Back to Journals » OncoTargets and Therapy » Volume 12
Progressive study of effects of erianin on anticancer activity
Authors Zhang Y, Zhang Q, Wei F, Liu N
Received 2 January 2019
Accepted for publication 18 May 2019
Published 9 July 2019 Volume 2019:12 Pages 5457—5465
DOI https://doi.org/10.2147/OTT.S200161
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr XuYu Yang
Yuying Zhang,1 Qianqian Zhang,1 Fanhua Wei,2 Na Liu1
1School of Biological Science and Technology, University of Jinan, Jinan 250022, People’s Republic of China; 2College of Agriculture, Ningxia University, Yinchuan 750021, People’s Republic of China
Abstract: Erianin is the major bisbenzyl compound extracted from the traditional Chinese medicine Dendrohium chrysotoxum Lindl. Erianin possesses many biological properties relevant to cancer prevention and therapy. The previous studies confirmed that antitumor effects of erianin are regulated with multiple signaling pathways. The mechanisms of erianin are numerous, and most of them induce cancer cell apoptosis that may be intrinsic or extrinsic and modulate the ROS/JNK signaling pathways. Invasion, migration, and angiogenesis represent emerging targets of erianin and support its anticancer properties. This review aimed to summarize the recent advances in the antitumor activity of erianin and to provide a rationale for further exploring the potential application of erianin in overcoming cancer in the future.
Keywords: Erianin, antitumor, apoptosis, cell cycle arrest, molecular mechanism
Introduction
Cancer is a critical illness whose cell division and growth are uncontrolled after gene mutation. It has been estimated that around 13.2 million cancer patients will die annually by 2030 worldwide.1 There are 14 million new cases and this number is expected to increase up to 22 million within the next two decades according to the statistical data from the WHO in 2012.2 The incidence of cancer is increasing because of the growth and aging of human population as well as because of an increasing prevalence of established risk factors such as obesity, smoking, physical inactivity, and changing reproductive patterns associated with economic development.3 The resistance of malignant cells to cancer therapeutics is the top reason for cancer treatment failure and makes cancer malignancies refractory and challenging.4,5 In addition, side effects and high costs of cancer therapies limit their clinical applications.6,7 Therefore, the development of novel anticancer drugs or therapeutics without side effects or in combination with chemotherapeutic agents is helpful for cancer therapy. Nowadays, natural products have attracted more attention because of their multi-target characteristics and their ability to bind to specific cellular targets.8
Medicinal plants are important sources of novel therapeutic drugs that are promising for cancer treatment.9 A number of bioactive constituents from Dendrobium plants, such as polysaccharides,10 bibenzyls,11 phenanthrones,12 alkaloids,13 sesquiterpenoids,14 fluorenone,15 coumarins,16 triterpene glycosides,17 and volatile oil chemical composition, are obtained and show diverse pharmacological functions including anticancer, neuroprotective, antidiabetic, and immune-modulating activities. Erianin, which is a natural bibenzyl compound, is the most noteworthy constituent and has been used as an anti-pyretic and an analgesic in traditional Chinese medicine.18 In recent years, several studies have proved that erianin has strong anticancer activities in a variety of human cancer cells, including breast cancer cell line T47D,19 human hepatocarcinoma Bel7402 and melanoma A375 cells,20 human promyelocytic leukemia HL-60 cells,21 and human osteosarcoma cells,22 as shown in Table 1.
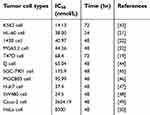 |
Table 1 Summary of anti-proliferation of various types of cells by erianin |
This review summarizes the progress of erianin as a model of natural product in anticancer effects in recent years and expounds the molecular mechanism related to anticancer activity. Finally, we will provide future perspectives for erianin as a novel cancer therapeutic remedies in both preventing and overcoming cancer.
Structure and bioactivities of erianin
Erianin (2-methoxy-5-[2-(3,4,5-trimethoxy-phe-nyl)-ethyl]-phenol) is a low-molecular-weight natural product which is first isolated from the Eria coronaria of Orchidaceae in India23 and is also extracted from Dendrobium chrysotoxum Lindl.24 The erianin structure consists of two substituted aromatic (aryl) rings linked by a two-carbon alkene bridge with several methoxyl substitutions on the phenyl rings (Figure 1) and belongs to the bibenzyl derivatives. Many compounds of the bibenzyl derivatives have shown antiviral,25 antibacterial,26,27 and anti-inflammatory response28 and anticancer activities.29,30
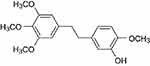 |
Figure 1 The chemical structure of erianin. |
At present, multiple pharmacological effects of erianin have been elucidated, including antioxidative and antitumor activity. It is worth mentioning that erianin shows therapeutic potential to inhibit multiple cancers in vivo and in vitro. Erianin has been reported to inhibit cell proliferation and induce apoptosis in human osteosarcoma cells22 and reverse multidrug resistance in B16/hMDR-1 cells.31 Erianin treatment inhibits tumor growth in mice with HePA (tumor suppressor rate: 50.82%) and ESC (tumor suppressor rate: 51.96%).32 In addition, some stilbene33 and phenanthrene derivatives34 that have a structure similar to erianin, also display potent antitumor activity. For instance, combretastatin A-4 (CA-4),35 isolated from a South African tree Combretum caffrum, is one of the most naturally potent antitumor agent with activity in the low-nanomolar range. Previous research showed that CA-4 inhibits tubulin polymerization at the colchicine-binding site of β-tubulin and leads to cytoskeletal instability, resulting in morphological changes of immature or proliferating endothelial cells.36–38 Moreover, disodium phosphate CA-4P is undergoing several advanced clinical trials.39
Despite remarkable anticancer properties of erianin, its application in pharmaceutical industries is limited for a variety of reasons, including low bioavailability, water solubility, and chemical stability; indeed, this molecule is rapidly and extensively decomposed and excreted.40 To overcome these, various attempts by medicinal chemistry have been made to improve the bioavailability of erianin. A previous study reported that the 1,1-ethane bridge encountered in isoerianin derivatives can replace the 1,2-ethane bridge of natural erianin with no loss of activity.41 Several compounds exhibited excellent antiproliferative activity at nanomolar concentrations against a panel of human cancer cell lines. Next, research have demonstrated a new class of isoerianin derivatives, named azaisoerianins, in which the two aromatic rings are connected through a nitrogen atom in place of the carbon linker in isoerianin compounds.42 Azaisoerianins showed excellent antiproliferative activity with mean GI50 values at a nanomolar level in a diverse set of human cancer cells. These compounds also inhibited tubulin assembly at a micromolar range, arrested the cellular cycle in the G2/M phase, and induced apoptosis at very low concentrations. Therefore, new formulations were designed and synthesized as a novel class of erianin analogs may be a way to improve erianin’s bioavailability and its druggability.
Anticancer bioactivities and mechanisms of erianin
Apoptosis
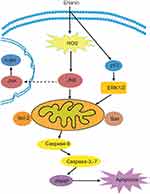
Apoptosis plays an important role in cancer treatment as most anticancer therapies primarily act by activating the apoptosis pathways, which are the final goals of anticancer therapies.51 There are two types of apoptosis pathways. One is the intrinsic pathway that occurs through mitochondrial pathway, which increases mitochondrial permeability, mitochondrial release of cytochrome C, and subsequent cysteinyl aspartate-specific proteinase (caspase) activations. Caspase is a kind of proteinase family, which is divided into three categories, apoptosis initiation factors, apoptosis effect factors, and inflammatory mediators. Apoptosis initiation factors initiate programmed apoptosis, including caspase-2, caspase-8, caspase-9, caspase-10, and so on, which are cleaved by other proteins for activation of downstream caspases. For example, almost all caspases cascade reaction can be activated by caspase-8 to induce apoptosis.52,53 Apoptosis effect factors are located downstream of apoptosis cascade reaction, including caspase-3, caspase-6, and caspase-7, which act on their specific substrates. Caspase-3 is one of the most important apoptosis executors in the caspase family and is the main effector factor in apoptosis. In normal cells, caspase exists in an inactive zymogen form, but it is activated when cells are stimulated by apoptosis induction factors such as physical or chemical regulators. Extrinsic pathway involves activation of death receptors by ligand binding leading to the formation of the death-inducing signaling complex and activation of caspase-8, and final induction of the mitochondrial pathway.
The elevating evidence suggest that targeting apoptosis of cancer cells is feasible.54 Several proapoptotic and antiapoptotic proteins are attractive targets for anticancer therapies. Antiapoptotic proteins inhibit cancer cell apoptosis such as Bcl-2, Bcl-XL, Bcl-W, Mcl-1, Bfl1/A1, Bcl-G, and so on. The proapoptotic signal promotes cancer cell apoptosis including Bax, Bak, Bad, Bim, Bik, Puma, Noxa, and so on. Erianin can induce the early apoptosis and late apoptosis in T47D human breast cancer cells, and the proapoptotic effect of erianin depends on reducing Bcl-2 expression and activating caspase signaling.19 Erianin decreases human osteosarcoma cells viability in a time- and dose-dependent manner.22 HL-60 cells exposed to erianin of 12.5–81.9 nM/L show evident antiproliferative effect after 24, 48, and 72 hrs in a concentration-dependent manner, and erianin treatment induces cancer cell apoptosis by downregulating the expression of Bcl-2 gene while upregulating the expression of Bax gene.21 In EJ bladder cancer cells, erianin significantly inhibits cell proliferation in a dose-dependent manner, and the IC50 value is 65.04 nM/L after 48 hrs of exposure to erianin.44 Meanwhile, erianin-mediated inhibitory effect on proliferation of EJ cells is largely attenuated through the knockdown of Bim gene or over-expression of Bcl-2 gene.45 In gastric cancer SGC-7901 cells,46 erianin inhibits cell proliferation, and the IC50 value is 175.9 nM/L after treatment with erianin for 48 hrs, which probably depends on inhibition of telomerase activity. Furthermore, the effects of apoptosis by erianin in combination with 5-fluorouracil are more remarkable than alone by regulating the expressions of Bax, caspase-3, and Bcl-2.47
The proapoptotic proteins in the Bcl-2 family normally act on the mitochondrial membrane to promote permeabilization and release of cytochrome C and reactive oxygen species (ROS), which are important signals in the apoptosis cascade.55 Basic levels of ROS may function as signals to promote cell proliferation and survival, whereas high levels of ROS can damage cellular components such as DNA, protein, and lipids. Hence, ROS plays a crucial role in cancer cell apoptosis and autophagy, which activates the JNK/c-jun signal pathway.56 Erianin induces an increase in ROS production, while pretreatment with ROS inhibitor NAC remarkably reverses erianin-induced inhibition of cell proliferation, apoptosis and autophagy in osteosarcoma cells. Erianin induces apoptosis and autophagy in human osteosarcoma cell lines by activating ROS-dependent JNK/c-jun pathway.22 Osteosarcoma cells treated with erianin leads to activation of JNK and c-Jun in a concentration-dependent manner, whereas when co-treated with JNK inhibitor sp600125 or dominant negative mutant of c-Jun, it markedly blocks erianin-induced growth-inhibitory and apoptotic effects, suggesting that JNK activation is required for the modulation of erianin-induced cancer cell apoptosis and autophagy. Furthermore, the JNK/stress-activated protein kinase (SAPK) signal is also involved in erianin-induced decreasing in acidification rate.57 JNK/SAPK inhibition can effectively abolish erianin-induced decreasing in ATP levels, and the effect on endothelial metabolism is JNK/SAPK-dependent. JNK/SAPK activation is closely related with mitochondrial permeability, which plays a crucial role in cancer cell apoptosis. Exploring the mechanism involved in directly or indirectly regulating mitochondrial respiration is helpful for understanding the antitumor and antiangiogenic actions of erianin. Meanwhile, erianin-induced mitochondrial-based cancer cell apoptosis through the inhibition of ERK1/2 (extracellular signal-regulated kinase, ERK) signaling and the activation of p53 is also examined.50
Caspase signaling plays an important role in the initiation and process of cell apoptosis. Caspase-3 is the main effector in cell apoptosis, which is activated in apoptotic cells both by extrinsic and by intrinsic pathways.58 In bladder cancer cells, erianin activates caspase cascade reaction and finally triggers the breakage of poly (ADP-ribose) polymerase (PARP) by many caspases in vitro and by caspase-3 in vivo and results in the PARP carboxy-terminal catalytic domain (89 kD) separated from the amino-terminal DNA-binding domain (24 kD), so that PARP abolishes its enzymatic activity and cannot be combined with other proteins to repair damaged DNA. In erianin-treated tumor tissues, erianin increases cleaved caspase-3 level and in apoptosis proportion by the TUNEL assay. In breast cancer cells, erianin treatment significantly upregulates the expression of cleaved-caspase-3, caspase-7, and caspase-9 and alters the ratio of Bcl-2/Bax. In conclusion, erianin can induce tumor cell apoptosis as shown in Figure 2.
Cell cycle
Dysregulation of cell cycle checkpoint is an initiating event in cancer development allowing for cancer cell growth due to changes in intracellular signaling pathways that cause cells to enter the cell cycle without external stimuli.59 The growth factors bind to receptors on the cell membrane, resulting in signals transmitted through the membrane into the cytoplasm led to the activation of downstream target genes which stimulate cells to enter the cell cycle. Erianin has been found to arrest cell cycle at G2/M phase in a variety of cancer cells (except sgc-7091 cell S phase of gastric cancer and caco-2 cell G2 phase of colon cancer). Erianin treatment significantly increases amounts of cells at G2/M phase in a concentration-dependent and time-dependent manner in T47D cells19 and in HL-60 cells.21 In human osteosarcoma cells, erianin treatment increases cell number at G2/M phase after 24 hrs of treatment with elevating concentration, accompanied by decreased cell numbers at S and G0/G1 phases in 143B and MG63.2 cells.22 However, the molecular mechanisms involved in cell cycle arresting by erianin remain not very clear, although the modulation of multiple cell cycle regulatory proteins seems to be involved. Erianin treatment significantly upregulates the expression of p53, cyclin B1, p21, and p27, but significantly downregulates the mRNA levels of CDK1 and CDK7. It illustrates that erianin can upregulate the expression of p21, p27, and other genes to inhibit the activation of cyclin-CDK complexes and to cause G2/M phase arrest, as shown in Figure 3.
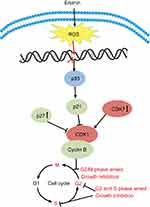 |
Figure 3 The effects of erianin on the tumor cell cycle arrest. Abbreviation: CDK, cyclin-dependent kinases. |
Metastasis
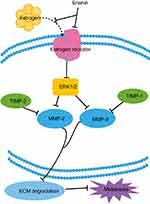
Cancer therapy is easy to relapse mainly due to a cancer diagnosis in later stages and when metastasis has occurred. The migration consists of three processes, including chemotactic migration of tumor cells, guidance of cell membrane elongation activity, between cells and their extracellular matrix adhesion ability, and protease hydrolysis. Therefore, how to effectively using its regulation process to inhibit the invasion and migration of tumor cells is also an urgent topic in anticancer strategies. Matrix metalloproteinases (MMPs) play a crucial role in tumor metastasis. MMPs regulate extracellular matrix degradation and cell adhesion processes to enhance tumor cell invasion and metastasis. Among them, MMP-2 and MMP-9 are key regulators of tumor migration and are capable of degrading type IV collagen which is the most abundant component of the basement membrane. In tumor cells, MMPs are mainly regulated by MAPK (mitogen-activated protein kinases, MAPKs)/ERK signaling pathways and the expression of tissue inhibitor of matrix metalloproteinases (TIMPs). Erianin treatment inhibits the migration of T47D cells by decreasing expression of ERα, p-ERK1/2, MMP2, and MMP9 and increasing expression of TIMP1 and TIMP2.19 In addition, erianin treatment leads to extensive tumor necrosis, growth delay, and rapid vascular shutdown in xenografted human hepatoma Bel7402 and melanoma A375 tumors by inducing endothelial cytoskeletal disorganization.20 Furthermore, erianin also inhibits the movement of tumor cells by upregulating the expression of E-cadherin and downregulating the expression of N-cadherin and fibronectin.
Overall, these results suggest that the migration of tumor cells is largely inhibited by erianin, which further acts on JAK2/STAT3 and ERK1/2 pathway and its downstream gene MMP-2 and MMP-9 to inhibit the tumor metastasis, as shown in Figure 4.
Angiogenesis
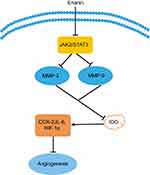
Tumor angiogenesis is the growth of new blood vessels around the tumor tissue to meet the needs of oxygen and nutrients for tumor cells. Indoleamine 2, 3-dioxygenase (IDO) is a rate-limiting enzyme of tryptophan metabolism along the kynurenine pathway.61 IDO can promote tumor cell invasion, metastasis, vasculogenic mimicry, and angiogenesis. Several inflammatory factors are involved in IDO regulation and angiogenesis such as COX-2, hypoxia-inducible factor 1 (HIF-1), IL-6, and so on. Furthermore, JAK (Janus kinase, JAK)/STAT (signal transducers and activators of transcription, STAT) pathway is closely related with IDO in tumor angiogenesis. Erianin effectively inhibits the expression of IDO and its enzyme activity in mice Lewis lung cancer 2LL cells.62 And erianin effectively decreases the expression of IDO-mediated inflammatory factors COX-2, HIF-1α, and IL-6. Meanwhile, erianin treatment decreases the phosphorylation of JAK2 and STAT3 levels and the expression of MMP2 and MMP9. As shown in Figure 5, erianin regulates the expression of key molecules in tumor angiogenesis and inflammatory microenvironment. Furthermore, erianin exhibits potent antiangiogenic activities in vitro by abrogating spontaneous or basic fibroblast growth factor-induced neovascularization, disrupting endothelial tube formation, inhibiting proliferation of human umbilical vein endothelial cells, and abolishing migration across collagen and adhesion to fibronectin.20 Erianin also induces endothelial cytoskeletal disorganization by depolymerizing both F-actin and β-tubulin.20 In addition, erianin inhibits HG-induced vascular endothelial growth factor (VEGF) expression, HIF-1α translocation into nucleus, and ERK1/2 activation in choroid-retinal endothelial cells RF/6A and microglia cells BV-2.63 In RF/6A cells, erianin inhibits VEGF-induced activation of VEGF receptor 2 and its downstream signaling pathways such as cRaf-MEK1/2-ERK1/2 and PI3K-AKT.63 Interestingly, ZJU-6, a derivative of erianin, has a significant antiangiogenesis capability to pancreatic cancer cells MiaPaCa-2, breast cancer cells MDA-468, and colon cancer cell line HCC-2998.64 The erianin derivative and its glycoside analog both have a potent inhibitory effect on HL-60 and lung cancer cells A549.
Autophagy
Dysregulated autophagy in cancer is reported in 1999.65 Autophagy causes a specific form of programmed cell death that is different from caspase-mediated apoptosis, which is characterized by the presence of autophagosomes in the cytoplasm and then the lysosome degrades its contents. Autophagy, which is regarded as a promising strategy for enhancing the antitumor efficacy of chemotherapy drugs, has been under extensive investigation. Erinian treatment increases the number of autophagic vesicles and enhanced conversion of LC3B-I to LC3B-II.22 However, inhibition of autophagy by treatment with 3-MA increases erianin-induced cancer cell apoptosis, indicating that erianin-induced autophagy contributes to the cancer cell survival. In addition, growing evidence demonstrates that activation of the JNK pathway transduces oxidative stress signal to promote cell apoptosis and autophagy in response to various stress signals. Treatment with erianin induced a significant increase in JNK and c-Jun phosphorylation. The use of the JNK inhibitor or JNK activation is associated with the regulation of erianin-induced apoptosis and autophagy. Furthermore, ROS accumulation is involved in the activation of JNK/c-Jun pathway. Together, these data showed that erianin induced autophagy through activation of ROS-dependent JNK/c-Jun pathway, as shown in Figure 6. The role of autophagy in tumor suppression and progression is more complicated, since elevating evidence demonstrate a dual role of autophagy for therapeutic purposes in cancer. Therefore, it is necessary to future investigate the relationship between autophagy and apoptosis in erianin-mediated inhibition of tumor progression.
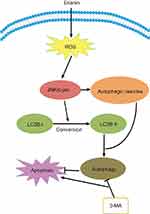 |
Figure 6 Erianin induces autophagy via the activation of ROS/JNK pathway. Abbreviations: ROS, reactive oxygen species; JNK, Jun N-terminal kinase. |
Other mechanisms
An inflammatory state is essential for promoting cancer progression and for achieving the full malignant phenotype. Thus, much effort need to be made to investigate whether erianin has a potential use in cancer immunotherapy, for example, enhancing innate immune cells activation, promoting the differentiation of monocytes into antigen-presenting cells, increasing the activation of IL-10 signaling, etc.
Conclusion and future perspectives
The occurrence and development of cancer is caused by many factors and the disorder of multiple gene regulation. Erianin, a natural product, has shown therapeutic potential for regulating multiple cancer-related pathways including apoptosis, cell cycle arrest, invasion, migration, angiogenesis, and autophagy in vivo and in vitro. The antitumor effect of erianin is the result of the interaction of several mechanisms and include some unknown signals. At present, there have been only a few dozen studies on erianin since it was discovered in 1984. And there are few studies on the toxicity of erianin in vivo. Because of the complexity and a number of cellular processes involved, more studies must be performed to completely understand how erianin could be used to prevent the development of cancer. Therefore, future research should focus on in vivo preclinical research to document the effects of erianin at different stages of carcinogenesis and improve in vitro basic research to better support the design and execution of clinical trials. Furthermore, future studies on detailed regulation mechanism will be helpful for conducting a molecular approach in finding the scientific basis of a natural product (Chinese characteristic and precious Chinese medicinal materials resources) in the future.
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China (No. 31501104).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wong AS, Che CM, Leung KW. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat Prod Rep. 2015;32:256–272. doi:10.1039/c4np00080c
2. Martino E, Casamassima G, Castiglione S, et al. Vinca alkaloids and analogues as anti-cancer agents: looking back, peering ahead. Bioorg Med Chem Lett. 2018;28:2816–2826. doi:10.1016/j.bmcl.2018.06.044
3. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi:10.1002/ijc.29210
4. Al-Dimassi S, Abou-Antoun T, El-Sibai M. Cancer cell resistance mechanisms: a mini review. Clin Transl Oncol. 2014;16:511–516. doi:10.1007/s12094-014-1162-1
5. Kachalaki S, Ebrahimi M, Mohamed Khosroshahi L, Mohammadinejad S, Baradaran B. Cancer chemoresistance; biochemical and molecular aspects: a brief overview. Eur J Pharm Sci. 2016;89:20–30. doi:10.1016/j.ejps.2016.03.025
6. Alsaied OA, Sangwan V, Banerjee S, et al. Sorafenib and triptolide as combination therapy for hepatocellular carcinoma. Surgery. 2014;156:270–279. doi:10.1016/j.surg.2014.04.055
7. Li CJ, Chu CY, Huang LH, et al. Synergistic anticancer activity of triptolide combined with cisplatin enhances apoptosis in gastric cancer in vitro and in vivo. Cancer Lett. 2012;319:203–213. doi:10.1016/j.canlet.2012.01.006
8. Titov DV, Gilman B, He QL, et al. XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat Chem Biol. 2011;7:182–188. doi:10.1038/nchembio.522
9. Kudumela RG, McGaw LJ, Masoko P. Antibacterial interactions, anti-inflammatory and cytotoxic effects of four medicinal plant species. BMC Complement Altern Med. 2018;18:199. doi:10.1186/s12906-018-2317-3
10. Zhang Y, Wang H, Wang P, Ma C, He G, Rahman MR. Optimization of PEG-based extraction of polysaccharides from Dendrobium nobile Lindl. and bioactivity study. Int J Biol Macromol. 2016;92:1057–1066. doi:10.1016/j.ijbiomac.2016.07.034
11. Li Y, Wang CL, Zhao HJ, Guo SX. Eight new bibenzyl derivatives from Dendrobium candidum. J Asian Nat Prod Res. 2014;16:1035–1043. doi:10.1080/10286020.2014.967230
12. Gong YQ, Yang H, Liu Y, et al. Studies on chemical constituents in stem of Dendrobium chrysotoxum. Chin J Chin Mater Med. 2006;31:304–306.
13. Xu YY, Xu YS, Wang Y, et al. Dendrobium nobile Lindl. alkaloids regulate metabolism gene expression in livers of mice. J Pharm Pharmacol. 2017;69(10):1409–1417. doi:10.1111/jphp.12778
14. Meng CW, He YL, Peng C, Ding XJ, Guo L, Xiong L. Picrotoxane sesquiterpenoids from the stems of Dendrobium nobile and their absolute configurations and angiogenesis effect. Fitoterapia. 2017;121:206–211. doi:10.1016/j.fitote.2017.07.017
15. Ye QH, Zhao WM, Qin GW. New fluorenone and phenanthrene derivatives from Dendrobium chrysanthum. Nat Prod Res. 2003;17(3):201–205.
16. Zheng Y, Xu L, Wang Z, Zhang C. Distribution and dynamic change of coumarins in leaf and root of Dendrobium thyrsiflorum. Chin J Chin Mater Med. 2009;34(9):1071–1074.
17. Zhao W, Ye Q, Ta X, et al. Three new sesquiterpene glycosides from Dendrobium nobile with immunomodulatory activity. J Nat Prod. 2001;64(9):1196–1200.
18. Ng TB, Liu F, Wang ZT. Antioxidative activity of natural products from plants. Life Sci. 2000;66:709–723.
19. Sun J, Fu X, Wang Y, et al. Erianin inhibits the proliferation of T47D cells by inhibiting cell cycles, inducing apoptosis and suppressing migration. Am J Transl Res. 2016;8(7):3077–3086.
20. Gong YQ, Fan Y, Wu DZ, Yang H, Hu ZB, Wang ZT. In vivo and in vitro evaluation of erianin, a novel anti-angiogenic agent. Eur J Cancer. 2014;40:1554–1565. doi:10.1016/j.ejca.2004.01.041
21. Li YM, Wang H, Liu GQ. Erianin induces apoptosis in human leukemia HL-60 cells. Acta Pharmacol Sin. 2001;22(11):1018–1022.
22. Wang H, Zhang T, Sun W, et al. Erianin induces G2/M-phase arrest, apoptosis, and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2016;7(6):e2247. doi:10.1038/cddis.2016.138
23. Majumder PL, Joardar M. Structure of erianin, a new bibenzyl derivative from the Orchid Eria-Carinata. Indian J Chem B. 1984;23:1040–1042.
24. Ma GX, Xu GJ, Xu LS, Wang ZT and Kickuchi T. Studies on chemical constituents of Dendrobium chrysotoxum Lindl. Acta Pharm Sin. 1994;29:763–766.
25. Manfredi KP, Vallurupalli V, Demidova M, Kindscher K, Pannell LK. Isolation of an anti-HIV diprenylated bibenzyl from Glycyrrhiza lepidota. Phytochemistry. 2001;58:153–157.
26. Kamory E, Keseru GM, Papp B. Isolation and antibacterial activity of marchantin-a, a cyclic bis(Bibenzyl) constituent of Hungarian marchantia-polymorpha. Planta Med. 1995;61:387–388.
27. Ouyang P, He X, Yuan ZW, et al. Erianin against Staphylococcus aureus infection via inhibiting sortase A. Toxins (Basel). 2018;23(10):10.
28. Baek JM, Kim JY, Ahn SJ, et al. Dendrobium moniliforme exerts inhibitory effects on both receptor activator of nuclear factor kappa-B ligand-mediated osteoclast differentiation in vitro and lipopolysaccharide-induced bone erosion in vivo. Molecules. 2016;21(3):295. doi:10.3390/molecules21030295
29. Bai HZ, Sun SR, Zhang YM, Sun S, Cao ZW. Inhibitory effect of Dendrobium officinale polysaccharide on growth of human breast cancer MCF-7 cells and the related mechanism. Biomed Res-India. 2017;28:1922–1926.
30. Dekanski JB. Anti-prostatic activity of bifluranol, a fluorinated bibenzyl. Brit J Pharmacol. 1980;71:11–16. doi:10.1111/j.1476-5381.1980.tb10903.x
31. Ma G-X, Gerald A. LeBlanc the activity of erianin and chrysotoxine from Dendrobium chrysotoxum to reverse multidrug resistance in B16/h MDR-1 cells. J Chin Pharm Sci. 1998;7(3):142–146.
32. Guoxiang M, Guojun X, Luoshan X. Inhibitory effects of Dendrobium chrysotoxum and its constituents on the mouse HePA and ESC. J Chin Pharm Univ. 1994;25(3):188–189.
33. Cushman M, Nagarathnam D, Gopal D, Chakraborti AK, Lin CM, Hamel E. Synthesis and evaluation of stilbene and dihydrostilbene derivatives as potential anticancer agents that inhibit tubulin polymerization. J Med Chem. 1991;34:2579–2588.
34. Lee YH, Park JD, Baek NI, Kim SI, Ahn BZ. In-vitro and in-vivo antitumoral phenanthrenes from the aerial parts of dendrobium nobile. Planta Med. 1995;61:178–180. doi:10.1055/s-2006-958043
35. Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D. Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Experientia. 1989;45(2):209–211.
36. Nam NH. Combretastatin A-4 analogues as antimitotic antitumor agents. Curr Med Chem. 2003;10:1697–1722.
37. Simoni D, Romagnoli R, Baruchello R, et al. Novel combretastatin analogues endowed with antitumor activity. J Med Chem. 2006;49:3143–3152. doi:10.1021/jm0510732
38. West CML, Price P. Combretastatin A4 phosphate. Anti-Cancer Drug. 2004;15:179–187. doi:10.1097/00001813-200403000-00001
39. Nathan P, Zweifel M, Padhani AR, et al. Phase I trial of combretastatin A4 phosphate (CA4P) in combination with bevacizumab in patients with advanced cancer. Clin Cancer Res. 2012;18(12):3428–3439. doi:10.1158/1078-0432.CCR-11-3376
40. Zhou H, Yang B, Hong M, Ma R, Sheng L. Liquid chromatographic-mass spectrometry analysis and pharmacokinetic studies of erianin for intravenous injection in dogs. Arzneimittelforschung. 2009;59(3):141–145. doi:10.1055/s-0031-1296377
41. Messaoudi S, Hamze A, Provot O, et al. Discovery of isoerianin analogues as promising anticancer agents. Chem Med Chem. 2011;6(3):488–497. doi:10.1002/cmdc.201000456
42. Soussi MA, Provot O, Bernadat G, et al. Discovery of azaisoerianin derivatives as potential antitumors agents. Eur J Med Chem. 2014;78:178–189. doi:10.1016/j.ejmech.2014.03.032
43. Tianshan W, Yueming L, Guoxiang M, et al. In vitro inhbition activities of leukemia K562 cells growth of constituents from D. Chrystooxum. Nat Prod Res Develop. 1997;2:1–3.
44. Zhu Q, Sheng Y, Li W, Wang J, Ma Y, Du B, Tang Y. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits bladder cancer cell growth via the mitochondrial apoptosis and JNK pathways. Toxicol Appl Pharmacol. 2019;371:41–54. doi:10.1016/j.taap.2019.03.027
45. Hong W, Shenglin M, Lingshan D, et al. Experimental study of erianin inducing apoptosis in gastric carcinoma SGC-7901. China Cancer. 2008;6:499–501.
46. Mingkun L. Effect on Erianin and 5-Fluorouracil on Cell Proliferation and Apoptosis in Hunmangastric cancerMGC-803 [master's thesis]. Hengyang: University of South China; 2016.
47. Peng S, Jing W, Junxia A, Qiyu Z, Xiaoxia L, Yaxiong T. Inhibitory effect of erianin on hepatocellular carcinoma (HCC) Huh7 Cells. Chin J Appl Environ Biol. 2011;17(05):662–665.
48. Cui XQ, Su P, Zhu QY, et al. Molecular mechanism of apoptosis of human colorectal cancer SW480 cells induced by erianin. Chin J Appl Environ Biol. 2011;17(04):512–516.
49. Cui MY, Kang DD, He L, Shi YJ, Ren JW. Erianin induces apoptosis of hunman colorectal cancer Caco-2 cells. Sci Technol Food Ind. 2016;37(16):352–356.
50. Li M, He Y, Peng C, Xie X, Hu G. Erianin inhibits human cervical cancer cell through regulation of tumor protein p53 via the extracellular signal-regulated kinase signaling pathway. Oncol Lett. 2018;16:5006–5012. doi:10.3892/ol.2018.9267
51. Mohammad RM, Muqbil I, Lowe L, et al. Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol. 2015;35:S78–S103. doi:10.1016/j.semcancer.2015.03.001
52. Scatena R, Bottoni P, Botta G, Martoran GE, Giardina B. The role of mitochondria in pharmacotoxicology: a reevaluation of an old, newly emerging topic. Am J Physiol Cell Physiol. 2007;293:C12–C21. doi:10.1152/ajpcell.00314.2006
53. Hacker G, Paschen SA. Therapeutic targets in the mitochondrial apoptotic pathway. Expert Opin Ther Targets. 2007;11:515–526. doi:10.1517/14728222.11.4.515
54. Wong RSY. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Canc Res. 2011;30. doi:10.1186/1756-9966-30-87
55. Hardwick JM, Soane L. Multiple functions of BCL-2 family proteins. Csh Perspect Biol. 2013;5. doi:10.1101/cshperspect.a008722
56. Okon IS, Zou MH. Mitochondrial ROS and cancer drug resistance: implications for therapy. Pharmacol Res. 2015;100:170–174. doi:10.1016/j.phrs.2015.06.013
57. Gong YQ, Fan Y, Liu L, Wu D, Chang Z, Wang Z. Erianin induces a JNK/SAPK-dependent metabolic inhibition in human umbilical vein endothelial cells. In Vivo. 2004;18:223–228.
58. Walters J, Pop C, Scott FL, et al. A constitutively active and uninhibitable caspase-3 zymogen efficiently induces apoptosis. Biochem J. 2009;424:335–345. doi:10.1042/BJ20090825
59. Hartwell L. Defects in a cell-cycle checkpoint may be responsible for the genomic instability of cancer-cells. Cell. 1992;71:543–546.
60. Yu JP, Sun J, Wang SE, et al. Upregulated expression of indoleamine 2, 3-dioxygenase in primary breast cancer correlates with increase of infiltrated regulatory T cells in situ and lymph node metastasis. Clin Dev Immuno. 2011. doi:10.1155/2011/469135
61. Kronberger IE, Troestar B, Amberger A, et al. Elevation of the immunomodulatory enzyme indoleamine 2,3-dioxygenase in tumor cells facilitates immune escape and leads to enhanced metastases. Inflamm Res. 2007;56:S285–S285. doi:10.1007/s00011-006-6151-6
62. Su C, Zhang P, Liu JW, Cao YO. Erianin inhibits indoleamine 2, 3-dioxygenase - induced tumor angiogenesis. Biomed Pharmacother. 2017;88:521–528. doi:10.1016/j.biopha.2017.01.090
63. Yu Z, Zhang T, Gong C, et al. Erianin inhibits high glucose-induced retinal angiogenesis via blocking ERK1/2-regulated HIF-1alpha-VEGF/VEGFR2 signaling pathway. Sci Rep. 2016;6:34306. doi:10.1038/srep34306
64. Lam F, Bradshaw TD, Mao H, Roberts S, Pan Y, Wang S. ZJU-6, a novel derivative of Erianin, shows potent anti-tubulin polymerisation and anti-angiogenic activities. Invest New Drug. 2012;30:1899–1907. doi:10.1007/s10637-011-9755-9
65. Liang XH, Jackson S, Seaman M, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi:10.1038/45257
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.