Back to Journals » Cancer Management and Research » Volume 10
Programmed death ligand 1 expression in human intrahepatic cholangiocarcinoma and its association with prognosis and CD8+ T-cell immune responses
Authors Zhu Y , Wang XY, Zhang Y, Xu D, Dong J , Zhang Z, Yi CH, Jia HL, Yang X
Received 1 May 2018
Accepted for publication 22 June 2018
Published 2 October 2018 Volume 2018:10 Pages 4113—4123
DOI https://doi.org/10.2147/CMAR.S172719
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Ying Zhu,1,2,* Xiang-Yu Wang,1,2,* Yu Zhang,1,2,* Da Xu,1,2,* Jian Dong,3,4 Ze Zhang,1,2 Chen-He Yi,1,2 Hu-Liang Jia,1,2 Xin Yang1,2
1Department of General Surgery, Huashan Hospital, Fudan University, Shanghai 200040, China; 2Institutes of Cancer Metastasis, Fudan University, Shanghai 200040, China; 3Department of Hepatobiliary Surgery, First Affiliated Hospital of Medical College, Xi’an Jiaotong University, Xi’an 710061, China; 4Institute of Advanced Surgical Technology and Engineering, First Affiliated Hospital of Medical College, Xi’an Jiaotong University, Xi’an 710061, China
*These authors contributed equally to this work
Background: Agents targeting the programmed death ligand 1 (PD-L1)/programmed death receptor 1 immune checkpoint exhibited promising clinical outcomes in a variety of malignant tumors, including intrahepatic cholangiocarcinoma (ICC). However, the relationship between PD-L1 expression and CD8+ T-cell immune responses is not well defined in ICC.
Patients and methods: We investigated PD-L1 expression immunohistochemistry in formalin-fixed, paraffin-embedded tissues from 192 ICC patients undergoing curative resection and correlated our results with the clinicopathologic features and prognosis. We also quantified CD8+ T-cell infiltration in ICC specimens and evaluated the relationship between PD-L1 expression and CD8+ T-cell infiltration. After incubating human ICC cell lines (HCCC9810 and RBE) with interferon (IFN)-γ, we measured the PD-L1 expression of these ICC cells by Western blot and flow cytometry.
Results: Only 34 patients (17.7%) showed ≥5% membranous PD-L1 expression on tumor cells, and tumoral PD-L1 overexpression (≥5%) was significantly associated with superior overall survival (P=0.012) and disease-free survival (P=0.018). A significant positive association was found between PD-L1 expression and the presence of CD8+ T-cells. In fresh frozen ICC specimens, IFN-γ was found to be significantly correlated with PD-L1 and CD8A gene expression, as evaluated by reverse transcription-polymerase chain reaction. Moreover, stimulation of the HCCC9810 and RBE cells with recombinant IFN-γ, secreted by CD8+ T-cells rapidly induced PD-L1 upregulation in these cell lines in vitro.
Conclusion: Tumor PD-L1 overexpression is mainly stimulated by activated CD8+ T-cells pre-existing in the ICC microenvironment, and PD-L1 is a favorable prognostic factor for the patients. These observations suggest that anti-PD-L1/programmed death receptor 1 therapy may benefit ICC patients with tumor cell PD-L1 expression and the presence of CD8+ T-cells.
Keywords: tumor microenvironment, adaptive immune resistance, PD-L1, CD8+ T-cell, IFN-γ
Introduction
Intrahepatic cholangiocarcinoma (ICC) is a highly malignant subtype of biliary cancers originating from the epithelium of the intrahepatic bile duct. It is also the second most common primary hepatic malignancy following hepatocellular carcinoma.1,2 In recent years, the incidence and mortality of ICC are on the rise in almost all countries.1–3 Clinically, surgical resection is the only curative treatment for early-stage ICC. However, the prognosis of ICC after curative resection remains extremely dismal because of high recurrence rates. There is, therefore, an urgent need to develop alternative systemic therapies to improve patients’ outcomes.4
Recently, drugs blocking programmed death ligand 1 (PD-L1)/programmed death receptor 1 (PD-1) have provided a significant survival benefit and have good prospect of application in clinical trials of a variety of malignant tumors, such as advanced melanoma, renal cell carcinoma, urothelium carcinoma, non-small-cell lung cancer and others.5–7 The PD-L1/PD-1 immune checkpoint pathway has been identified as a critical mediator of immunosuppression within the tumor microenvironment.8 PD-L1, one of the PD-1 ligands, is expressed not only on tumor cells but also on tumor-infiltrating stroma cells, and its ligation to PD-1 has indeed been shown to induce activated tumor-specific T-cell apoptosis and impair T-cell-mediated antitumor immune responses, leading to local immune suppression, thus favoring tumor growth and metastasis.8,9
However, the objective response rate of this novel immune checkpoint blockade is limited to <40%, and the underlying mechanisms of resistance remain obscure.10 These findings have stimulated interest in characterizing the host’s immune response and understanding the mechanisms controlling PD-L1 expression in the tumor microenvironment.
Accumulating evidence suggests that the presence of antitumor tumor-infiltrating lymphocytes is important for the activity of immunotherapies, including checkpoint blockade.11,12 Early research showed that a high degree of CD8+ T-cell infiltration in ICC correlated with better prognosis after curative resection, suggesting that the tumor-specific T-cell immune responses play a role in the clinical disease course.13
Currently, there are two mechanisms of PD-L1 expression on tumor cells. Some previous studies have suggested that the constitutive expression of PD-L1 can be driven by genetic alterations or activation of certain intrinsic signaling pathways, leading to immune evasion through an innate immune resistance and poor prognostic factor for tumor.14 Other studies have considered tumors upregulating PD-L1 as an induced adaptive immune response to inflammatory signals and as a favorable prognostic factor for tumor.15,16
To the best of our knowledge, there is very limited information on the expression of PD-L1 in ICC, and its relationship with immune responses in ICC microenvironment remains unknown. In the present study, we aimed to analyze the characteristics of PD-L1 expression in ICC and determine its relationship with clinicopathologic features. We also explored the relationship between PD-L1 expression and CD8+ T-cell antitumor immunity.
Patients and methods
Cell lines
Two ICC cell lines, HCCC9810 and RBE, were purchased from the American Type Culture Collection and cultured at 37°C, 5% CO2 in RPMI 1640 supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin.
Patients and tumor samples
A total of 192 patients who underwent initial hepatectomy of ICC were included in the study. The surgically resected specimens were confirmed histopathologically and classified according to the eighth American Joint Committee on Cancer/tumor–node–metastasis classification.17 This research was verified and ethically approved by the Ethics Committee of Huashan Hospital of Fudan University (No. 2018-033). All patients provided written informed consent for the use of surgical specimens for pathological testing with operation consents. Patients did not receive any neoadjuvant radiotherapy and/or chemotherapy prior to surgery. Clinical and follow-up details were analyzed for all patients. The clinicopathologic characteristics of the study population are presented in Table 1.
Immunohistochemistry
Immunohistochemical (IHC) staining was performed as described previously.18 Briefly, following deparaffinization, rehydrating and antigen retrieval, primary antibodies were applied to slides, incubated at 4°C overnight, followed by incubation with secondary antibody (Dako Denmark A/S, Glostrup, Denmark) at 37°C for 30 minutes. A rabbit anti-human PD-L1 monoclonal antibody (1:50 dilution, SP142; Spring Bioscience, Inc., CA, USA) and antihuman CD8 monoclonal antibody (1:50 dilution; BD Bioscience, Franklin Lakes, NJ USA) were used as specific markers for PD-L1 and CD8+ T-cells, respectively. Staining was carried out with DAB and counterstaining was performed with hematoxylin.
Quantification of PD-L1 and CD8 density
Two independent pathologists evaluated all specimens. The proportion of PD-L1–positive cells was evaluated as the percentage of total tumor cells, and PD-L1 tumor positivity was defined by membrane staining of ≥5% of tumor cells, in accordance with previous studies.15,16 CD8+ T-cells were defined by their expression of CD8. For quantification of infiltrating CD8+ T-cells, the five most representative areas (200× magnification) were selected, and positive cells were counted manually and expressed as the mean number of cells of every specimen.
Reverse transcription-polymerase chain reaction
Total RNA was extracted from cells and frozen samples by using Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA) and then reverse transcribed into cDNA. Real-time polymerase chain reaction (PCR) was performed using SYBR Green PCR Master Mix (DBI® Bioscience, Ludwigshafen, Germany) and ABI PRISM 7900 Sequence Detection System (Thermo Fisher Scientific). Results were normalized to β-actin for mRNA measurement. All the primer sequences used in this study were listed as follows: PD-L1 (forward: TGGCATTTGCTGAACGCATTT, reverse: TGCAGCCAGGTCTAATTGTTTT); CD8A (forward: ATGGCCTTACCAGTGACCG, reverse: AGGTTCCAGGTCCGATCCAG); interferon (IFN)-γ (forward: TCGGTAACTGACTTGAATGTCCA, reverse: TCGCTTCCCTGTTTTAGCTGC); β-actin (forward: CATGTACGTTGCTATCCAGGC, reverse: CTCCTTAATGTCACGCACGAT).
Western blot assay
Western blot was performed as described previously.18 Briefly, whole cell lysis was performed in RIPA buffer containing protease inhibitor (Roche Diagnostics, Indianapolis, IN, USA). Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. After blocking with 5% non-fat milk, membranes were incubated with the primary antibody. Rabbit monoclonal anti-PD-L1 (1:1,000, SP142; Spring Bioscience, Inc.) and anti-GAPDH (1:3,000; Cell Signaling Technology, Beverly, MA, USA) were used as primary antibodies. Primary antibodies were applied, followed by horseradish-peroxidase-conjugated secondary antibodies. Antibody binding was detected by enhanced chemiluminescence assays, and each band was detected with Image Acquisition using ImageQuant™ LAS 4000 (GE Healthcare Life Sciences, San Diego, CA, USA).
Statistical analysis
Survival analysis, univariate analysis and Kaplan–Meier curves were generated using SPSS 16.0 statistical software. Comparison of categorical and continuous variables was performed using the chi-squared test and the Fisher’s exact test, respectively. Survival data were compared with the log-rank test. For the gene expression analysis, the correlations between the mRNA expression of PD-L1 and CD8A and INF-γ were analyzed using Spearman’s rank correlation. A difference was considered significant for P<0.05.
Results
PD-L1 expression in ICC specimens
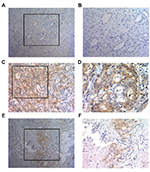
IHC analysis of all ICC specimens showed that tumor PD-L1 expression occurred in a membranous pattern on cancer cells with variable degrees of staining intensity in the cytoplasm. As shown in Figure 1, three major PD-L1 expression patterns were observed – absence (Figure 1A, B), diffuse expression (Figure 1C, D) and regional expression (Figure 1E, F). Based on the features of PD-L1 expression on cancer cells, we scored the ICC specimens as negative (defined as <5% PD-L1+ cells) for 158 patients (82.3%) and positive for 34 patients (17.7%).
Correlation between PD-L1 expression and clinicopathologic features
We performed univariate analysis with the expression of PD-L1 and clinicopathologic parameters of ICC patients. Detailed results are shown in Table 1. Tumor PD-L1 expression was significantly associated with decreased CA19-9 and CEA levels at diagnosis (P<0.01 and P<0.05, respectively). In addition, tumor PD-L1 expression was significantly associated with the common markers of tumor aggressiveness, including higher number of tumors (P<0.01) and vascular invasion (P<0.05), as shown in Table 1. No other associations were found between other clinicopathologic parameters and expression of PD-L1.
Prognostic significance of PD-L1 expression
The median follow-up period was 24 months (range 0.4–85 months), and a total of 114 patients (59.4%) died during the follow-up. Median times to disease-free survival (DFS) and overall survival were 19.4 and 24 months, respectively.
Survival analysis suggested that favorable overall survival and DFS intervals were highlighted by Kaplan–Meier curves and log-rank test (P=0.012 and P=0.018, respectively; Figure 2A, B) for the patients with positive PD-L1 expression compared to those with negative expression. These data clearly demonstrate the strong correlation between PD-L1 overexpression and superior clinical outcome of the patients with ICC.
PD-L1 expression was associated with CD8+ T-cell infiltration in the ICC immune microenvironment
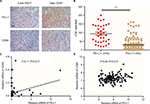
As CD8+ T-cells are central to adaptive antitumor immune responses and immune evasion associated with PD-L1 expression in tumors, we quantified CD8+ T-cell infiltration in ICC specimens to determine the association between tumor PD-L1 expression and CD8+ T-cell immune responses. Interestingly, we found that PD-L1 expression was proportional to the CD8 infiltrate (Figure 3A) and that the density of CD8+ T-cells was significantly higher in tumors with positive rather than those with negative PD-L1 expression (P<0.001; Figure 3B).
To confirm the association between elevated expression of PD-L1 and abundant CD8+ T-cell infiltration in ICC, we also measured the mRNA levels of PD-L1 and CD8A by quantitative PCR using the screened 54 ICC patients with available frozen tissue samples. As shown in Figure 3C, the PD-L1 mRNA within tumors was positively associated with the CD8A mRNA level in ICC tissues (P=0.017). We also analyzed the relationship between PD-L1 and CD8A expression in publicly available human ICC gene expression from the National Center for Biotechnology Information Gene Expression Omnibus database (GSE33327)19 and found that the PD-L1 levels were significantly correlated with CD8A in ICC patients (P=0.012; Figure 3D). Together, these results clearly revealed a significant correlation between PD-L1 expression and CD8+ T-cell infiltration in ICC. In addition, we analyzed the relationship between PD-L1 and other markers of defective cells expression in publicly available human ICC gene expression from GSE33327 and found that there was a negative correlation between PD-L1 expression and regulatory T-cell infiltration (FoxP3, P=0.039; Figure S1A). However, the expression of PD-L1 was not associated with any markers of other immune cells in this cohort (Figure S1B–D). We also analyzed the relationship between PD-L1 and FoxP3 expression in our screened 54 ICC patients with available frozen tissue samples and found that the PD-L1 expression was significantly negatively correlated with Foxp3 in ICC patients (P=0.028; Figure S1E). Together, these results clearly revealed a significantly negative correlation between PD-L1 expression and regulatory T-cell infiltration in ICC.
Interaction between tumor PD-L1 expression and INF-γ in ICC
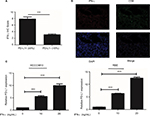
Tumor PD-L1 expression was associated with the favorable prognosis of ICC patients. Also, the strongly positive association of PD-L1 expression by tumor cells with CD8+ T-cell infiltration suggested that, tumor PD-L1 expression was induced by an adaptive antitumor immune response that secreted cytokines, particularly IFN-γ. We evaluated the mRNA levels of INF-γ in the same frozen ICC specimen shown in Figure 3C and found that INF-γ mRNA was associated with PD-L1 (P<0.001; Figure 4A) and CD8A expression (P<0.001; Figure 4A), respectively. Consistent with the mRNA results, IHC staining revealed a positive correlation between PD-L1 expression and INF-γ expression or CD8 infiltration (Figure S2A, B; Table S1). Similarly, the GSE33327 data also confirmed the significant positive correlation among the expression of CD8A, PD-L1 and INF-γ (Figure 4B).
In addition, we further evaluated the impact of INF-γ on PD-L1 in ICC cell lines in vitro (HCCC9810 and RBE). Stimulation of the HCCC9810 and RBE cells with recombinant INF-γ for 24 hours led to a significant increase in ICC cell surface expression of PD-L1 by Western blot and quantitative PCR (Figure 4C and Figure S2C). Furthermore, flow cytometric analysis also revealed that incubation with INF-γ resulted in a significant increase of PD-L1 protein in ICC cells (Figure 4D).
Discussion
ICC remains one of the most aggressive human malignancies, with limited treatment options.3 To develop effective therapeutic strategies for advanced ICC, substantial efforts have focused on the interaction of ICC with immune cells in the tumor microenvironment.
Emerging evidence has demonstrated that the expression of PD-L1 on a number of solid tumors, including ICC, plays a major role in immune escape within the microenvironment and in suppressing the effector function of local antitumor CD8+ T-cells in tumors.9,20–22 Durable responses have been shown with agents targeting PD-L1/PD-1 immune checkpoint in patients with various solid malignancies. Moreover, clinical efficacy with these agents is strongly related to PD-L1 expression in the tumors, as assessed by IHC.23 However, analyses of various tumors have shown conflicting results on whether PD-L1 expression is related to poor prognosis, better prognosis or has no association with prognosis.24–26 Conventional view suggests that the constitutive expression of PD-L1 can be driven by oncogenic signaling pathways in tumor cells, leading to immune escape through an innate immune resistance, and is associated with worse survival in patients.14 Sabbatino et al reported that high PD-L1 expression is a poor prognostic factor for ICC.20 However, they did not provide direct evidence demonstrating the association of high PD-L1 expression with suppression of local antitumor CD8+ T-cell–specific immune response. Interestingly, we observed a positive association between high PD-L1 expression and favorable prognosis in ICC. This seemingly conflicting result may be because tumors expressing high level of PD-L1 were more likely to be infiltrated with abundant CD8+ T-cells (Figure 2A, B), which is consistent with the new concept that tumors express PD-L1 as an adaptive resistance mechanism to an active antitumor immune response.15,16 In addition, the use of various PD-L1 antibodies with different detection methods and positive criteria make different studies confusing and challenging to arrive at a consistent conclusion. In the future, efforts should be made to standardize PD-L1 evaluation.
INF-γ is secreted by CD8+ T-cells, and this cytokine rapidly induces PD-L1 expression within the tumor microenvironment. A previous study found that the expression of the inflammatory cytokine INF-γ was detected specifically at the interface of PD-L1+ melanoma cells and infiltrating immune cells, but not in PD-L1- melanoma.15 Coincidentally, we also demonstrated a strong relationship between tumor cell surface PD-L1 expression with both CD8+ T-cells infiltration and intratumoral IFN-γ expression in human ICC specimens. Moreover, recombinant IFN-γ also rapidly induced PD-L1 upregulation in vitro in ICC cell lines. Together, these results collectively indicated that specific CD8+ T-cell infiltration in tumors could secrete inflammatory cytokine driving PD-L1 expression as a negative feedback mechanism, leading to what could be considered an adaptive immune resistance mechanism by ICC. Although the present study associates IFN-γ production with PD-L1 overexpression in vitro and in vivo in ICC, other secreted cytokines in the tumor microenvironment, such as transforming growth factor-β1, interleukin (IL)-10 and IL-6, may also be involved.27,28 Further studies are required to clarify the molecular mechanisms responsible for regulation of PD-L1 expression within the tumor microenvironment.
As effective as immunotherapy can be, only a minority of people exhibit durable responses, with the objective response rate of this novel immune checkpoint anti-PD-L1/PD-1 antibodies ranging from 10% to 40%, depending on the individual’s indication.10 Responses to some other forms of immunotherapy, including IFN-α, high-dose IL-2 and vaccine, are even lower.29–31 Accumulating evidence shows that clinical responses to anti-PD-L1/PD-1 therapy occur most often in patients with inflamed tumor microenvironment. The inflamed tumors, characterized by the presence of both CD8+ T-cells and PD-L1 expression in the tumor parenchyma, are viewed as reflecting the presence of a pre-existing antitumor immune response among tumor patients who are candidates for immunotherapy.32 Nevertheless, our study revealed a highly significant concordance of expression of PD-L1 with the presence of CD8+ T-cell infiltrates, but only rarely ICC patients exhibited positive PD-L1 expression, possibly indicating that only a small proportion of ICC patients will respond to anti-PD-L1/PD-1 therapy. It is worth studying whether this series of ICC patients with tumor cell PD-L1 expression and the presence of CD8+ T-cells correlate generally with higher response rates to anti-PD-L1/PD-1 therapy.
There are some limitations in the present study. One of the main drawbacks is that inevitable selection bias may exist in our retrospective analysis. Another drawback is that IHC staining is unable to precisely distinguish PD-L1 expression on the macrophages and other immunosuppressed cells such as myeloid-derived suppressor cells from PD-L1 cancer cell expression within the tumor microenvironment.
Finally, the determination of cutoff values for the PD-L1-positive tumor cells was difficult, and the absence of optimal positivity cutoff might be correlated with divergent results in previous studies.
Conclusion
Our study showed that tumor PD-L1 expression is mainly stimulated by activated CD8+ T-cells pre-existing in the ICC microenvironment, rather than be constitutively expressed by the tumor cells, and PD-L1 is a favorable prognostic factor for ICC patients. A deep understanding of the landscape of infiltrating CD8+ T-cells and the mechanisms leading to overexpression of PD-L1 in ICC microenvironment will provide better strategies for the anti-PD-L1/PD-1 therapy.
Acknowledgment
This work was generously sponsored by China National Natural Science Foundation (81672365 and 81700560) and China National Key Projects for Infectious Disease (2017ZX10203207).
Disclosure
The authors report no conflicts of interest in this work.
References
Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40(3):472–477. | ||
Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):115–125. | ||
Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268–1289. | ||
Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248(1):84–96. | ||
Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. | ||
Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. | ||
Balar AV, Weber JS. PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol Immunother. 2017;66(5):551–564. | ||
Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. | ||
Chen L, Gibbons DL, Goswami S, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun. 2014;5:5241. | ||
Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4. | ||
Harlin H, Meng Y, Peterson AC, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69(7):3077–3085. | ||
Ji RR, Chasalow SD, Wang L, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61(7):1019–1031. | ||
Goeppert B, Frauenschuh L, Zucknick M, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer. 2013;109(10):2665–2674. | ||
Atefi M, Avramis E, Lassen A, et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res. 2014;20(13):3446–3457. | ||
Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. | ||
Spranger S, Spaapen RM, Zha Y. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. | ||
Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. | ||
Zhu Y, Gao XM, Yang J, et al. C–C chemokine receptor type 1 mediates osteopontin-promoted metastasis in hepatocellular carcinoma. Cancer Sci. 2018;109(3):710–723. | ||
Sia D, Losic B, Moeini A, et al. Massive parallel sequencing uncovers actionable FGFR2–PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma. Nat Commun. 2015;6:6087. | ||
Sabbatino F, Villani V, Yearley JH, et al. PD-L1 and HLA class I antigen expression and clinical course of the disease in intrahepatic cholangiocarcinoma. Clin Cancer Res. 2016;22(2):470–478. | ||
Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, Huynh TG, Mino-Kenudson M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol. 2016;29(9):1104–1112. | ||
Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104(9):3360–3365. | ||
Patel SP, Kurzrock R. PD-L1 Expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. | ||
Thompson ED, Zahurak M, Murphy A, et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2017;66(5):794–801. | ||
Karim R, Jordanova ES, Piersma SJ, et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009;15(20):6341–6347. | ||
Xie QK, Zhao YJ, Pan T, et al. Programmed death ligand 1 as an indicator of pre-existing adaptive immune responses in human hepatocellular carcinoma. Oncoimmunology. 2016;5(7):e1181252. | ||
Wölfle SJ, Strebovsky J, Bartz H, et al. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol. 2011;41(2) :413–424. | ||
Kinter AL, Godbout EJ, McNally JP, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181(10):6738–6746. | ||
Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–5458. | ||
Sun HC, Tang ZY, Wang L, et al. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: a randomized clinical trial. J Cancer Res Clin Oncol. 2006;132(7):458–465. | ||
Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359(6382):1355–1360. | ||
Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. |
Supplementary materials
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.