Back to Journals » Cancer Management and Research » Volume 11
Programmed cell death 4 (PDCD4) as a novel prognostic marker for papillary thyroid carcinoma
Authors Galuppini F, Fassan M, Bertazza L , Barollo S , Cascione L , Watutantrige-Fernando S , Lazzarin V, Simonato P, Vianello F, Rugge M, Mian C, Pennelli G
Received 13 November 2018
Accepted for publication 22 July 2019
Published 20 August 2019 Volume 2019:11 Pages 7845—7855
DOI https://doi.org/10.2147/CMAR.S194344
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rituraj Purohit
Francesca Galuppini,1 Matteo Fassan,1 Loris Bertazza,2 Susi Barollo,2 Luciano Cascione,3 Sara Watutantrige-Fernando,4 Vanni Lazzarin,1 Paolo Simonato,1 Federica Vianello,4 Massimo Rugge,1 Caterina Mian,2 Gianmaria Pennelli1
1Pathology Unit, Department of Medicine (DIMED), University of Padova, Padova 35121, Italy; 2Endocrinology Unit, Department of Medicine (DIMED), University of Padova, Padova 35121, Italy; 3Università Della Svizzera Italiana, Institute of Oncology Research and Swiss Institute of Bioinformatics, Bellinzona 6500, Switzerland; 4Department of Radiotherapy, Istituto Oncologico del Veneto, Padova 35128, Italy
Correspondence: Gianmaria Pennelli
Surgical Pathology Unit, Department of Medicine (DIMED), University of Padova, Via Aristide Gabelli, Padua 61 35121, Italy
Tel +39 049 821 8996
Fax +39 049 821 7655
Email [email protected]
Background: The primary goal of papillary thyroid cancer (PTC) management was to stratify patients at pre- and post-surgical level to identify the small proportion of cases with potentially aggressive disease.
Purpose: The aim of our study is to evaluate the possible role of programmed cell death 4 (PDCD4) and BRAF status as prognostic markers in PTC.
Patients and methods: We investigate programmed cell death 4 (PDCD4) immunohistochemical expression in 125 consecutive PTCs with median follow-up of 75.3 months (range, 15–98 months) to verify the possible correlation between BRAF status and correlate the classical clinicopathological prognostic factors and PTC outcome with PDCD4 expression. To further support the data, miR-21 expression was tested (by quantitative real-time PCR and in situ hybridization) in a different series of 30 cases (15 PTCs BRAFwt and 15 PTCs BRAFV600E). Moreover, we validated our results using TGCA thyroid carcinoma dataset.
Results: We found that 59.8% of the patients showed low-grade PDCD4 nuclear expression and low-grade expression correlated with BRAF V600E. Compared with BRAF 15 wild-type tissue samples, a significant miR-21 up-regulation was associated with BRAF V600E mutations. Low-grade PDCD4 resulted, and was associated with aggressive histological variants, higher cancer size, extra-thyroidal extension, multifocality, lymph-node metastasis and lymph nodal ratio at the diagnosis. Concerning the outcome, the low-grade PDCD4 expression correlated at univariate and multivariate analysis, with lower levels of recurrence-free survival rate (RFS) and with poor outcome. Moreover, there was significant association between BRAF V600E patients with PDCD4 nuclear loss and lower RFS, whilet here was significant association between BRAF wild-type patients with PDCD4 nuclear expression and better outcome.
Conclusion: These results showed that PDCD4 could predict PTC outcome and that the sum of PDCD4 and BRAF alterations increases the prognostic power of BRAF mutation alone.
Keywords: papillary thyroid cancer, PDCD4, BRAF, outcome
Introduction
Papillary thyroid cancer (PTC) is the most common thyroid cancer subtype and usually carries a favorable prognosis, with a survival rate of around 90% at 10 years. 80% of low-risk patients are successfully treated with primary surgery followed by radio-iodine (131I) ablation.1,2 Although PTC is generally curable with a good prognosis, 5–15% of patients show local recurrences and/or distant metastases. One in three recurrences lose the ability to trap 131I, and furthers therapies such as as surgery and external radiotherapy become necessary. The prognosis of these patients is unfavorable, due to the lack of effective therapies.
Consequently, the primary goal of PTC management is to stratify patients at pre- and post-surgical level in terms of prognosis. It is aimed at identifying the small proportion of cases with potentially aggressive disease who require tailored treatment and specific follow-up programs.
The classical and well-known clinical-prognostic factors for PTCs are older age at the time of diagnosis, large tumor size, aggressive histological variants, extrathyroidal invasion, lymph node metastasis, and distant metastasis. In the last time, molecular cancer profiling has promise as a novel tool for improving patient risk stratification and prognostics. The largest studies of preoperative molecular markers in patients with indeterminate fine needle aspiration (FNA) cytology have, respectively, evaluated a panel of genetic mutations and rearrangements. The new American Thyroid Association (ATA) management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer identify at pre-surgical levels a mutational panel including multiple mutations and translocations as BRAF, NRAS, HRAS, and KRAS point mutations, as well as RET/PTC1, RET/PTC3 and PAX8/PPARc rearrangements.3–6 In indeterminate cytology thyroid nodules, the sensitivity of the seven genes mutational panel testing is variable, with reports ranging from 44% to 100%. This algorithm employing seven-gene mutational testing as a means to inform decision making on extent of primary thyroid surgery (i.e., lobectomy or total thyroidectomy)5 were developed at a time when the ATA guidelines favored total thyroidectomy for most PTCs >1 cm in diameter.7 However, this does not reflect recommendations in these guidelines. The role of BRAF in PTC is debated. At the time, there are no strong evidence of the correlation between BRAF mutations and outcome,8 but the majority of the studies reported an association between BRAF mutation and advanced age at the diagnosis, extrathyroid extension, advanced stages (III–IV), lymph nodes metastasis and recurrences of the disease.9–11
In PTC diagnosis, different immunohistochemical markers were identified such as Galectin-3,12 but none were distinctly correlated to the outcome.
Recently, programmed cell death 4 (PDCD4)–miR21 pathways were evaluated in a series of follicular cells-derived carcinomas (PDCD4) and resulted in down-regulating neoplastic tissues. PDCD4 is a tumor suppressor gene involved in the apoptotic machinery and in cell transformation and invasion, and tumor progression through its interaction with the translation initiation factors eIF4A and eIF4G.13,14 Several mechanisms are involved in PDCD4 dysregulation and the oncogenic microRNA miR-21 has been shown to specifically target the PDCD4 3′-untranslated region, which negatively regulates PDCD4 expression.13–17 In spite of this data, no previous study evaluated PDCD4 expression specifically in PTC and correlated its expression with the outcome.
The aims of the present study were to 1) investigate PDCD4 expression in a larger series of PTCs; 2) verify the possible correlation between BRAF status and nuclear PDCD4 expression; 3) correlate the classical clinicopathological prognostic factors for PTC with PDCD4 expression and BRAF status; and 4) evaluate the possible role of PDCD4 and BRAF status as prognostic markers in PTC.
Materials and methods
Patient’s selection
The study concerned a consecutive series of 125 patients with PTC (51 men [40.8%] and 74 women [59.2%]; median age, 45 years; range, 11–84 years) collected from 2007 to 2011 with a median follow-up of 75.3 months (range, 15–98 months). At our institution, BRAF mutation analysis in fine-needle aspiration biopsies (FNAB) is a standard procedure in patients with single thyroid nodules, and/or nodules showing suspect features on ultrasound, so for all the patients included in the study, we had available FNABs on which BRAF status had been explored. Later, all the patients underwent to total or hemi-thyroidectomy and histological diagnoses and staging were done according to the TNM classification.18 The cases considered were retrospectively selected from the electronic archives of the Surgical Pathology & Cytopathology Unit at Padua University. All patients involved in this study gave their informed written consent together with confirmation of parental/legal guardian written informed consent for patients under the age of 18 years in compliance with the Declaration of Helsinki and the Ethical Committee on Research on Human Tissues of the Padua University approved this study.
DNA extraction and BRAF status detection
DNA from FNAB material was isolated using the QIAamp DNA Micro kit (Qiagen, Milan, Italy) according to the manufacturer’s protocol. The BRAF status of exon 15 was assessed both by direct sequencing and by mutant allele-specific PCR amplification (MASA) for the T to A substitution at nucleotide 1799 (V600E), based on descriptions in the literature.19 We performed our statistical analysis on the direct sequencing results; in the event of discordant results (sequencing versus MASA), we confirmed the findings by assessing BRAF status in surgical specimens.
Quantitative real-time PCR and miR-21 detection
Tissue samples from 15 BRAF wild-type PTCs and 15 BRAF V600E PTCs were deparaffinized with xylene at 50°C for 3 mins. Total RNA was extracted using the RecoverAll kit (Ambion, Austin, TX, USA) according to the manufacturer’s instructions. The NCode™ miRNA quantitative real-time PCR method (Invitrogen, Carlsbad, CA, USA) was used to detect and quantify mature hsa-miR-21 (miR-21; primer sequence: 5′-CGG TAG CTT ATC AGA CTG ATG TTG A-3′) on real-time PCR instruments according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA, USA) as previously described.20 Normalization was done with the small nuclear RNA U6B (RNU6B; Invitrogen). PCR reactions were run in triplicate, including no-template controls. The data were analyzed using the comparative CT method.
Histology and PDCD4 immunohistochemistry
Tissue specimens were fixed in 10% buffered formalin and embedded in paraffin. Serial histology sections 4–6-μm thick were stained with hematoxylin and eosin, and assessed by two pathologists (M.F. and G.P.). Immunohistochemical staining for PDCD4 was done automatically (Ventana Benchmark XT System, Touchstone, AZ, USA) (catalog# HPA001032; Atlas Antibodies, Stockholm, Sweden; 1:100) according to the manufacturer’s instructions.20 Immunohistochemistry (IHC)IHC sections were lightly counterstained with hematoxylin. Appropriate positive and negative controls were run concurrently. PDCD4 expression in stromal/inflammatory cells and in coexisting non-neoplastic epithelia served as a positive internal control. Only PDCD4 nuclear expression was evaluated by two pathologists (F.G. and G.P.), unaware of any clinical information. PDCD4 nuclear staining was scored on a four-tiered scale (score 0: no stain; score 1: ≥1–≤30% positive nuclei; score 2: >30–≤70%; score 3: ≥71%).21,22 The nuclear immunostaining score was then dichotomized as low-grade versus high-grade (for values of 0 and 1 versus values of 2 and 3).
miR-21 in situ hybridization
Tissue samples from 15 BRAF wild-type PTCs and 15 BRAF V600E PTCs were considered for the in situ hybridization (ISH) study. ISH was performed using the GenPoint® Catalyzed Signal Amplification System (DakoCytomation, Carpinteria, CA, USA) according to the manufacturer’s protocol. Briefly, slides were incubated at 60°C for 30 mins and deparaffinized, as described elsewhere.23–25 Sections were treated with Proteinase K (DakoCytomation) for 30 mins at room temperature, rinsed several times with dH2O, and immersed in 95% ethanol for 10 s before air-drying. The slides were prehybridized at 49–56°C for 1 hr with mRNA ISH buffer (Ambion, Carlsbad, CA, USA) before incubation overnight at 49–56°C in buffer containing the 5ʹ-biotin-labeled hsa-miR-21 miRCURY® LNA detection probe (Exiqon, Woburn, MA, USA) or the scrambled negative control probe (U6; Exiqon) at a final concentration of 200 nM. The slides were washed in both Tris-buffered saline Tween-20 (TBST) and GenPoint® stringent wash solution (54°C for 30 mins), then exposed to H2O2 blocking solution (DakoCytomation) for 20 mins, and then further blocked in a blocking buffer (DakoCytomation) for 30 mins before they were exposed to primary streptavidin–horseradish peroxidase (HRP) antibody, biotinyl tyramide, secondary streptavidin–HRP antibody, and 3,3ʹ-diaminobenzidine chromogen solutions, according to the manufacturer’s protocol. The slides were then briefly counterstained with hematoxylin and rinsed with TBST and water before mounting.
TGCA dataset analysis
TCGA Thyroid carcinoma dataset (validation set/cohort http://cancergenome.nih.gov/cancersselected) was used as an independent set. PDCD4 and miR-21 expression data for 136 tumors from patients with PTC were downloaded and processed. The expression levels have been normalized and logged (base 2). The gene and microRNA of interest have been correlated using Pearson correlation method and the plots were created with R.
Statistical analysis
All statistical analyses were performed using the MedCalc Statistical Software version 16.4.3 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2016). Categorical data were summarized using frequencies and percentages. Distributions of the continuous variables were assessed and data were summarized accordingly. Group comparisons of categorical variables were performed using the χ2-test or Fisher’s exact test. Mann–Whitney test for nonparametric data was used to correlate lymph node ratio (LNR) with BRAF status and PDCD4 immunohistochemical expression. A multivariate analysis was performed using Cox proportional-hazards regression with Enter method. A P<0.05 was considered statistically significant.
Results
Clinicopathological features
In this study, we evaluated 125 consecutive patients with cytologic and histological diagnosis of PTCs. The 59.2% (n=74) of all the patients were female and the 40.8% (n=52) were male, accordingly with the prevalence reported in the literature. The mean age was 45 years (range, 11–84 years) and the median age was 44. The histological variants of the 125 PTCs were as follows: classical in 51.2% of cases (64/125), follicular in 22.4% (28/125), oxyphilic in 16.8% (21/125), tall cell in 4.0% (5/125), hobnail in 2.4% (3/125) and columnar in 3.2% (4/125). According to the TNM classification, 80.8% (101/125) patients were in stage I, 18.4% (23/125) were in stage II and 0.8% (1/125) were in stage III. Considering the dimensional criteria, 64.5% (80/125) resulted in T1, 14.5% (18/125) in T2; 17.7% (22/125) in T3 and 3.2% (4/125) in T4. 52.8% of the patients (55/125) was found to have lymph node metastasis at the diagnosis.
Correlation between nuclear PDCD4 expression, BRAF status and miR-21 levels
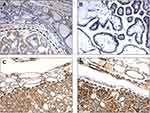
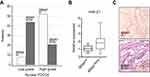
Among all cases, 52% (65/125) resulted in BRAF V600E mutations, according to literature data26. At the immunohistochemical analysis, 59.8% (74/125) of the patients showed low-grade PDCD4 nuclear expression (Figure 1). Moreover, low-grade PDCD4 nuclear expression correlated with BRAF V600E mutation (P<0.0001) (Figure 2A).
Compared with BRAF wild-type tissue samples, a significant miR-21 up-regulation (quantitative real-time PCR) was associated with BRAF V600E mutations (P=0.026) (Figure 2B). miR-21 up-regulation was confirmed in the same neoplastic samples by the miR-21 over-expression revealed by ISH (brown granular cytoplasmic staining; Figure 2C).
Correlation between PDCD4 expression, BRAF status, and clinic-pathological features
At univariate statistical analysis, the loss of nuclear PDCD4 expression resulted associated with aggressive histological variants of PTC as hobnail (66.7%), tall cell (80%) and columnar variants (100%) (P=0.0002) (Figure 3A) and correlated with higher cancer size (P=0.01) (Figure 3B), extra-thyroidal extension (P=0.002), multifocality (P=0.016), lymph node metastasis (P=0.003) (Figure 3C) and lymph nodal ratio (P=0.01) (Figure 3D) at the diagnosis (Table 1).
 |
Table 1 Correlation between PDCD4, clinicopathological features and outcome in patients with PTC |
At diagnosis, BRAF mutation was significantly associated with aggressive histological variants (P=0.03) and staging (P=0.04).
Correlation between PDCD4 expression, BRAF status, and outcome
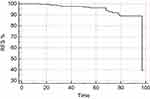
Information about the outcome was available for 111 patients. At the end of follow-up, 11 patients (9.9%) resulted with recurrent/persisted disease, 99 patients (89.9%) resulted cured and only one patient (0.9%) resulted dead for progressive PTC disease. At the statistical analysis, the nuclear loss of PDCD4 expression correlated with lower levels of recurrence-free survival rate (RFS) (P=0.0008) and with poor outcome (evaluated as recurrence/persisted disease status at the end of follow-up) (P=0.003). (Figure 4A and C). BRAF mutation resulted did not correlated with RFS or outcome at the end of follow-up, but interestingly, BRAF V600E patients with PDCD4 nuclear loss resulted significantly associated with lower RFS (0.04) (Figure 4B), while BRAF wild-type patients with PDCD4 nuclear expression resulted significantly associated with better outcome (0.004) (Figure 4D).
At multivariate analysis, only the loss of nuclear PDCD4 expression resulted to correlate with RFS (P=0.002) (Figure 5).
Validation analysis
TCGA Thyroid carcinoma dataset analysis confirmed that PDCD4 has a lower nuclear expression in PTC, in particular in classical and tall-cell variant compared to follicular one. Moreover, miR-21 expression inversely correlated with PDCD4 in the considered cohort (P<0.05) (Figure 6A–C).
Discussion
PTC is generally a curable disease with a good prognosis, but the 5–15% of patients show local recurrences and/or distant metastases. One in three recurrences lose the ability to trap 131I, and the prognosis of these patients is unfavorable, due to the lack of effective therapies. In recent years, the primary goal of PTC management was to stratify patients at pre- and post-surgical level to identify the small proportion of cases with potentially aggressive disease who require tailored treatment and specific follow-up programs. In addition to classical risk factors for PTC (age at the time of diagnosis, large tumor size, aggressive histological variants, extrathyroidal invasion, lymph node metastasis, and distant metastasis), nowadays many studies have identified at pre-surgical levels a seven-genes mutational panel including multiple mutations and translocations as BRAF, NRAS, HRAS, and KRAS point mutations, as well as RET/PTC1, RET/PTC3 and PAX8/PPARc rearrangements,3–6 able to increase the diagnostic predictive value for PTC. While the molecular profile has improved the diagnostic yield of needle aspiration cytology, on the other hand, there are not yet enough data to fully understand its possible use in the prognosis and follow-up of patients with PTC. The BRAF mutations have already been taken into account as a pre-operative reliable prognostic factor, in order to optimize the choice of surgical treatment. In fact, this mutation is very frequent in PTC, with values ranging from 14% to 64%.27–30 Most of these gene variants cause the substitution of valine with the glutamine at codon 600 of this protein (V600E). This mutation causes constitutively activated mitogen-activated protein kinase (MAPK) pathway. BRAF V600E is found more frequently in the classical variant and the tall-cell variants,9,30,31 while BRAF K601E is more associated with follicular variant.32 The same transgenic mouse models, BRAF V600E mutated, develop PTC with classical architecture or tall cell, with a tendency to dedifferentiation and extrathyroid invasiveness. Numerous studies in the literature have attempted to correlate the classical prognostic parameters listed above with the BRAF mutation, in an attempt to validate its preoperative prognostic value. Among these parameters, the extrathyroid invasion, the advanced stage, and the presence of lymph node metastases seem to be more related to outcome of the patient and associated with the presence of the BRAF mutation.33–35 Lymph node status, however, shows an association weaker than the other two, this is probably due to the approach to the different lymph node dissection offered in the various centers. The correlation between the mutation and the other parameters such as gender, age and size of the primary tumor is not yet clear in the literature to date. These differences appear to be imputable to the different approaches of the various centers in the inclusion of patients in the studies, the interpretation of histological specimens and in various strategies on the treatment and follow-up of these patients.
Also, the role of PDCD4 was investigated in thyroid tumors along with the possibility of its use as a marker of aggressiveness. PDCD4 is a protein that, when expressed at the nuclear level, acts facilitating the process of apoptosis. In many tumors, it was seen to be significantly down-regulated in the nucleus increasing its cytoplasmic positivity, although the significance of this increase at cytosolic level is not yet known. Studies have been conducted on both esophageal carcinomas,33,36 medullary carcinomas of the thyroid gland37 and seminal studies on follicular tumors of the thyroid.20
PDCD4 has been reported to be able to inhibit protein translation through binding to the translation initiation factor eIF4A and inhibiting its RNA helicase activity. Different mechanisms in turn are involved in PDCD4 dysregulation in human cancer; as well as the miR-21 activity, the MAPK pathway and the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) cascade also cooperate in PDCD4 proteasome-dependent protein degradation.37–39
This is the first study in which PDCD4 was investigated in a large series of PTC tissues and correlated with clinicopathological features, the main prognostic factors and outcome.
Our results showed that PDCD4 is down-regulated in the 59.8% of the considered series and that the loss of nuclear PDCD4 resulted significantly correlated with BRAF V600E mutation (P<0.0001) and with the classic prognostic factors for PTC as aggressive histological variants (tall cells, hobnail and columnar; P=0.0002), greater cancer size (P=0.01), extra-thyroidal extension (P=0.002), multifocality (P=0.016) and lymph node metastasis at the diagnosis (P=0.003) also expressed as a ratio of the number of positive versus the number of removed lymph nodes (LNR; P=0.03). We evaluated lymph node metastasis also as LNR because in some tumors such as breast cancer, LNR categorization was found as a better predictor for cancer relapse than categorization based on the number of positive lymph nodes.40 To increase the association between the loss of PDCD4 nuclear expression and BRAF V600E, we also analyzed the well known upstream regulator of PDCD4, the microRNA miR-21. Our previous studies20,25,37 demonstrated a significant miR-21 up-regulation accompanied by a marked loss of nuclear PDCD4 protein in some thyroid cancer as familial and sporadic medullary thyroid carcinomas, and in a few cases of different thyroid tumors of follicular origins. In this work, we found that in PTC, miR-21 up-regulation is not only associated with a loss of PDCD4, but also to the BRAF V600E mutation. These data allow us to hypothesize that the BRAF mutation might down-regulate the PDCD4 protein through the inhibitory mechanism of miR-21.
The most interesting data regard the association between PDCD4 and outcome. PTC is not usually a lethal carcinoma and, in our series, only one patient died of progressive disease, so to verify the prognostic significance of PDCD4 expression, we used endpoint as not only the survival information but also the recurrence of disease during the follow-up. Thus, we found that at the end of the follow-up, the nuclear loss of PDCD4 expression resulted also correlated with lower values of RFS (P=0.0008) and with a poor outcome (P=0.003). Therefore, PTC patients with nuclear loss of PDCD4 not only show a recurrence/persistence of the disease at the end of the observational period, but the recurrence also occurs earlier. This data allows us to hypothesize that PDCD4 as well as being a negative prognostic factor for PTC could also provide information on performing a closer follow-up of the case with the loss of nuclear expression.
BRAF V600E mutation resulted not correlated with RFS or outcome at the end of follow-up. As previously described,41 there is still debate about whether or not the BRAF V600E mutation could be a poor prognosis predictor of PTC outcome. Some studies found that there was no significant relationship between the BRAF V600E mutation and PTC prognosis.1 But other researches implied that the BRAF V600E mutation predicts poor overall survival and/or RFS of PTC patients [9]. In our previous study, we showed that BRAF-mutated patients needed a second treatment earlier than patients with BRAF wild type, although the difference did not completely reach the statistical significance.8
Interestingly, in the current study, BRAF V600E patients with PDCD4 nuclear loss resulted significantly associated with lower RFS (P=0.04), while BRAF wild-type patients with PDCD4 nuclear expression resulted significantly associated with better outcome (P=0.004). This data support the hypothesis that the presence of both alterations represents a negative prognostic factor for the outcome of the disease. In clinical practice, we could suppose a combined use of the two markers, maybe even at pre-surgical level. This type of dual analysis could also add to the high diagnostic value of BRAF mutated-status for PTC, even to the negative prognostic value of PDCD4.
In conclusion, our study showed that in PTC, the loss of nuclear PDCD4 is associated with more adverse clinicopathological features such as aggressive histological variants, BRAF mutation and lymph node metastases. In addition, the loss of PDCD4 is associated with a low value of RFS and a poor outcome. Thus, the sum of PDCD4 and BRAF alterations increases the prognostic power of BRAF mutation alone.
Acknowledgment
The authors are grateful to Vincenza Guzzardo and Mariangela Balistreri for their technical assistance.
Disclosure
All the authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
1. Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–1427. doi:10.1093/jnci/dji080
2. Mazzaferri EL, Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86:1447–1463. doi:10.1210/jcem.86.4.7407
3. Ohori NP, Schoedel KE. Variability in the atypia of undetermined significance/follicular lesion of undetermined significance diagnosis in the Bethesda system for reporting thyroid cytopathology: sources and recommendations. Acta Cytol. 2011;55:492–498. doi:10.1159/000334218
4. Nikiforov YE, Ohori NP, Hodak SP, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96:3390–3397. doi:10.1210/jc.2011-1469
5. Nikiforov YE, Steward DL, Robinson-Smith TM, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092–2098. doi:10.1210/jc.2009-0247
6. Beaudenon-Huibregtse S, Alexander EK, Guttler RB, et al. Centralized molecular testing for oncogenic gene mutations complements the local cytopathologic diagnosis of thyroid nodules. Thyroid. 2014;24:1479–1487. doi:10.1089/thy.2013.0640
7. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi:10.1089/thy.2015.0373
8. Galuppini F, Pennelli G, Vianello F, et al. BRAF analysis before surgery for papillary thyroid carcinoma: correlation with clinicopathological features and prognosis in a single-institution prospective experience. Clin Chem Lab Med. 2016;54:1531–1539. doi:10.1515/cclm-2015-0218
9. Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–6379. doi:10.1210/jc.2005-0987
10. Chakraborty A, Narkar A, Mukhopadhyaya R, et al. BRAF V600E mutation in papillary thyroid carcinoma: significant association with node metastases and extra thyroidal invasion. Endocr Pathol. 2012;23:83–93. doi:10.1007/s12022-011-9184-5
11. Kim TH, Park YJ, Lim JA, et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer. 2012;118:1764–1773. doi:10.1002/cncr.26523
12. Bartolazzi A, Orlandi F, Saggiorato E, Italian Thyroid Cancer Study Group (ITCSG), et al. Galectin-3-expression analysis in the surgical selection of follicular thyroid nodules with indeterminate fine-needle aspiration cytology: a prospective multicentre study. Lancet Oncol. 9;2008:543–549. doi:10.1016/S1470-2045(08)70132-3
13. Yasuda M, Schmid T, Rübsamen D, et al. Downregulation of programmed cell death 4 by inflammatory conditions contributes to the generation of the tumor promoting microenvironment. Mol Carcinog. 2010;49:837–848. doi:10.1002/mc.20608
14. Fassan M, Realdon S, Pizzi M, et al. Programmed cell death 4 nuclear loss and miR-21 or activated Akt overexpression in esophageal squamous cell carcinogenesis. Dis Esophagus. 2012;25:263–268. doi:10.1111/j.1442-2050.2011.01236.x
15. Frankel LB, Christoffersen NR, Jacobsen A, et al. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026–1033. doi:10.1074/jbc.M707224200
16. Sheedy FJ, Palsson-McDermott E, Hennessy EJ, et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2009;11:141–147. doi:10.1038/ni.1828
17. Yang HS, Jansen AP, Nair R, et al. A novel transformation suppressor, Pdcd4, inhibits AP-1 transactivation but not NFkappaB or ODC transactivation. Oncogene. 2001;21:669–676. doi:10.1038/sj.onc.1204137
18. UICC. Thyroid gland. In: Brierly JD, Gospodarowicz MK, Wittekind C editors, TNM Classification of Malignant Rumours,
19. Sapio MR, Posca D, Troncone G, et al. Detection of BRAF mutation in thyroid papillary carcinomas by mutant allele-specific PCR amplification (MASA). Eur J Endocrinol. 2006;154:341–348. doi:10.1530/eje.1.02072
20. Pennelli G, Fassan M, Mian C, et al. PDCD4 expression in thyroid neoplasia. Virchows Arch. 2013;462:95–100. doi:10.1007/s00428-012-1352-6
21. Fassan M, Pizzi M, Battaglia G, et al. Programmed cell death 4 (PDCD4) expression during multistep Barrett’s carcinogenesis. J Clin Pathol. 2010;63:692–696. doi:10.1136/jcp.2010.078253
22. Mudduluru G, Medved F, Grobholz R, et al. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer. 2007;110:1697–1707. doi:10.1002/cncr.22812
23. Fassan M, Pizzi M, Giacomelli L, et al. PDCD4 nuclear loss inversely correlates with miR-21 levels in colon carcinogenesis. Virchows Arch. 2011;458:413–419. doi:10.1007/s00428-011-1046-5
24. Fassan M, Volinia S, Palatini J, et al. MicroRNA expression profiling in human Barrett’s carcinogenesis. Int J Cancer. 2011;129:1661–1670. doi:10.1002/ijc.25823
25. Mian C, Pennelli G, Fassan M, et al. MicroRNA profiles in familial and sporadic medullary thyroid carcinoma: preliminary relationships with RET status and outcome. Thyroid. 2012;22:890–896. doi:10.1089/thy.2012.0045
26. Zatelli MC, Trasforini G, Leoni S, et al. BRAF V600E mutation analysis increases diagnostic accuracy for papillary thyroid carcinoma in fine-needle aspiration biopsies. Eur J Endocrinol. 2009;161:467–473. doi:10.1530/EJE-09-0353
27. Namba H, Nakashima M, Hayashi T, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88:4393–4397. doi:10.1210/jc.2003-030305
28. Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95:625–627. doi:10.1093/jnci/95.8.625
29. Fukushima T, Suzuki S, Mashiko M, et al. BRAF mutations in papillary carcinomas of the thyroid. Oncogene. 2003;22:6455–6457. doi:10.1038/sj.onc.1206739
30. Nikiforova MN, Kimura ET, Gandhi M, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88:5399–5404. doi:10.1210/jc.2003-030838
31. Frattini M, Ferrario C, Bressan P, et al. Alternative mutations of BRAF, RET and NTRK1 are associated with similar but distinct gene expression patterns in papillary thyroid cancer. Oncogene. 2004;23:7436–7440. doi:10.1038/sj.onc.1207444
32. Trovisco V, Soares P, Preto A, et al. Type and prevalence of BRAF mutations are closely associated with papillary thyroid carcinoma histotype and patients’ age but not with tumour aggressiveness. Virchows Arch. 2005;446:589–595. doi:10.1007/s00428-005-1236-0
33. Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. doi:10.1210/er.2007-0007
34. Damiani L, Lupo S, Rossi R, et al. Evaluation of the role of BRAFV600E somatic mutation on papillary thyroid cancer disease persistence: a prospective study. Eur Thyroid J. 2018;7(5):251–257. doi:10.1159/000490699
35. Shen G, Kou Y, Liu B, Huang R, Kuang A. The BRAFV600E mutation in papillary thyroid microcarcinoma with intermediate-risk to high-risk features: does the mutation have an effect on clinical response to radioiodine therapy? Nucl Med Commun. 2019;40(1):8–13. doi:10.1097/MNM.0000000000000930
36. Fassan M, Cagol M, Pennelli G, et al. Programmed cell death 4 protein in esophageal cancer. Oncol Rep. 2010;24:135–139. doi:10.3892/or_00000838
37. Pennelli G, Galuppini F, Barollo S, et al. The PDCD4/miR-21 pathway in medullary thyroid carcinoma. Human Pathol. 2015;46:50–57. doi:10.1016/j.humpath.2014.09.006
38. Chen X, Wu W, Chen X, Gong X. Roles of phosphatidylinositol 3-kinase regulatory subunit alpha, activator protein-1, and programmed cell death 4 in diagnosis of papillary thyroid carcinoma. Tumour Biol. 2016;37(5):6519–6526. doi:10.1007/s13277-015-4476-x
39. Wei C, Song H, Sun X, et al. miR-183 regulates biological behavior in papillary thyroid carcinoma by targeting the programmed cell death 4. Oncol Rep. 2015;34(1):211–220. doi:10.3892/or.2015.3971
40. Vinh-Hung V, Verkooijen HM, Fioretta G, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009;27:1062–1068. doi:10.1200/JCO.2008.18.6965
41. Li J, Zhang S, Zheng S, et al. The BRAF V600E mutation predicts poor survival outcome in patients with papillary thyroid carcinoma: a meta analysis. Int J Clin Exp Med. 2015;8:22246–22253.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.