et al reported that 41.6% of pTHA patients experienced at least two recurrent dislocations within two years, and patients revised due to dislocation had a 19.8% incidence of new dislocation.14,26 Recurrent dislocations result in marked and persistent deterioration of health-related quality of life.5,27 These high rates of recurrent dislocation warrant efforts to reduce the incidence of first-time rTHA due to dislocation.
Strategies to avoid the initial rTHA dislocation episode for pTHA patients with SPP include use of dual-mobility implants, anterior-based surgical approaches, and technology-assistance.4,8–10 The Australian Orthopaedic Association National Joint Replacement Registry 2021 Annual Report showed that dual mobility prostheses have half the risk of being revised for dislocation/instability compared to other acetabular prostheses; and the anterior approach has half the risk of rTHA for dislocation/instability compared to both the posterior and lateral approaches.10
Technology-assistance is also a plausible means of reducing revision risk in a patient population at higher risk of revision.28 Investigators have reported that pre-surgical planning, computer navigation, and robotic-assistance can potentially lower the risk of rTHA due to dislocation among patients with SPP. Vigdorchik et al found that preoperative planning for rTHA due to recurrent instability resulted in 97% (108/111) survival-free dislocation at two years compared to 84% (93/111) for historical controls whereby 77% of the inappropriately positioned acetabular components would have been unrecognized without biplanar radiographs or stereoradiographic images to identify spinal deformity and spinal stiffness, which suggests an RRR of 75%.9 Further, among patients with a diagnosis of sagittal spinal deformity who were evaluated preoperatively using stereoradiographic imaging, DelSole et al reported a pTHA dislocation rate of 8.0% (11/139).4 Furthermore, among patients with stiff spine and flatback syndrome who were undergoing pTHA, investigators reported a dislocation rate of 6.8% (10/147) with use of technology-assistance (pre-surgical planning with computer navigation and/or robotic-assistance).8 While not an exhaustive literature review, these studies suggest that the RRR associated with use of technology-assistance among pTHA patients with SPP may fall between 45–75% at tertiary referral centers.
Given the uncertainty in the “true” RRR nationally, we explored a wide range from 0–50% (in 5% increments), where the RRR reflects the reduction in risk of rTHA due to dislocation conferred by these strategies intended to reduce revision risk. Referring to Figures 3 and 4, for example, lowering the risk of rTHA due to dislocation by 25% would result in approximately 25,000 rTHA averted and nearly $1 billion in all-payer projected savings over a 10-year period. These cost-savings may be slightly offset by additional expenditures incurred by the payer, if any; whereas, providers will incur additional costs by using these strategies (for example, for navigation, robots, and implants).29,30 With increasing adoption of these strategies the risk of rTHA due to dislocation will decrease, thus reducing the effective RRR.30 Contract negotiations with private payers present an opportunity for recognizing value-based healthcare associated with risk-mitigation strategies that reduce the incidence of rTHA due to dislocation.
The target population size and associated financial burden reported herein are likely underestimated for several reasons. First, the projected growth in the target population size over the 10-year period (CAGR of 4.14%) was estimated using NIS 2016–2019 data.1,18 The CAGR is likely higher from 2022–2031 due to increased demand for pTHA as a result of the aging population with more active lifestyles and the expansion of insurance coverage from the Affordable Care Act.31,32 Second, we conservatively assumed that Medicare FFS and MA payments were similar, and we estimated private payer expenditures for rTHA as 123.2% of Medicare FFS payments for DRG 468 (Revision of Hip or Knee Replacement without Complication or Comorbidity/Major Complication or Comorbidity).22–24 In contrast, based on reimbursement for DRG 470 (Major Hip and Knee Joint Replacement without Major Complication or Comorbidity) from the American Hospital Utilization Database (474 hospitals), Toren et al reported that MA and private insurance payments were 115.2% and 138.7%, respectively, of Medicare FFS payments.33 Consequently, our estimated economic burden and 10-year projected savings may be underestimated.
Scenario analyses were performed to estimate the range in target population size. Yang et al found that spinopelvic fixation patients had a 3.5-fold increase in hip dislocation risk relative to pTHA patients without lumbar spinal fusion.21 Applying this 3.5-fold risk to those with SPP likely results in overestimating the target population size because most THA patients with stiff spine have not undergone an instrumented fusion.34 On the other hand, the second scenario analysis was based on Medicare beneficiaries who underwent hospital inpatient pTHA. Yet some patients with private insurance undergo pTHA in the outpatient setting. Consequently, applying the hospital inpatient payer mix when grossing up the number of Medicare pTHA patients to all-payer does not include rTHAs following pTHAs performed on private payer patients in the hospital outpatient setting, resulting in underestimating the target population size. Despite these limitations, the target population size from the base case and scenario analyses converged, which nonetheless merits further research to more precisely define the true population size.
There are several limitations to this study. First, spinopelvic pathology is not explicitly listed as a diagnosis code on health insurance claims. Thus, to define the target population size we conducted base case and scenario analyses using three sets of published clinical studies, which yielded a fairly narrow range. Further, DRGs 466–470 (major joint replacement or revision) do not distinguish between hip or knee; therefore, we applied the percentage of hip (pTHA+rTHA) discharges calculated from NIS. In addition, recognizing that not every rTHA will be successful,14,26 all rTHA due to dislocation among pTHA patients with SPP were included in our analysis given that available data sources do not distinguish between first and subsequent rTHA. Lastly, we did not consider the fiscal impact associated with lost productivity for rTHA patients covered by self-insured employers who have a vested interest in maintaining a healthy workforce. Despite this exclusion, our approach is conservative in that it underestimates the projected savings.
Conclusions
Among pTHA patients with spinopelvic pathology, a modest reduction in the risk of rTHA due to dislocation could achieve substantial cumulative savings while improving healthcare quality for Medicare and private payer beneficiaries. Value-based payment models should reward strategies using advanced technologies and techniques that reduce the risk of rTHA due to dislocation. Additional research is warranted to further quantify the relative risk reduction associated with these strategies.
Abbreviations
DRG, diagnosis-related group; EoC, Episode of Care; FFS, fee-for-service; MA, Medicare Advantage; MEDPAR, Medicare Provider Analysis and Review; N/n, number; NIS, National Inpatient Sample; pTHA, primary total hip arthroplasty; pTKA, primary total knee arthroplasty; RRR, relative risk reduction; rTHA, revision total hip arthroplasty; rTKA, revision total knee arthroplasty; SPP, spinopelvic pathology; THA, total hip arthroplasty; TKA, total knee arthroplasty; US, United States.
Acknowledgments
Research supported in part by Alphatec Spine (Carlsbad, CA, USA). The content is solely the responsibility of the authors.
Disclosure
SJA is a paid consultant to Alphatec Spine; SI-BONE; and Advise, Connect, Inspire. JMV reports royalties from DePuy Synthes; paid consultant to DePuy and Stryker; and stock or stock options in Corin USA, Intellijoint Surgical, Motion Insights, and OrthoAI. BRS reports no disclosures. JMG reports royalties from OrthoGrid; paid consultant to OrthoGrid, Stryker, and Enovis; stock or stock options in OrthoGrid, Connextions, and MiCare Path; and research support from Stryker, Zimmer Biomet, and Medacta. PKS is a paid consultant to Alphatec Spine. DWP reports royalties from SI-BONE, Globus Medical, and Springer; paid consultant to SI-BONE and Globus Medical; and research support from Medtronic and Mizuho OSI. The authors report no other conflicts of interest in this work.
References
1. Agency for Healthcare Research and Quality (AHRQ), Healthcare Cost and Utilization Project (HCUP), National Inpatient Sample (NIS); 2019. Clinical Classifications Software Refined (CCSR) for ICD-10-PCS procedure codes. Available from: https://datatools.ahrq.gov/hcup-fast-stats.
2. van der Gronde BA, Schlösser TPC, van Erp JHJ, et al. Current evidence for spinopelvic characteristics influencing total hip arthroplasty dislocation risk. JBJS Rev. 2022;10(8). doi:10.2106/JBJS.RVW.22.00038
3. Buckland AJ, Ayres EW, Shimmin AJ, Bare JV, McMahon SJ, Vigdorchik JM. Prevalence of sagittal spinal deformity among patients undergoing total hip arthroplasty. J Arthroplasty. 2020;35(1):160–165. doi:10.1016/j.arth.2019.08.020
4. DelSole EM, Vigdorchik JM, Schwarzkopf R, Errico TJ, Buckland AJ. Total hip arthroplasty in the spinal deformity population: does degree of sagittal deformity affect rates of safe zone placement, instability, or revision? J Arthroplasty. 2017;32(6):1910–1917. doi:10.1016/j.arth.2016.12.039
5. Enocson A, Pettersson H, Ponzer S, Törnkvist H, Dalén N, Tidermark J. Quality of life after dislocation of hip arthroplasty: a prospective cohort study on 319 patients with femoral neck fractures with a one-year follow-up. Qual Life Res. 2009;18(9):1177–1184. doi:10.1007/s11136-009-9531-x
6. American Academy of Orthopaedic Surgeons (AAOS). American Joint Replacement Registry (AJRR) 2021 annual report. Available from: https://connect.registryapps.net/2021-ajrr-annual-report.
7. Ackerman SJ, Knight T, Wahl PM. Projected Medicare savings associated with lowering the risk of total hip arthroplasty revision: an administrative claims data analysis. Orthopedics. 2019;42(1):e86–e92. doi:10.3928/01477447-20181120-03
8. Vigdorchik JM, Sharma AK, Buckland AJ, et al. 2021 Otto Aufranc Award: a simple hip-spine classification for total hip arthroplasty: validation and a large multicentre series. Bone Joint J. 2021;103-B(7 Supple B):17–24. doi:10.1302/0301-620X.103B7.BJJ-2020-2448.R2
9. Vigdorchik J, Eftekhary N, Elbuluk A, et al. Evaluation of the spine is critical in the workup of recurrent instability after total hip arthroplasty. Bone Joint J. 2019;101-B(7):817–823. doi:10.1302/0301-620X.101B7.BJJ-2018-1502.R1
10. Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Hip, knee & shoulder arthroplasty: 2021 annual report. Adelaide: AOA; 2021:1–432. Available from: https://aoanjrr.sahmri.com/annual-reports-2021.
11. Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5–14. doi:10.1016/j.jval.2013.08.2291
12. Medicare Inpatient Hospitals by Geography and Service dataset. Medicare provider analysis and review (MEDPAR) file (CY2019). Available from: https://data.cms.gov/provider-summary-by-type-of-service/medicare-inpatient-hospitals/medicare-inpatient-hospitals-by-geography-and-service/data.
13. Bureau of Labor Statistics, Division of Consumer Prices and Price Indexes. CPI-all urban consumers (current series). Available from: https://data.bls.gov/cgi-bin/surveymost?cu.
14. Hermansen LL, Viberg B, Overgaard S. Risk factors for dislocation and re-revision after first-time revision total hip arthroplasty due to recurrent dislocation - a study from the Danish Hip Arthroplasty Register. J Arthroplasty. 2021;36(4):1407–1412. doi:10.1016/j.arth.2020.10.004
15. Tarazi W, Welch WP, Nguyen N, et al. Medicare beneficiary enrollment trends and demographic characteristics. (Issue Brief No. HP- 2022-08). Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services; 2022. Available from: https://aspe.hhs.gov/sites/default/files/documents/f81aafbba0b331c71c6e8bc66512e25d/medicare-beneficiary-enrollment-ib.pdf.
16. Martino SC, Elliott MN, Dembosky JW, et al. Racial, ethnic, and gender disparities in health care in Medicare Advantage. Baltimore, MD: CMS Office of Minority Health; 2021. Available from: https://www.cms.gov/files/document/racial-ethnic-gender-disparities-health-care-medicare-advantage.pdf.
17. Thirukumaran CP, Cai X, Glance LG, et al. Geographic variation and disparities in total joint replacement use for Medicare beneficiaries: 2009 to 2017. J Bone Joint Surg Am. 2020;102(24):2120–2128. doi:10.2106/JBJS.20.00246
18. Agency for Healthcare Research and Quality (AHRQ), Healthcare Cost and Utilization Project (HCUP), National Inpatient Sample (NIS). Clinical Classifications Software Refined (CCSR) for ICD-10-PCS procedure codes; 2016. Available from: https://datatools.ahrq.gov/hcup-fast-stats.
19. Vigdorchik JM, Madurawe CS, Dennis DA, Pierrepont JW, Jones T, Huddleston JI. High prevalence of spinopelvic risk factors in patients with post-operative hip dislocations. J Arthroplasty. 2023;38(4):706–712. doi:10.1016/j.arth.2022.05.016
20. Novikov D, Mercuri JJ, Schwarzkopf R, Long WJ, Bosco Iii JA, Vigdorchik JM. Can some early revision total hip arthroplasties be avoided? Bone Joint J. 2019;101-B(6_Supple_B):97–103. doi:10.1302/0301-620X.101B6.BJJ-2018-1448.R1
21. Yang DS, McDonald CL, DiSilvestro KJ, et al. Risk of dislocation and revision following primary total hip arthroplasty in patients with prior lumbar fusion with spinopelvic fixation. J Arthroplasty. 2023;38(4):700–705.e1. doi:10.1016/j.arth.2022.03.061
22. Fang CJ, Shaker JM, Ward DM, Jawa A, Mattingly DA, Smith EL. Financial burden of revision hip and knee arthroplasty at an orthopedic specialty hospital: higher costs and unequal reimbursements. J Arthroplasty. 2021;36(8):2680–2684. doi:10.1016/j.arth.2021.03.044
23. Moore BJ, Liang L. Medicare Advantage versus the traditional Medicare program: costs of inpatient stays, 2009–2017: statistical brief #262. 2020 Aug 4. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562007/.
24. Maeda JLK, Nelson L. How do the hospital prices paid by Medicare Advantage plans and commercial plans compare with Medicare fee-for-service prices? Inquiry. 2018;55:46958018779654. doi:10.1177/0046958018779654
25. Congressional Budget Office. How the CBO prepares cost estimates; 2018. Available from: https://www.cbo.gov/publication/53519.
26. Hermansen LL, Viberg B, Hansen L, Overgaard S. “True” cumulative incidence of and risk factors for hip dislocation within 2 years after primary total hip arthroplasty due to osteoarthritis: a nationwide population-based study from the Danish Hip Arthroplasty Register. J Bone Joint Surg Am. 2021;103(4):295–302. doi:10.2106/JBJS.19.01352
27. Hermansen LL, Viberg B, Overgaard S. Patient-reported outcome after dislocation of primary total hip arthroplasties: a cross-sectional study derived from the Danish Hip Arthroplasty Register. Acta Orthop. 2022;93:29–36. doi:10.1080/17453674.2021.1983973
28. Hickey MD, Masri BA, Hodgson AJ. Can technology assistance be cost effective in TKA? A simulation-based analysis of a risk-prioritized, practice-specific framework. Clin Orthop Relat Res. 2022. doi:10.1097/CORR.0000000000002375
29. Kirchner GJ, Lieber AM, Haislup B, Kerbel YE, Moretti VM. The cost of robot-assisted total hip arthroplasty: comparing safety and hospital charges to conventional total hip arthroplasty. J Am Acad Orthop Surg. 2021;29(14):609–615. doi:10.5435/JAAOS-D-20-00715
30. Emara AK, Zhou G, Klika AK, et al. Is there increased value in robotic arm-assisted total hip arthroplasty? : a nationwide outcomes, trends, and projections analysis of 4,699,894 cases. Bone Joint J. 2021;103-B(9):1488–1496. doi:10.1302/0301-620X.103B9.BJJ-2020-2411.R1
31. Affordable Care Act; 2022. Available from: https://www.healthcare.gov/glossary/affordable-care-act/.
32. Dy CJ, Salter A, Barker A, Brown D, Keller M, Olsen MA. Increased utilization of total joint arthroplasty after Medicaid expansion. J Bone Joint Surg Am. 2021;103(6):524–531. doi:10.2106/JBJS.20.00303
33. Toren L, Fronsdal TL, Bhattacharya J, Tamang S. Variation in health care prices across public and private payers. National Bureau of Economic Research. Working Paper 27490; 2020. Available from: http://www.nber.org/papers/w27490.
34. Vigdorchik JM, Sharma AK, Dennis DA, Walter LR, Pierrepont JW, Shimmin AJ. The majority of total hip arthroplasty patients with a stiff spine do not have an instrumented fusion. J Arthroplasty. 2020;35(6S):S252–S254. doi:10.1016/j.arth.2020.01.031
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.