Back to Journals » Journal of Inflammation Research » Volume 16
Progress in Research on the Mechanisms and Interventions of Phlebitis from the Perspective of Vascular Endothelial Cell and Signaling Pathway
Authors Zhu LL, Wang YH, Zhou Q
Received 2 December 2023
Accepted for publication 22 December 2023
Published 29 December 2023 Volume 2023:16 Pages 6469—6481
DOI https://doi.org/10.2147/JIR.S450149
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Ling-Ling Zhu,1 Yan-hong Wang,2 Quan Zhou2
1VIP Geriatric Ward, Division of Nursing, the Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, People’s Republic of China; 2Department of Pharmacy, the Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang Province, People’s Republic of China
Correspondence: Quan Zhou, Department of Pharmacy, the Second Affiliated Hospital, School of Medicine, Zhejiang University, Jiefang Road No. 88, Hangzhou, Zhejiang Province, 310009, People’s Republic of China, Email [email protected]
Background: Phlebitis is a common complication of intravenous administration and greatly affects clinical outcomes, patient satisfaction, and health-care expenditure. Numerous studies have revealed venous injuries only through visual and histopathological examination. Although sporadic studies have explored the cellular and molecular biological mechanisms of phlebitis and the outcomes of pharmacological interventions, an updated review over the last decade is not available.
Methods: Progress in research on the mechanisms and interventions of phlebitis was summarized from the perspective of endothelial cells and signaling pathways by retrieving the PubMed, Web of Science Core Collection, MEDLINE, Embase, and CNKI.
Results: Phlebitis involves multiple signaling pathways (eg, nuclear factor kappa B, Wnt/β-catenin, focal adhesion kinase/protein kinase B, Toll-like receptor, protein kinase C beta/NADPH oxidase, PI3K/AKT/TNF, and JAK2/STAT3), upregulation of E-selectin, GBP5/NLRP3 inflammasome axis, cell apoptosis, intracellular ROS generation, SOD reduction, stimulation of angiogenesis, and induction of autophagy-associated cell death. Preventive and curative interventions included α-solanine, baicalein, escin, intermedin, Y15, micro-ribonucleic acid-223, sotrastaurin, cimetidine, aescin, resveratrol, α-chaconine, Chahuang ointment, QingLuoTongMai, Mailuo Shutong, and N-acetylcysteine. Laboratory models included vascular endothelial cells, real-time cell-monitoring analysis, network pharmacology analysis and experimental verification in vivo, animal models of phlebitis (rat, rabbit, and mouse), rabbit models with peripherally inserted central catheters (PICC) catheterization, models of PICC/central venous catheter indwelling with combined drugs in human umbilical vein endothelial cells, and compatibility with endothelial cells. Factors affecting vascular endothelial cell injury include difference in the same class of drugs, concentration and exposure time of precipitant, and infusion strategy.
Conclusion: Phlebitis is accompanied by endothelial dysfunction and may involve multiple molecular and cellular mechanisms. These findings improve our understanding of the molecular targets of interventions and help identify effective candidates for the prophylaxis and treatment of phlebitis. Vascular health and risk management should be considered when initiating intravenous administration.
Keywords: endothelial cells, intravenous infusion therapy, phlebitis, pharmacological interventions, peripherally inserted central catheters, signaling pathway
Introduction
Infusion-related phlebitis (IRP) is a common complication of intravenous administration, and greatly affects clinical outcomes, patient satisfaction, and health-care expenditure.1 It is clinically relevant to reduce the incidence of IRP by adopting appropriate interventions.
Non-pharmacological interventions to alleviate phlebitis include careful selection and placement of vascular access, modification of the infusion strategy (concentration, infusion rate, sequence), rotation of infusion site, and application of inline filters to reduce pollutant particulates. For example, implanted port catheter has more advantages than peripherally inserted central catheter (PICC) in reducing phlebitis.2 A randomized trial revealed that PICC placement could reduce the incidence rate of phlebitis in patients with heart failure compared to peripheral venous access indwelling.3 Pharmacological interventions to relieve symptoms of phlebitis usually refer to the topical and systemic uses of anti-inflammatory analgesics.
Numerous studies have revealed venous injuries only through visual and histopathological examination. Although some sporadic studies have explored the cellular and molecular biological mechanisms of phlebitis as well as the outcomes of pharmacological interventions, an updated review in the last decade is not available. Here, we summarize research progress in the mechanisms and drug interventions for phlebitis from the perspective of vascular endothelial cell injury and cell signaling pathways.
Methods
Search Strategy
Potentially relevant literature with “phlebitis and endothelial” in the last 10 years until the end of October 2023 was obtained by retrieving PubMed, Web of Science Core Collection, MEDLINE, and Embase. The CNKI database was also searched for papers with titles including “endothelial cells and phlebitis” or “endothelial cells and PICC” or “endothelial cells and central venous catheter (CVC)” during the same period.
Selection Criteria
ZLL and WYH independently searched the documents and screened related studies. QZ was consulted in the event of disagreement on the inclusion or exclusion of a paper. There were 41 documents were identified in PubMed, 46 from the Web of Science Core Collection and MEDLINE, 154 from Embase, and six from CNKI. After excluding duplicate studies, 160 papers were subjected to further assessment. After reviewing abstracts, documents were excluded because they were not closely related to phlebitis or endothelial cells (n=129). The full text of these articles was evaluated to determine eligibility. Two case reports and studies that did not involve cellular or molecular mechanisms were excluded. Twenty-seven papers were finally selected based on the inclusion and exclusion criteria (Figure 1). Key information is summarized in this review.
 |
Figure 1 Flow chart showing selection of literature. |
Results
Nuclear Factor Kappa B Signaling Pathway
α-Solanine
α-solanine is one of the main glycoalkaloids in potatoes. Wang et al examined whether α-solanine had impacts on endothelial inflammation via the nuclear factor kappa B (NF-κB) signaling.4 Human umbilical vein endothelial cells (HUVECs) overexpressing tumor necrosis factor alpha (TNF-α) were constructed. Compared to control cells, TNF-α-overexpressing cells showed significantly less viability and higher levels of TNF-α, interleukin 6 (IL-6), p-P65, p-IκBα, and IKKα/β. Treatment with α-solanine in TNF-α-overexpressing HUVECs for 24 h decreased the levels of TNF-α and IL-6 while lowering the relative protein levels of p-P65, p-IκBα, and IKKα/β. These findings show the role of α-solanine in inhibiting endothelial inflammation via NF-κB signaling, and the possibility of a new candidate for the prophylaxis and treatment of phlebitis.
Baicalein
Ge et al investigated the mechanism of baicalein in alleviating vinorelbine-triggered phlebitis and vascular endothelial cell injury.5 Baicalein could reduce edema, blood clots, vascular endothelial cell loss, inflammatory cell infiltration, and serum levels of TNF-α, IL-1β, IL-6, and intercellular adhesion molecule-1 (ICAM-1) in the rabbit model of phlebitis. In vitro experiments further revealed the ability of baicalein to reduce cell apoptosis, intracellular reactive oxygen species (ROS), p38 phosphorylation, and activation of the NF-κB signaling. It indicated the protective effects of baicalein against vinorelbine-induced endothelial injury through inhibiting ROS generation, p38/NF-κB pathway, and formation of pro-inflammatory cytokines.
Escin
A study demonstrated the molecular mechanism for the pharmacological effect of escin.6 The shear-stress assays in HUVECs revealed that escin could inhibit inflammatory factors, intracellular calcium levels, and Yoda1-induced aortic ring relaxation. In addition, escin alleviated inflammation and suppressed the activity of NF-κB in HUVECs subjected to mechanical stretching, whereas this phenomenon was not observed when Piezo1 was knocked out. Also, escin reduced inflammation in HUVECs under mechanical stretching; however, this effect disappeared after treatment with the NF-κB antagonist SN50. This indicates that escin could inhibit the inflammatory response evoked by mechanical stretch through NF-κB pathway mediated by Piezo1.
Wnt/β-Catenin Signaling Pathway
Wang and colleagues investigated the protective effect of intermedin in amiodarone-induced phlebitis.7 Compared to control, treatment with intermedin could obviously attenuate apoptosis, endothelial exfoliation, and inflammatory cell infiltration in the rabbit model of amiodarone-induced phlebitis. In vitro tests further showed that the role of intermedin involved activating Wnt/β-catenin signaling pathway, which could be inhibited by amiodarone.
Focal Adhesion Kinase Signaling Pathway
The focal adhesion kinase (FAK)-protein kinase B (AKT) pathway seems to be a promising pharmacological target for treating CVC-related phlebitis. Wang et al explored the role of the FAK inhibitor Y15 (1,2,4,5-benzenetetramine tetrahydrochloride) in CVC-induced oxidative damage. EA.hy926 cells were divided into three groups: normal control, CVCs combined with scratches (truncated CVC catheter cultured with scratched endothelial cells), and CVCs combined with scratches and Y15 (50 μmol/L). New Zealand rabbits were randomly assigned to three groups: normal control, CVC, and CVC pre-soaked with Y15 (50 μmol/L) before insertion. Results showed that the presence of Y15 significantly reduced the levels of ROS and malondialdehyde (MDA) while increasing the cell viability and concentrations of nitric oxide and superoxide dismutase (SOD) in rabbit serum and cell culture fluid in a time-dependent manner. Also, Y15 efficiently alleviated the CVC-triggered pathological changes in injured vascular tissues and decreased the contents of p-FAK Tyr 397 and p-AKT Ser 473 in endothelial cells and injured external jugular vein. It indicated that Y15 could not only reduce CVC-induced oxidative stress injury to blood vessels but also prevent phlebitis and thrombosis caused by CVC through inhibiting the activation of FAK-AKT pathway.8
Wang et al also confirmed that Y15 had anti-inflammatory effects against vascular endothelial injury triggered by CVC combined with 5-FU by regulating the FAK-NF-κB signaling pathway.9 EA.hy926 cells were randomly assigned to three groups: normal control, model group [ie, a cell inflammatory injury model induced by three scratches (2 cm each), three CVC (1 cm) segments, and 1 g/L 5-FU 80 μL], and experimental group (ie, 50 μL of 2 mmol/L Y15 was added to the model group). After 48 h, the model group exhibited statistically significant higher mRNA level of IL-6 and expression levels of pY397 FAK and NF-κB than normal control; however, the experimental group showed a great decline in the above indices compared with the model group (P<0.001).
Another study investigated the molecular mechanism by which Y15 reduced the CVC-induced adhesion of vascular endothelial cells and monocytes. Three groups of EA. hy926 cells were as follows: normal control, model [scratched cells co-cultured with intercepted CVCs, THP-1 cells, and 5-FU (40 mg/L)], and experimental group (Y15 50 μmol/L was added to the model group). The results showed that Y15 reduced expression levels of zinc finger transcription factor 4 and inhibited THP-1 cell adhesion by suppressing FAK activation.10
Toll-Like Receptor Signaling Pathway
Li et al confirmed that the protective effect of micro-ribonucleic acid (miR)-223 in rat model of thrombophlebitis was through the regulation of toll-like receptor (TLR) signaling.11 The rats were divided into miR-223 inhibitor, miR-223 mimic, and normal control groups. The miR-223 inhibitor group exhibited remarkably higher concentrations of serum inflammatory factors (IL-6, IL-1β, and TNF-β), and fibrinolytic index (plasminogen activator inhibitor 1) than the mimic group. In the inhibitor group, the inferior vena cava wall disappeared owing to complete necrosis, while in the mimic group, the vascular lumen was slightly dilated and the vascular wall profile was intact. The inhibitor group witnessed obvious increases in the mRNA and protein levels of TLR2 and myeloid differential protein-88 (MyD88).
Qian et al investigated the value of TLR4 in vascular endothelial injury caused by vinorelbine. HUVECs were treated with vinorelbine for 60 min and then cultured for 6 and 12 h following the washing away of vinorelbine. The cells treated with vinorelbine exhibited stretched, lengthened and irregular shapes, whereas the control cells showed normal morphology. Vinorelbine-treated HUVECs had significantly higher mRNA and protein levels of TLR4, and nuclear translocation of NF-κB p65 than the blank control group, indicating that TLR4 was involved in the progression of vinorelbine-triggered endothelial injury through the effect on NF-κB translocation.12
Protein Kinase C Beta/NADPH Oxidase Pathway
Docetaxel can also cause vessel damage and phlebitis. Huang et al investigated the mechanisms of docetaxel-triggered endothelial injury. HUVECs were stimulated with docetaxel (2.5, 5, or 10 nmol/L) for 24 h. The presence of docetaxel concentration- and time-dependently reduced cell viability, causing endothelial apoptosis and DNA damage. Docetaxel increased activities of caspase-3 and NADPH oxidase and levels of protein kinase C (PKC) β phosphorylation; meanwhile, it facilitated the production of ROS and mitochondrial dysfunction. Interestingly, such changes induced by docetaxel could be abolished by inhibition of PKCβ and NADPH oxidase. In addition, PKCβ inhibitor sotrastaurin could alleviate the oxidative injury caused by docetaxel in HUVECs. The results indicated that docetaxel could cause endothelial dysfunction via PKCβ/NADPH oxidase pathway, which might provide a promising method for prophylaxis of docetaxel-induced vascular injury.13
Upregulation of E-Selectin
E-selectin is a precondition for the adhesion of leukocytes to endothelial cells and the migration of leukocytes through the vessel wall in an inflammatory state. Wang et al investigated the expression of E-selectin in mouse and rabbit models of vinorelbine-triggered phlebitis.14 Vinorelbine-treated mice had higher pathological scores than cimetidine-pretreated mice (P<0.05). Rabbits with vinorelbine administration had significantly more prominent serum concentrations of E-selectin than vehicle controls (P<0.05); however, such a change was significantly weakened by pretreatment of cimetidine (P<0.05). Vinorelbine treatment greatly improved the rate of neutrophil adhesion to endothelial cells compared to vehicle control; however, this phenomenon disappeared in the presence of cimetidine (P<0.01). In addition, tail swelling was similar in the vinorelbine-treated and cimetidine plus vinorelbine-treated E-selectin knockout mice. Taken together, cimetidine attenuated the severity of vinorelbine-triggered phlebitis in mice through suppressing the upregulation of E-selectin.
Guanylate Binding Protein-5/NLR Family Pyrin Domain-Containing 3 (GBP5/NLRP3) Inflammasome Axis
Patients with chemotherapy-induced phlebitis (CIP) have up-regulated GBP5 expression in human peripheral blood mononuclear cells. Genetic deletion of GBP5 in macrophages significantly reduced vinorelbine-triggered CIP in experimental murine models. GBP5 contributed to inflammatory responses through the activation of NLRP3 inflammasome and the promotion of IL-1β production. Aescin is a mixture of triterpene saponins extracted from seeds of horse chestnut trees. Evidence has shown the role of aescin in suppressing GBP5/NLRP3 axis and alleviating CIP using a mouse model. Therefore, aescin seems to be a promising candidate for CIP treatment.15
Cell Apoptosis and Intracellular ROS Generation
Resveratrol, a well-known polyphenol that harbours many health beneficial properties, could alleviate vessel injuries caused by vinorelbine.16 Human vascular endothelial cells (ECV-304) were treated with vinorelbine for 10 min, followed by cell culture with a serum-free medium in the presence or absence of resveratrol for 24 h. Vinorelbine exposure resulted in significant decline in the following indicators (cell viability, apoptosis, intracellular ROS production, and intracellular SOD activity) in a dose-dependent manner. However, this phenomenon was dose-dependently reversed by resveratrol treatment for 2 h.
Stimulator of Angiogenesis
A multicenter randomized controlled trial assessed the effectiveness of a Chinese herbal formulation (Chahuang ointment) in the prophylaxis of PICC-related phlebitis.17 The incidence of phlebitis, edema, and microthrombus after 72 h of PICC insertion was significantly lower in the herbal treatment group than in the control group. Moreover, Chahuang ointment treatment for 3 days greatly suppressed the expression levels of vascular endothelial growth factor (VEGF) and endothelin-1. There was no statistical difference in the effectiveness between this herbal ointment and mucopolysaccharide polysulfate. Thus, Chahuang ointment could provide an effective tool in preventing mechanical phlebitis induced by PICC.
Zhou and colleagues confirmed the beneficial effect of mashed potato and creams containing alkaloids (α-solanine, α-chaconine) on the expression of endothelial growth factors [basic fibroblast growth factor (bFGF) and VEGF] and PKC-α in the ear vein and surrounding tissues of rabbit model with phlebitis induced by intravenous vincristine.18 Additionally, cream of high-concentration had a stronger enhancing effect than cream of low-concentration, and α-chaconine had higher enhancing effect than α-solanine.
Induction of Autophagic Cell Death and Autophagy Inhibition
Nicardipine hydrochloride injection is a first-line agent for emergency control of hypertension despite potentially serious peripheral vascular injuries. Ochi and colleagues used human microvascular endothelial cells (HMVECs) to explore the mechanism of nicardipine-triggered vascular injury.19 Autophagosome existed in HMVECs after 6 h of exposure to nicardipine (30 mg/L), but not in HMVECs without nicardipine treatment. Autophagy induction occurs in cell injury induced by nicardipine. However, 3-methyladenine significantly prevented nicardipine-induced reduction in cell viability through inhibition of autophagosome formation. Data demonstrate that autophagy is the primary mechanism of nicardipine-triggered vascular injury and autophagy inhibition may alleviate cell death.
New Laboratory Models
Real-Time Cell-Monitoring Analysis
Hazekawa and colleagues applied a real-time cell analyzer (RTCA) system to determine the responses of HUVECs to paclitaxel.20 After culture of HUVECs in an E-plate for 24 h, paclitaxel and carboplatin were injected into each well and impedance changes were documented for 72 h. The effects of chemotherapeutic agents on HUVEC viability were easily monitored in a time-dependent manner by cell index (CI) values calculated from electrical impedance. Paclitaxel could cause a more obvious decrease in the CI values than carboplatin in a concentration- and time-dependent manner. The results were consistent with clinical case report (ie, taxanes could induce angialgia and phlebitis more commonly than platinum). Additionally, the CI values were associated with elevated concentrations of inflammatory markers [ICAM-1, vascular cell adhesion molecule-1 (VCAM-1), and phosphorylated NF-κB]. On the whole, the RTCA technology creates a practical in vitro platform for studying angialgia and phlebitis induced by chemotherapy.
Network Pharmacology Analysis and Experimental Verification in vivo
Yu and colleagues reported that QingLuoTongMai Pills (QLTMP) could mitigate CIP by the anti-inflammatory roles in regulation of PI3K/AKT/TNF signaling pathway.21 In their study, 165 bioactive compounds in QLTMP were identified by network pharmacology analysis, and 19 core therapeutic targets for CIP were clarified by the protein–protein interaction network, followed by biological function and pathway enrichment analysis. The final step was to confirm the reliability of network pharmacology analysis by evaluating the curative effect of QLTMP in a CIP model evoked by vinorelbine. Most recently, Cao et al explored the mechanism of Mailuo Shutong Pills (MLSTW) against superficial thrombophlebitis through network pharmacology in combination with animal experimental validation in vivo.22 The researchers obtained a total of 1112 compound targets of MLSTP, 837 disease targets, 84 common targets, and 31 key targets. The enrichment analysis revealed the main targets involved in the following processes [positive regulation of smooth muscle cell proliferation, inflammatory response, apoptotic process, signaling pathways such as PI3K/AKT and Janus kinase/signal transducer and activator of transcription (JAK/STAT)]. The in vivo experimental results indicated that the MLSTW treatment could greatly improve the pathological damage to rabbit ear tissues, with obvious reductions in the serum concentrations of cytokines (eg, IL-1β, TNF-α, and IL-6) in rabbits and phosphorylation levels of AKT, PI3K, JAK2, and STAT3 in rabbit ear tissues. Combining these findings, the anti-superficial thrombophlebitis role of MLSTP was attributed to inhibiting inflammation and thrombosis by down-regulating the expression of proteins related to signaling pathways (ie, PI3K/AKT and JAK2/STAT3).
Animal Model of Phlebitis Evoked by Carbomer/Vinorelbine Gel
Zhang and colleagues established an animal model to investigate the pathogenic mechanism of phlebitis by in situ carbomer/vinorelbine ditartrate gel application and assessed the curative effect of N-acetylcysteine against vinorelbine-evoked phlebitis.23 Carbomer/vinorelbine gel induced phlebitis quickly in rabbits and rats and increased ROS production in venous endothelial cells; however, N-acetylcysteine could alleviate the oxidative stress triggered by vinorelbine and the expression of cytokines (IL-6 and TNF-α) by reducing mitochondrial injury in vascular endothelial cells and thus prevent the generation of phlebitis. Therefore, N-acetylcysteine is a promising therapeutic agent for phlebitis.
Construction of the PICC Rabbit Model
Huang and colleagues established a rabbit model of vinorelbine dosing via PICC and dynamically monitored biomarker changes associated with phlebitis and microthrombosis. This platform enables nurses to early predict and timely prevent and treat PICC-associated antineoplastic therapy complications.24 Phlebitis could be triggered by vinorelbine administered through PICC, but it is slowly alleviated during the first chemotherapy cycle of vinorelbine. The rabbit model had significantly higher levels of P-selectin, E-selectin, and IL-6 on the second day after chemotherapy than on the first day after PICC catheterization. However, the levels of three biomarkers obviously reduced in the first, second, and third weeks after PICC catheterization compared with the second day. In addition, the levels of inflammation- and thrombosis-related factors were remarkably increased following vinorelbine dosing on the 23rd day of PICC insertion compared with those on day 21, but this phenomenon was reversed on day 24.
HUVECs Model of PICC Indwelling Combined Drugs
Zhao et al explored the mechanism of PICC indwelling and injury of 5-FU stimuli on HUVECs (EA.HY926).25 Sterile PICC which was tailored to the proper length to put into EA.HY926 cell culture fluid, followed by the addition of 5-FU at a concentration of 40 mg/L for co-culture with EA.HY926 cells. The methyl thiazole tetrazolium (MTT) method was used to examine activity changes in EA.HY926 cells at different time intervals in the control group and in each model group (PICC, 5-FU, and PICC-combined 5-FU injury group). Scratch tests were performed to detect changes in cell mobility at different time intervals. Immunoassays were used to measure the expression levels of three inflammatory cytokines [von Willebrand factor (vWF), IL-6, and fibroblasts (FIB]) in the culture supernatants and EA.HY926 cells. The results showed that indwelling PICC combined with 5-FU reduced cell activity, inhibited cell mobility, and caused cell injury by releasing vWF and increasing IL-6 and FIB levels. This model enables clinicians to explore the mechanism of vascular injury caused by PICC-indwelling and combined drugs with a risk of chemical phlebitis.
Endothelial Cell Compatibility
In vitro model of HUVECs is a useful supplement to animal models and can be used to evaluate the biocompatibility of intravenous drugs. Cellular impairment during incubation with drugs is reflected by cell viability or intracellular levels of purine nucleotides. For example, incubating cells with azithromycin (2 g/L) for 60 min caused a rapid decline in intracellular adenosine 5’-triphosphate, whereas 60 min-incubation of azithromycin (0.5 g/L) did not result in significant changes in the levels of high-energy phosphate in HUVECs. The results indicated that azithromycin solution would cause local adverse reactions at 2 g/L rather than 0.5 g/L.26 Drouet and colleagues investigated the endothelial toxicity of vancomycin by evaluating cell viability with AlamarBlue assay following a 24 h incubation with antibiotic solutions.27
Discussion
Phlebitis from Different Types of Drug Infusion
Extravasated drugs are classified according to their potential for causing damage as vesicant, irritant, and non-vesicant.28 The extent of local tissue injury depends mainly on the chemical structure of the extravasated substance, which may be affected by other factors. Vesicant drugs can cause blistering of the skin/mucous membranes, severe and/or irreversible tissue injury, and necrosis. Irritant drugs can cause tissue inflammation or irritation without associated blister formation. In order from most likely to least likely to cause damage when extravasation occurs: vesicants (most likely), exfoliants, irritants, inflammitants, and neutrals (least likely). It is also important to note that some drugs are categorized differently by different references.29,30 For cisplatin infusion, it belongs to vesicant at concentrations greater than or equal to 0.5 g/L, while it is irritant at concentrations less than 0.5 mg/mL.
Typically, vesicants are as follows: vinca alkaloids (vinorelbine, vindesine, vincristine, and vinblastine), anthracyclines (idarubicin, epirubicin, doxorubicin, and daunorubicin), mitomycin, dactinomycin, amsacrine, and mechlorethamine. Paclitaxel and docetaxel are proposed to be vesicants.31 Irritant drugs are as follows: alkylating agents (carmustine, dacarbazine, ifosfamide, melphalan, and thiotepa), carboplatin (usually classified as irritants but reported to be mild vesicants), cisplatin (concentration-dependent), oxaliplatin, topoisomerase II inhibitors (teniposide, etoposide), liposomal doxorubicin, liposomal daunorubicin, and topoisomerase I inhibitors (irinotecan, topotecan).28,29 It is interesting to have a high-throughput comparison of consequences of different types of drug infusion from the perspective of vascular endothelial cell and signaling pathway.
The Difference in the Same Class of Molecules
Cimetidine, a classical H2-receptor antagonist, efficiently inhibited the expression of E-selectin and mitigated the severity of phlebitis triggered by vinorelbine in mice. However, two other more potent H2-receptor antagonists (ie, ranitidine and famotidine) did not have the same significant benefit as cimetidine.14 This indicates that cimetidine might exert its role via pathways beyond H2-receptors. Although both α-solanine and α-chaconine are steroidal glycoalkaloids, α-chaconine is more potent than α-solanine in promoting the expression of endothelial growth factors in venous vessels and surrounding tissues in rabbit model of vincristine-induced phlebitis.18
Concentration and Exposure Time of Precipitant
Wang and coworkers revealed that HUVECs injury can be significantly enhanced by increasing the concentration and exposure time of amiodarone.32 The viability of HUVECs incubated with amiodarone for 24 h rapidly decreased with increasing drug concentrations in the range of 10–60 μmol/L (P<0.01). After incubation with 30 μmol/L amiodarone for 6, 12, 24, 36, or 48 h, the cell viability exhibited a significant and time-dependent decline. Also, the protective role of resveratrol in vinorelbine-induced HUVECs model exhibited a dose-dependent manner.16 In HMVECs model, nicardipine could dose-dependently decrease cell viability and increase percent of dead cells.19 Guo and coworkers investigated the time to occurrence of phlebitis caused after continuous infusion of total nutrient admixture through a peripheral vein by measuring the levels of inflammatory markers and the degree of inflammatory cell infiltration within 6 hr. Results showed that 4 hr was the time limit for continuous infusion in the same peripheral vein before significant inflammatory changes occur.33 This also suggests the importance of TPN infusion via PICC rather than peripheral veins.
Infusion Strategy
Phlebitis is a common complication of vancomycin administered via a peripheral intravenous access. HUVECs were stimulated with vancomycin at clinically used doses over a period of 1–3 days via intermittent or continuous infusion. Vancomycin toxicity in HUVECs was observed and exhibited in a concentration-dependent and time-dependent manner. For example, the lethal dose 50 (LD50) was 5 g/L after 24 h, but it decreased to 2.5 g/L after 48 and 72 h of treatment. If daily vancomycin dose is fixed, continuous infusion could trigger more cell toxicities than intermittent infusion at a dose of more than 1 g/day. Additionally, intermittent infusion triggers significantly less dose-dependent toxicity compared to continuous infusion.34
Additionally, vancomycin is usually infused together with miscellaneous intravenous antimicrobial agents via the same Y-site. Drouet et al evaluated the local toxicity of such combinations. HUVECs were treated with various vancomycin doses for 24 h. The AlamarBlue assay indicated an excessive cell mortality when vancomycin was administered with erythromycin (a macrolide antibiotic) or gentamicin (an aminoglycoside antibiotic) via the same Y-tube; however, this phenomenon was not observed for imipenem-cilastatin combined with vancomycin.35
New Horizon and Further Opportunities
There is a new horizon for clinicians to focus on IRP- related practice and research. The molecular and cellular mechanisms of phlebitis and laboratory models are presented in Table 1, and a summary of studies identified for this review is illustrated in Table 2. We also draw on signalling pathways and pathophysiology of phlebitis (Figure 2). The latest knowledge of the mechanisms and drug interventions will sharpen the awareness of new pharmacological interventions against phlebitis.
 |
Table 1 Molecular and Cellular Mechanisms of Phlebitis and Laboratory Models |
 |
Table 2 Summary of Studies Identified for This Review |
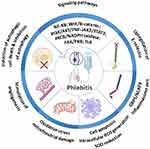 |
Figure 2 The signalling pathways and pathophysiology of phlebitis. |
It contains a lot of research opportunities in clinical nursing. The first is intravenous infusion regimens (eg, seeking the optimal fluid concentration and infusion time). Second, new laboratory models are being utilized to rapidly screen intravenous drugs with a high risk of IRP, and promising drugs with the potential to prevent and treat phlebitis based on core therapeutic targets. It would be interesting to perform phlebitis-relevant studies using RTCA and network pharmacology analysis in combination with experimental verification in vivo. Third, the model of PICC or CVC indwelling combined drugs in HUVECs and/or animals would make it possible to perform the following studies: 1) assess the possibility and severity of IRP in patients receiving intravenous infusion via different types of venous access (eg, silicone catheters versus polyurethane catheters, PICC versus CVC, midline, or peripheral vascular access) and 2) observe the pathological process of phlebitis and venous thrombosis after test drug interventions, which is beneficial to early forecast and timely prophylaxis of infusion therapy complications.
Conclusion
IRP is accompanied by endothelial dysfunction and may involve multiple molecular and cellular mechanisms. Progress in the last decade has improved our understanding of the molecular targets of intervention drugs or natural compounds with confirmed effects on vascular endothelial injury and phlebitis. More effective candidates should be identified for the prophylaxis and treatment of phlebitis. Vascular health and risk management should be considered when initiating intravenous administration. Nurses and pharmacists can collaborate to reduce the negative effect of IRP on medication administration and patients’ quality of life.
Acknowledgments
This work was supported by the research projects from Zhejiang University (project codes 491020-5215F6 and 491020-I4154A). We would also like to thank all core members of the intravenous infusion therapy team in our institution.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Johnson JL, Norton C, Fryfogle E, Fincher TK, Burmeister MA. The pharmacist’s role in reducing infusion-related phlebitis. Am J Health Syst Pharm. 2023;80(15):974–983. doi:10.1093/ajhp/zxad090
2. Sun Y, Wu X. Complications of implanted port catheters and peripherally inserted central catheters in chemotherapy-treated cancer patients: a meta-analysis. Adv Clin Exp Med. 2023;32(5):523–532. doi:10.17219/acem/156346
3. Silva EVC, Ochiai ME, Vieira KRN, Pereira Barretto AC. The use of peripherally inserted central catheter reduced the incidence of phlebitis in heart failure patients: a randomized trial. J Vasc Access. 2021;11297298211059650. doi:10.1177/11297298211059650
4. Wang N, Jiang D, Zhou C, Han X. Alpha-solanine inhibits endothelial inflammation via nuclear factor kappa B signaling pathway. Adv Clin Exp Med. 2023;32(8):909–920. doi:10.17219/acem/158781
5. Ge GF, Shi WW, Yu CH, et al. Baicalein attenuates vinorelbine-induced vascular endothelial cell injury and chemotherapeutic phlebitis in rabbits. Toxicol Appl Pharmacol. 2017;318:23–32. doi:10.1016/j.taap.2017.01.013
6. Wang Y, Chu T, Pan X, Bian Y, Li J. Escin ameliorates inflammation via inhibiting mechanical stretch and chemically induced Piezo1 activation in vascular endothelial cells. Eur J Pharmacol. 2023;956:175951. doi:10.1016/j.ejphar.2023.175951
7. Wang Y, Wang J, Yang J, et al. Study on protection of human umbilical vein endothelial cells from amiodarone-induced damage by intermedin through activation of Wnt/β-Catenin signaling pathway. Oxid Med Cell Longev. 2021;2021:8889408. doi:10.1155/2021/8889408
8. Wang Y, Lin S, Jiang P, Song Y, Zhao Y, Zheng Y. Focal adhesion kinase inhibitor inhibits the oxidative damage induced by central venous catheter via abolishing focal adhesion kinase-protein kinase B pathway activation. Biomed Res Int. 2021;2021:6685493. doi:10.1155/2021/6685493
9. Wang YR, Chang J, Jiang P, et al. Anti- inflammatory effects of focal adhesion kinase inhibitor Y15 on vascular endothelial cell injury induced by central venous catheter combined with 5-fluorouracil. Chin J Clin Pharmacol. 2022;38:2449–2453.
10. Wang YR, Zhao YJ, Zheng YJ, Lin SL. Molecular mechanism of focal adhesion kinase inhibitor reducing the adhesion of damaged vascular endothelial cells and monocytes induced by central venous catheter administration. Chin J Clin Pharmacol. 2021;37:2266–2269.
11. Li HL, Liu YH, Shang XB, Li Y, Feng L, Qi DL. Effect of miR-223 on thrombophlebitis rats through regulating Toll-like receptor signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24:2020–2027. doi:10.26355/eurrev_202002_20380
12. Qian W, Gao L, Chen C, Tan Y, Zhou Y, Li Z. Involvement of Toll-like receptor 4 in vinorelbine-induced vascular endothelial injury. Exp Ther Med. 2015;10(1):62–66. doi:10.3892/etm.2015.2494
13. Hung CH, Chan SH, Chu PM, Tsai KL. Docetaxel facilitates endothelial dysfunction through oxidative stress via modulation of protein kinase C beta: the protective effects of sotrastaurin. Toxicol Sci. 2015;145(1):59–67. doi:10.1093/toxsci/kfv017
14. Wang Z, Ma L, Wang X, et al. Cimetidine attenuates vinorelbine-induced phlebitis in mice by militating E-selectin expression. Cancer Chemother Pharmacol. 2014;74(2):239–247. doi:10.1007/s00280-014-2487-8
15. Liu P, Ye L, Ren Y, et al. Chemotherapy-induced phlebitis via the GBP5/NLRP3 inflammasome axis and the therapeutic effect of aescin. Br J Pharmacol. 2023;180(8):1132–1147. doi:10.1111/bph.16002
16. Zhang J, Tong N, Chen Y, Li P, Yang S, Zhao X. Resveratrol protects against vinorelbine-induced vascular endothelial cell injury. Toxicol Mech Methods. 2013;23(9):665–671. doi:10.3109/15376516.2013.837130
17. Wang X, Lv X, Zhang J, Wang Y. Effect of Chahuang ointment on prevention of phlebitis from peripherally inserted central catheter: randomized clinical trial. Rev Esc Enferm USP. 2021;55:e03680. doi:10.1590/S1980-220X2019008003680
18. Zhou C, Chen E, Jiang D, Wang N. Potato alkaloids α-solanine and α-chaconine promote bFGF and VEGF expression in vein and adjacent tissues in the rabbit model of phlebitis. Pharmacogn Mag. 2023;19(1):41–48. doi:10.1177/09731296221137381
19. Ochi M, Kawai Y, Tanaka Y, Toyoda H. Characterization of nicardipine hydrochloride-induced cell injury in human vascular endothelial cells. J Toxicol Sci. 2015;40(1):71–76. doi:10.2131/jts.40.71
20. Hazekawa M, Nishinakagawa T, Kawakubo-Yasukochi T, Nakashima M. New application of real-time cell-monitoring analysis system to detect responses of human umbilical vein endothelial cells to paclitaxel. SENSOR MATER. 2020;32(6):2127–2137. doi:10.18494/SAM.2020.2818
21. Yu N, Zhang SK, Chen J, et al. Mitigation of QingLuoTongMai Pills on chemotherapy-induced phlebitis: a network pharmacology study and experimental validation. Comb Chem High Throughput Screen. 2022. doi:10.2174/1386207325666220629121318
22. Cao NN, Li SR, Wang QG, et al. Mechanism of Mailuo Shutong Pills in treatment of superficial thrombophlebitis based on network pharmacology and experimental verification in vivo. Chin Traditional Herbal Drugs. 2023;54:1860–1869.
23. Zhang H, Gong J, Zhang S, et al. N-acetylcysteine attenuates the incidence of phlebitis induced by carbomer/vinorelbine gel. Heliyon. 2023;9(11):e21235. doi:10.1016/j.heliyon.2023.e21235
24. Huang L, Chen G, Hu Q, Hu B, Zhu L, Fang L. Construction of a rabbit model with vinorelbine administration via peripherally inserted central catheter and dynamic monitoring of changes in phlebitis and thrombosis. Exp Ther Med. 2022;23(3):212. doi:10.3892/etm.2022.11135
25. Zhao YJ. Study of PICC Combined 5-FU Induced Human Umbilical Vein Endothelial Cells Injury[D]. Xinjiang: Xinjiang Medical Univ; 2018.
26. Vorbach H, Armbruster C, Robibaro B, et al. Endothelial cell compatibility of azithromycin and erythromycin. J Antimicrob Chemother. 2002;49(2):407–409. doi:10.1093/jac/49.2.407
27. Drouet M, Cuvelier E, Chai F, Genay S, Odou P, Décaudin B. Disturbance of vancomycin infusion flow during multidrug infusion: influence on endothelial cell toxicity. Antibiotics. 2021;11(1):16. doi:10.3390/antibiotics11010016
28. Kim JT, Park JY, Lee HJ, Cheon YJ. Guidelines for the management of extravasation. J Educ Eval Health Prof. 2020;17:21. doi:10.3352/jeehp.2020.17.21
29. HemOnc.org LLC. [home page on the Internet]. Vesicant & irritant chemotherapy. Available from: https://hemonc.org/wiki/Vesicant_%26_irritant_chemotherapy.
30. Boschi R, Rostagno E. Extravasation of antineoplastic agents: prevention and treatments. Pediatr Rep. 2012;4(3):e28. doi:10.4081/pr.2012.e28
31. Barbee MS, Owonikoko TK, Harvey RD. Taxanes: vesicants, irritants, or just irritating? Ther Adv Med Oncol. 2014;6(1):16–20. doi:10.1177/1758834013510546
32. Wang J, Tian J, Kang J, et al. Study on the injury and its mechanism of amiodarone on human umbilical vein endothelial cells. Adverse Drug React J. 2021;23:461–467. doi:10.3760/cma.j.cn114015-20210322-00336
33. Guo JL, Yan XY, Zhao QL, et al. Time to occurrence of phlebitis after continuous infusion of total nutrient admixture through peripheral veins: an experimental animal study. J Inflamm Res. 2022;15:205–215. doi:10.2147/JIR.S346186
34. Drouet M, Chai F, Barthélémy C, et al. Influence of vancomycin infusion methods on endothelial cell toxicity. Antimicrob Agents Chemother. 2015;59(2):930–934. doi:10.1128/AAC.03694-14
35. Drouet M, Chai F, Barthélémy C, et al. Endothelial cell toxicity of vancomycin infusion combined with other antibiotics. Antimicrob Agents Chemother. 2015;59(8):4901–4906. doi:10.1128/AAC.00612-15
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
