Back to Journals » Journal of Inflammation Research » Volume 16
Proinflammatory and Immunomodulatory Gene and Protein Expression Patterns in Spinal Cord and Spleen Following Acute and Chronic High Thoracic Injury
Authors Michael FM, Patel SP, Bachstetter AD, Rabchevsky AG
Received 11 May 2023
Accepted for publication 4 August 2023
Published 8 August 2023 Volume 2023:16 Pages 3341—3349
DOI https://doi.org/10.2147/JIR.S417435
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Felicia M Michael,1,2 Samir P Patel,1,2 Adam D Bachstetter,2,3 Alexander G Rabchevsky1,2
1Department of Physiology, University of Kentucky, Lexington, KY, 40536-0509, USA; 2Spinal Cord & Brain Injury Research Center (SCoBIRC); University of Kentucky, Lexington, KY, 40536-0509, USA; 3Department of Neuroscience, University of Kentucky, Lexington, KY, 40536-0509, USA
Correspondence: Alexander G Rabchevsky, University of Kentucky, Department of Physiology, Spinal Cord & Brain Injury Research Center (SCoBIRC), B471, Biomedical & Biological Sciences Research Building, 741 South Limestone Street, Lexington, KY, 40536-0509, USA, Tel +1 859-323-0267, Fax +1 859-257-5737, Email [email protected]
Introduction: In addition to paralysis and loss of sensation, high-level spinal cord injury (SCI) causes sympathetic dysfunction that can lead to autonomic dysreflexia (AD) and chronic immune suppression involving splenic leukopenia. Evidence has shown that treatment with either gabapentin or blockade of TNFα mitigates maladaptive plasticity and the underlying hemodynamic dysfunction, spleen atrophy, and immune dysfunction associated with AD. Because significant improvements long term was noted following treatments only during acute stages of recovery, we sought to systematically examine changes in proinflammatory and immunomodulatory cytokines to ascertain the reason.
Methods: Adult female Wistar rats underwent complete T4 spinal transection before euthanasia at systematic intervals from 3 days to 8 weeks after injury. Using qRT-PCR and meso scale discovery (MSD) assays, the gene and protein expression of TNFα and IFNγ in the spleen, upper thoracic (T4-9) and lumbosacral (L5-S6) spinal cords were analyzed.
Results: We found that spleen atrophy occurs in a biphasic manner compared to naïve controls, with significant decreases in the spleen mass noted at 3 days and 8 weeks after injury. Splenic TNFα mRNA and protein levels did not change significantly over time, while IFNγ gene expression dipped acutely with trends for increased protein levels at more chronic time points. TNFα protein increased significantly only in thoracic spinal cord segments from 3 to 14 days post-injury. IFNγ mRNA and protein levels remained unelevated in injured spinal cords over time, with trends for increased protein levels at 2 and 8 weeks in the lumbosacral segments.
Discussion: Novel temporal-spatial cytokine expression profiles reveal that TNFα protein levels are increased solely in upper thoracic segments after high thoracic SCI, while IFNγ remains unaltered. Splenic leukopenia and latent systemic immunosuppression are not associated with altered TNFα or IFNγ expression in the spleen or spinal cord.
Keywords: autonomic dysreflexia, cytokine expression, spleen atrophy, inflammation
Introduction
Spinal cord injury (SCI) at the upper thoracic level or higher disrupts sensory and motor activities but, equally importantly, renders fulminant autonomic dysfunction. Disruption of supraspinal sympathetic regulation results in severe secondary complications such as autonomic dysreflexia and immunosuppression.1,2 The hypothalamic-pituitary-adrenal axis and sympathetic nervous system are two primary mediators of neuroimmune interactions,3 employing cytokines such as TNFα, interleukin-1β, and IL-6, as well as chemokines, to modulate neuronal and glial cell function in the central nervous system.4 In addition, neuroimmune modulation of the spleen is mediated by sympathetic innervation,5 primarily via norepinephrine (NE) actions that aid helper T cell maturation, and its turnover rate in the spleen is directly associated with immune suppression.6
Following particularly high levels of SCI, immune deficiency syndrome can develop in humans,7 rats,8 and mice,9 wherein injured individuals have a higher susceptibility to infections. In models of high (T3) versus lower thoracic (T9) spinal cord transection, the increased incidence and severity of autonomic dysreflexia and immune suppression are correlated with significant leukopenia and spleen atrophy within weeks after T3 SCI.2,10 It has recently been reported that the frequency and severity of autonomic dysreflexia, along with splenic leukopenia and atrophy in T3 injured mice, are chronically mitigated following acute treatment with gabapentin.11 While the mechanisms are noted to involve glutamatergic neurotransmission of spinal cord interneurons,10,12,13 it has similarly been reported that intrathecal blockade of soluble TNFα in T3 injured rats mitigates chronic fulminate autonomic dysreflexia and associated immunosuppression, along with leukopenia and spleen atrophy.14 However, such a blockade with a 2-week delay after injury is not equally effective chronically,15 indicating that acute maladaptive plasticity is critical. Spinal cord-splenic crosstalk via sympathetic innervation renders leukopenia after high thoracic SCI,16–18 so we characterized the spatial-temporal changes in gene and protein expression of proinflammatory and immunomodulatory cytokines, TNFα and IFNγ, in the spleen and both thoracic and lumbosacral spinal cord segments from 3 days to 8 weeks after high thoracic SCI in adult rats.
Materials and Methods
Ethics
All experimental procedures were approved by the Institutional Animal Care and Use Committee (approval number:00733M2004) at the University of Kentucky, and all animal handling techniques were performed in accordance with the National Institutes of Health guidelines for ethical treatment.
Animals
Adult female Wistar rats (n=77, 250–300 g) were obtained from Envigo Harlan and maintained in the Division of Laboratory Animal Resources at the University of Kentucky with access to food and water ad libitum under a 12 h light/dark cycle. The experiments were performed in two cohorts. Cohort I (n=4–7 per group) was assigned solely to qPCR, whereas both qPCR and meso scale discovery (MSD) assays were performed in Cohort II (n=6 per group). The rats were randomly assigned to undergo complete T4 spinal cord transection and subsequent euthanasia at 3 (n=12), 7 (n=13), 14 (n=10), 28 (n=10), or 56 (n=11) days post-injury (DPI), along with age-matched controls (n=18). Age-matched naïve rats were housed individually and euthanized in parallel with the injured animals. A total of n=3 injured animals were eliminated from the study due to premature deaths of unknown causes.
Spinal Cord Injury
Adult female Wistar rats (225–250 g) were anesthetized with ketamine (80 mg/kg, i.p., Zoetis, NJ, USA) and xylazine (10 mg/kg, i.p., Akorn, IL USA) before undergoing T3 laminectomy followed by complete T4 transection using a size 11 scalpel blade under aseptic conditions, as previously reported.19,20 A piece of gelfoam was placed between the cut edges to ensure complete transection and achieve hemostasis prior to sewing the surrounding muscles with absorbable 3–0 vicryl sutures and stapling a closed skin incision with Michel wound clips (Stoelting, Wood Dale, IL). Animals were housed in individual cages on heating pads until euthanasia.
Animal Care
Prior to surgery, the dorsal surface of each animal was shaved and sterilized with 70% isopropyl alcohol and povidone-iodine solution. Their eyes were protected from dryness by applying lubricating ophthalmic ointment. After surgery, animals received antisedan (0.5 mg/kg s.c., Zoetis Inc., Kalamazoo, MI) to reverse the effects of xylazine, and 5 mL of lactated Ringer’s solution (s.c.) to maintain hydration. Animals received buprenorphine (0.03 mg/kg s.c. Reckitt Benckiser Healthcare, Hull, UK) twice a day for 3 days post-surgery for pain management, as well as the antibiotic cephazolin (33.3 mg/kg s.c. WG Critical Care, NJ, USA) twice a day prophylactically for 5 days post-injury to prevent infections. Bladders of the spinal rats were manually emptied twice daily using the Crede maneuver during the entire study period.
Tissue Collection
At designated time points, the injured rats and age-matched controls were euthanized by CO2 narcosis, followed by cervical decapitation. The dissected spleens and pieces (~1.5 cm) of high thoracic (T4-9) and lumbosacral (L5-S6) spinal cords were weighed immediately and snap frozen in dry ice and stored at −80°C until use.18,21 Tissue samples were pulverized in a chilled cryo-cup, weighed, and divided into two tubes for protein and ribonucleic acid (RNA) isolation. The experimenters were blinded to the animal groups at the time of the tissue collection.
RNA Isolation and qPCR Analysis
RNA was isolated from the samples using TRIzol reagent (Invitrogen, California, USA) according to the manufacturer’s recommendations. Briefly, tissues were homogenized in a Trizol (1 mL/100 mg) and chloroform mixture (5:1). The homogenate was centrifuged at 4°C for 15 min at 12,000g. The aqueous phase containing RNA was collected and precipitated using chilled isopropanol and washed with 70% ethanol before being dissolved in RNAse-free water for storage at −80°C. The isolated RNA was quantified and assessed for purity by calculating the optical density at 260 and 280 nm using a Nanodrop 2000c (Thermo Fisher Scientific, Waltham, MA, USA).22 Isolated RNA (2 µg) was reverse-transcribed using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, USA) according to the manufacturer’s protocol. Complementary DNA (cDNA) was diluted 1:5 in RNAse-free water and was used as a template for qPCR. The PCR reaction (hold 2 min at 50°C, followed by 10 min at 95°C, followed by 40 cycles of 15s at 95°C and 1 min at 60°C) was set up in a QuantStudioTM 7 Flex Real-Time PCR system using TaqManTM Fast Advanced Master Mix and TaqMan gene expression assays (glyceraldehyde 3- phosphate dehydrogenase (Rn99999916_s1), TNFα (Rn99999017_m1), and IFNγ (Rn00594078) (Thermo Fisher Scientific; USA).22 The change in mRNA levels was expressed as fold-change normalized to naïve rats using glyceraldehyde 3- phosphate dehydrogenase as an internal control.23
Protein Isolation and Cytokine Measurements
Tissues were homogenized in lysis buffer (1:20 w/v) containing Phosphate buffered saline (PBS), 1 mM Ethylenediaminetetraacetic acid (EDTA), 1X Halt™ protease inhibitor cocktail (Thermo Fisher, MA, USA), and 1 mM Phenylmethanesulfonyl fluoride (PMSF) using Omni Beadrupter 24 (Omni International, GA, USA). The samples were centrifuged at 12,000 × g for 20 min at 4°C using Beckman Microfuge18 (Beckman Coulter, CA, USA). The supernatant was collected, aliquoted, and used for protein estimation and cytokine measurements. TNFα and IFNγ levels were measured by ELISA using kits from Meso Scale Discovery (MSD) (Meso Scale Diagnostics, MD, USA) according to the manufacturer’s instructions. Briefly, after blocking for 1 h with Blocker H provided in the kit, the supernatant containing ~100–200 μg of protein was loaded (50μL/well for spleen samples and 100μL/well for spinal cord samples) into the MSD plate and incubated overnight at 4°C with constant shaking at 700 rpm. The plate was rinsed with wash buffer (PBS containing 0.05% Tween 20) and subsequently incubated with the antibody cocktail for TNFα and IFNγ supplied with the kit for 3 h. The antibody cocktail was washed with wash buffer and replaced with proprietary 2X MSD read buffer prior to reading the signal using MESO QuickPlex SQ 120MM. Cytokine levels were normalized to the total amount of protein in the loaded sample, as determined by BCA Protein Assay (Thermo Fisher, MA, USA).
Statistical Analysis
Statistical analyses between control and injured rats were performed using Welch’s one-way analysis of variance among groups, and if significant, Dunnett T3 multiple comparison post-hoc tests were performed using GraphPad Prism Version 9. Statistical significance throughout all experiments was set at p<0.05. Data are presented as mean ± SD.
Results
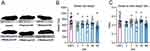
Although there were no gross morphological differences in the spleens between the groups (Figure 1A), there were significant reductions in spleen wet weights (W (5, 29.01) = 4.051, p=0.0065) at 3-days (p=0.0253, Dunnett’s test) and 56-days (p=0.0089, Dunnett’s test) post-injury (DPI) (Figure 1B) compared with control animals.
Unexpectedly, there was a high variability in age-matched naïve control spleen weights. Thus, we sought to determine whether the variance in spleen weight was due to differences in rat size. We investigated whether normalizing spleen weight to the animal’s body weight would highlight any differences. Although an overall difference between the groups was identified for spleen-to-body weight ratios (W (5, 28.62) = 3.773, p=0.0095), the only difference between the groups was at 3 days post-injury compared to 28 days post-injury (p=0.021, Dunnett’s test). The injured animals showed no change in the spleen-to-body weight ratio compared to control animals over time (Figure 1C).
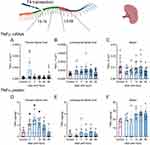
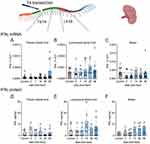
In injured spinal cords and spleens, TNF-α mRNA levels were not different among the groups (Figure 2A–C) according to the Welch test. However, TNF-α protein levels increased significantly in the thoracic segments (W (5,13.92) = 8.163, p=0.0009) at 3-days (p=0.0193, Dunnett’s test), 7-days (p=0.0142, Dunnett’s test), and 14-days (p=0.0091, Dunnett’s test) post-injury (Figure 2D) compared with control animals. In contrast, TNF-α protein levels were unchanged across the groups in the lumbosacral segments (W (5,13.82) = 0.5855, p=0.7111) (Figure 2E). Despite the overall difference in splenic TNF-α protein levels (W (5,13.61) = 3.771, p=0.0235) (Figure 2F), according to Welch’s test, there were no differences between the individual groups.
IFNγ mRNA levels were unaffected by SCI in the thoracic (Figure 3A) and lumbosacral segments (Figure 3B). Comparatively, the levels of splenic IFNγ mRNA (Figure 3C) were significantly affected by SCI (W (5,24.34) = 4.774, p=0.0035), with less IFNγ mRNA found at 3-days (p=0.0190, Dunnett’s test) post-injury. IFNγ protein levels remained unchanged after injury in the thoracic (Figure 3D) and lumbosacral segments (Figure 3E). In the spleen, IFNγ protein levels increased over time; however, this change was not statistically significant (W (5,13.34) = 2.942, p=0.0530) (Figure 3F).
Notably, we found no significant correlations between spleen weight and cytokine transcriptional levels in all tissues and timepoints assessed.
Discussion
We assessed temporal and spatial gene expression and protein changes in proinflammatory and immunomodulatory cytokines in injured spinal cord segments and spleens, simultaneously, at various time points during maladaptive plasticity in the development of autonomic dysreflexia in models of high thoracic SCI.20,24 Spinal regions below the injury were specifically used to determine the cytokine changes due to loss of supraspinal signals following injury. In addition, because sham laminectomies can induce changes in gene expression, we assessed injured and naïve spinal cord and spleen tissues using qPCR and MSD ELISA for TNF-α and IFN-γ in the same high thoracic SCI model.14,25
We previously reported that following the same T4 transection in adult rats, there was no injury effect on spleen size after four weeks.26 In the current study, we found for the first time that spleen weight decreased acutely at 3 DPI and then only after 8 weeks compared to naïve age-matched controls. Spleen size differences can be attributed to innate variability, irrespective of injury, and to different sources and strains of rats used in autonomic dysreflexia studies.1,14,19,24,27–29 The high variability among the naïve controls was unexpected. The factors that contributed might be varied with age, body length, nutritional status, pedigree, susceptibility to infections, etc. We accounted for body weight loss (including muscle atrophy) and found that the spleen-to-body weight ratio remained unaltered from to 1–8 weeks post-injury. While previous reports have noted a similar lack of spleen atrophy 2–20 weeks following low cervical spinal cord injury in adult rats,30 the current findings reveal a biphasic manner of spleen atrophy after SCI that has not yet been described. Such a biphasic response could be attributed to changes in the injured spinal cord due to primary and secondary cell death, altered phenotype of microglia/brain macrophages, reorganization of damaged axons, sprouting of new sympathetic propriospinal neurons, glial scar etc. Moreover, this could be a sex-dependent response as only female rats have been assessed.
Concomitantly, neither splenic TNFα mRNA nor protein levels changed significantly over time, with only trends for increased protein levels from to 4–8 weeks, whereas IFNγ gene expression significantly decreased 3 days before returning to control levels, followed by trends for increased protein levels from to 2–8 weeks. Such acute decreases in IFN-γ expression in the spleen may reflect splenocyte activation or leukopenia onset, thereby compromising immune function.2,10,11 Comparatively, TNF-α mRNA levels remained at control values in injured spinal cords, whereas TNFα protein levels increased significantly in thoracic spinal cord segments from 3 to 14 days post-injury before returning to control levels. This is at odds with reports of elevated soluble TNFα protein in the lumbosacral spinal cord one and four weeks following T3 SCI.14,25 Moreover, TNF-α mRNA levels in the injured spinal cord did not change significantly over time post-injury.
IFNγ is an immunomodulatory cytokine with a potential dual role in SCI-mediated inflammatory responses, wherein it helps to recruit both tissue-repairing and repair-inhibiting macrophages.31,32 Injured spleens showed an acute decline in IFNγ mRNA levels, whereas injured spinal cords maintain control levels over time and displayed minor chronic increases in thoracic segments, which were not statistically different from those in the control group. No statistically significant differences were found in IFNγ protein levels, but increasing trends chronically were observed in both the spleen and lumbosacral spinal cord. IFNγ is implicated in promoting synaptic plasticity33 and amplifying nociceptive synaptic transmission,34 so trends for increased expression in thoracic spinal cord segments at one- and two-weeks post-injury is associated with time-dependent increases in the severity of autonomic and neuroimmune dysfunction.2 In addition, prophylactic gabapentin treatment reduced synaptogenesis in the thoracic spinal cord during the development of autonomic dysreflexia.11
Conclusion
Following gene and protein expression analyses, we present novel inflammatory and immunomodulatory gene levels in the spleen and thoracic versus lumbosacral segments of the spinal cord in the days and weeks following complete upper thoracic SCI. While our study confirmed chronic splenic atrophy in this AD model, we are the first to report dynamic atrophy over time post-injury. We also found that elevations in inflammatory TNFα protein levels occurred in injured thoracic spinal cord segments overtime, but not in distal lumbosacral segments. These spatial-temporal findings reveal novel gene and protein expression patterns for TNF-α and IFNγ during periods of maladaptive intraspinal plasticity underlying the developing pathophysiology of autonomic dysreflexia. Additionally, our collective findings underscore careful consideration of species, sources, and innate variability regarding the sequential measurements of organ tissues, notably among uninjured naïve animals.
Abbreviations
cDNA, complementary deoxyribonucleic acid; FACS, Fluorescence-activated cell sorting; IFNγ, Interferon gamma; IL, interleukin; NE, norepinephrine; PCR, polymerase chain reaction; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction; RNA, ribonucleic acid; s.c., subcutaneous; SCI, spinal cord injury; sTNF, soluble tumor necrosis factor alpha; TNFα, tumor necrosis factor alpha; MSD, meso scale discovery.
Data Sharing Statement
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
All experimental procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee and all animal handling techniques were performed in accordance with the National Institutes of Health guidelines for ethical treatment.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
Craig H. Neilsen Postdoctoral Fellowship #651019 (FMM); University of Kentucky, SCoBIRC Chair #1 Endowment (AGR).
Disclosure
The authors declare that they have no competing interest in this work.
References
1. Eldahan KC, Rabchevsky AG. Autonomic dysreflexia after spinal cord injury: systemic pathophysiology and methods of management. Auton Neurosci. 2018;209:59–70. doi:10.1016/j.autneu.2017.05.002
2. Zhang Y, Guan Z, Reader B, et al. Autonomic dysreflexia causes chronic immune suppression after spinal cord injury. J Neurosci. 2013;33:12970–12981. doi:10.1523/JNEUROSCI.1974-13.2013
3. Nance DM, Macneil BJ. Immunoregulation by the sympathetic nervous system. Neuroimmune Biol. 2001;1:121–139.
4. Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6(10):775–786. doi:10.1038/nrn1765
5. Cano G, Sved AF, Rinaman L, Rabin BS, Card JP. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J Comp Neurol. 2001;439(1):1–18. doi:10.1002/cne.1331
6. Zha J, Smith A, Andreansky S, Bracchi-Ricard V, Bethea JR. Chronic thoracic spinal cord injury impairs CD8+ T-cell function by up-regulating programmed cell death-1 expression. J Neuroinflammation. 2014;11(1):65. doi:10.1186/1742-2094-11-65
7. Riegger T, Conrad S, Schluesener HJ, et al. Immune depression syndrome following human spinal cord injury (SCI): a pilot study. Neuroscience. 2009;158:1194–1199. doi:10.1016/j.neuroscience.2008.08.021
8. Riegger T, Conrad S, Liu K, Schluesener HJ, Adibzahdeh M, Schwab JM. Spinal cord injury-induced immune depression syndrome (SCI-IDS). Eur J Neurosci. 2007;25:1743–1747. doi:10.1111/j.1460-9568.2007.05447.x
9. Brommer B, Engel O, Kopp MA, et al. Spinal cord injury-induced immune deficiency syndrome enhances infection susceptibility dependent on lesion level. Brain. 2016;139:692–707. doi:10.1093/brain/awv375
10. Ueno M, Ueno-Nakamura Y, Niehaus J, Popovich PG, Yoshida Y. Silencing spinal interneurons inhibits immune suppressive autonomic reflexes caused by spinal cord injury. Nat Neurosci. 2016;19:784–787. doi:10.1038/nn.4289
11. Brennan FH, Noble BT, Wang Y, et al. Acute post-injury blockade of alpha2delta-1 calcium channel subunits prevents pathological autonomic plasticity after spinal cord injury. Cell Rep. 2021;34:108667. doi:10.1016/j.celrep.2020.108667
12. Maiorov DN, Krenz NR, Krassioukov AV, Weaver LC. Role of spinal NMDA and AMPA receptors in episodic hypertension in conscious spinal rats. Am J Physiol. 1997;273:H1266–H1274. doi:10.1152/ajpheart.1997.273.3.H1266
13. Noble BT, Brennan FH, Wang Y, et al. Thoracic VGluT2(+) spinal interneurons regulate structural and functional plasticity of sympathetic networks after high-level spinal cord injury. J Neurosci. 2022;42:3659–3675. doi:10.1523/JNEUROSCI.2134-21.2022
14. Mironets E, Osei-Owusu P, Bracchi-Ricard V, et al. Soluble TNFalpha signaling within the spinal cord contributes to the development of autonomic dysreflexia and ensuing vascular and immune dysfunction after spinal cord injury. J Neurosci. 2018;38:4146–4162. doi:10.1523/JNEUROSCI.2376-17.2018
15. O’Reilly ML, Mironets E, Shapiro TM, et al. Pharmacological inhibition of soluble tumor necrosis factor-alpha two weeks after high thoracic spinal cord injury does not affect sympathetic hyperreflexia. J Neurotrauma. 2021;38(15):2186–2191. doi:10.1089/neu.2020.7504
16. Monteiro S, Pinho AG, Macieira M, et al. Splenic sympathetic signaling contributes to acute neutrophil infiltration of the injured spinal cord. J Neuroinflammation. 2020;17:282. doi:10.1186/s12974-020-01945-8
17. Noble BT, Brennan FH, Popovich PG. The spleen as a neuroimmune interface after spinal cord injury. J Neuroimmunol. 2018;321:1–11. doi:10.1016/j.jneuroim.2018.05.007
18. Wu F, Ding XY, Li XH, et al. Cellular inflammatory response of the spleen after acute spinal cord injury in rat. Inflammation. 2019;42:1630–1640. doi:10.1007/s10753-019-01024-y
19. Cameron AA, Smith GM, Randall DC, Brown DR, Rabchevsky AG. Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia. J Neurosci. 2006;26:2923–2932. doi:10.1523/JNEUROSCI.4390-05.2006
20. Rabchevsky AG, Patel SP, Lyttle TS, et al. Effects of gabapentin on muscle spasticity and both induced as well as spontaneous autonomic dysreflexia after complete spinal cord injury. Front Physiol. 2012;3:329. doi:10.3389/fphys.2012.00329
21. Bansal S, Friedrichs WE, Velagapudi C, et al. Spleen contributes significantly to increased circulating levels of fibroblast growth factor 23 in response to lipopolysaccharide-induced inflammation. Nephrol Dial Transplant. 2017;32:960–968. doi:10.1093/ndt/gfw376
22. Michael FM, Chandran P, Chandramohan K, et al. Prospects of siRNA cocktails as tools for modifying multiple gene targets in the injured spinal cord. Exp Biol Med. 2019;244(13):1096–1110. doi:10.1177/1535370219871868
23. Zhang Z, Shen M, Gresch PJ, et al. Oligonucleotide-induced alternative splicing of serotonin 2C receptor reduces food intake. EMBO Mol Med. 2016;8:878–894. doi:10.15252/emmm.201506030
24. West CR, Popok D, Crawford MA, Krassioukov AV. Characterizing the temporal development of cardiovascular dysfunction in response to spinal cord injury. J Neurotrauma. 2015;32:922–930. doi:10.1089/neu.2014.3722
25. Mironets E, Fischer R, Bracchi-Ricard V, et al. Attenuating neurogenic sympathetic hyperreflexia robustly improves antibacterial immunity after chronic spinal cord injury. J Neurosci. 2020;40:478–492. doi:10.1523/JNEUROSCI.2417-19.2019
26. Eldahan KC, Williams HC, Cox DH, Gollihue JL, Patel SP, Rabchevsky AG. Paradoxical effects of continuous high dose gabapentin treatment on autonomic dysreflexia after complete spinal cord injury. Exp Neurol. 2020;323:113083. doi:10.1016/j.expneurol.2019.113083
27. Rivas DA, Chancellor MB, Huang B, Salzman SK. Autonomic dysreflexia in a rat model spinal cord injury and the effect of pharmacologic agents. Neurourol Urodyn. 1995;14:141–152. doi:10.1002/nau.1930140207
28. Trueblood CT, Iredia IW, Collyer ES, Tom VJ, Hou S. Development of cardiovascular dysfunction in a rat spinal cord crush model and responses to serotonergic interventions. J Neurotrauma. 2019;36:1478–1486. doi:10.1089/neu.2018.5962
29. Squair JW, West CR, Popok D, et al. High thoracic contusion model for the investigation of cardiovascular function after spinal cord injury. J Neurotrauma. 2017;34:671–684. doi:10.1089/neu.2016.4518
30. Ulndreaj A, Tzekou A, Siddiqui AM, Fehlings MG. Effects of experimental cervical spinal cord injury on peripheral adaptive immunity. PLoS One. 2020;15:e0241285. doi:10.1371/journal.pone.0241285
31. Ishii H, Tanabe S, Ueno M, et al. Ifn-gamma-dependent secretion of IL-10 from Th1 cells and microglia/macrophages contributes to functional recovery after spinal cord injury. Cell Death Dis. 2013;4:e710. doi:10.1038/cddis.2013.234
32. Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29(43):13435–13444. doi:10.1523/JNEUROSCI.3257-09.2009
33. Victorio SC, Havton LA, Oliveira AL. Absence of IFNgamma expression induces neuronal degeneration in the spinal cord of adult mice. J Neuroinflammation. 2010;7:77. doi:10.1186/1742-2094-7-77
34. Reischer G, Heinke B, Sandkuhler J. Interferon-gamma facilitates the synaptic transmission between primary afferent C-fibres and lamina I neurons in the rat spinal dorsal horn via microglia activation. Mol Pain. 2020;16:1744806920917249. doi:10.1177/1744806920917249
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.