Back to Journals » International Journal of General Medicine » Volume 15
Prognosticating 2-Year Survival Rate of Breast Cancer Patients Through Plasma miRNA-21 and Other Associating Factors
Authors Romadhon PZ , Prayoga AA, Bintoro SUY, Diansyah MN, Amrita PNA, Savitri M, Suryantoro SD, Prahasanti K, Wijaya AY, Hendrata WM, Windradi C , Mahdi BA , Widiyastuti KN, Agustin ED
Received 19 February 2022
Accepted for publication 24 May 2022
Published 9 June 2022 Volume 2022:15 Pages 5557—5566
DOI https://doi.org/10.2147/IJGM.S361934
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Pradana Zaky Romadhon,1– 3 Ami Ashariati Prayoga,1– 3 Siprianus Ugroseno Yudho Bintoro,1– 3 Muhammad Noor Diansyah,1– 3 Putu Niken Ayu Amrita,1– 3 Merlyna Savitri,1– 3 Satriyo Dwi Suryantoro,1,2 Kartika Prahasanti,4 Andi Yasmin Wijaya,5 Winona May Hendrata,5 Choirina Windradi,2 Bagus Aulia Mahdi,1 Krisnina Nurul Widiyastuti,1 Esthiningrum Dewi Agustin1
1Department of Internal Medicine, Faculty of Medicine, Airlangga University, Surabaya, Indonesia; 2Department of Internal Medicine, Universitas Airlangga Hospital, Surabaya, Indonesia; 3Hematology and Oncology Division, Department of Internal Medicine, Dr. Soetomo General Teaching Hospital, Surabaya, Indonesia; 4Department of Physiology, Faculty of Medicine, Muhammadiyah Surabaya University, Surabaya, Indonesia; 5Faculty of Medicine, Airlangga University, Surabaya, Indonesia
Correspondence: Pradana Zaky Romadhon, Department of Internal Medicine, Faculty of Medicine, Airlangga University, Jl. Mayjen Prof. Dr. Moestopo No. 47, Pacar Kembang, Kec. Tambaksari, Kota SBY, Surabaya, Jawa Timur, 60132, Indonesia, Email [email protected]
Background: miRNA-21, one of breast cancer (BC) predictive markers, is now gaining cardinal attention from researchers worldwide to evaluate BC patients’ survival rate. However, cancer staging, hormonal status, and other BC markers still have to be discussed. We aim to determine the relationship between miRNA-21 and associating factors such as BC staging, other tumor markers, and hormonal status to predict the 2-year survival rate of BC patients.
Methods: We conducted a prospective cohort study on 49 BC patients (26 early stage, 23 advanced stage). Apart from cancer staging, we also examined CEA, Ca15-3, and hormonal status (ER, PR, Her2) and correlated them with miRNA-21 to predict 2-year survival rate. We did bivariate, multivariate, and survival analyses to determine the link between miRNA-21 and those factors to prognosticate on 2-year survival rate.
Results: There are significances between advanced and loco-regional stage (p < 0.001); high and low miRNA-21 (p = 0.002) and CA 15– 3 (p = 0.001), and low survival rate in patients with ER/PR-Her2- status (p=0.0015). Cox proportional hazard showed miRNA-21 (Adjusted HR 1.41; 95% CI = 1.205– 1.632), cancer stage (Adjusted HR 9.5; 95% CI = 1.378– 20.683), and CA15-3 (Adjusted HR 4.64; 95% CI = 1.548– 13.931) affected patients’ mortality within 2 years.
Conclusion: Low two-year survival rate depends on miRNA-21, cancer stage, CA15-3, and ER/PR-Her2-. Cancer stage is robustly associated with miRNA-21 in predicting 2-year survival rate.
Keywords: breast cancer, miRNA-21, survival, stage, health
Introduction
Breast cancer (BC) is the most prevalent malignancy in women and is the second leading malignancy-related cause of death.1–4 Nearly 1.5 million new cases are diagnosed each year, and 460,000 of them die from chemoresistance and metastases.1 Since the incidence rockets every year, early diagnosis and appropriate treatment remain the prime key to reduce the mortality. Clinical considerations involving prognostic factors are indispensable in determining appropriate therapy.5 BC’s pathological and clinical characteristics including patients’ age, histologic subtype, tumor size, lymph node enlargement, hormone receptor status, and human epidermal growth factor receptor-2 (Her-2) have been used as prognostic factors foretelling survival rate and post-therapy clinical outcome. In addition, BC patients with similar characteristics often have distinct clinical outcomes. Moreover, microscopic metastases have already occurred once they were initially diagnosed. This indicates that the clinicopathological picture of BC has limitations in determining the diagnosis, prognosis, and prediction of clinical outcomes; therefore, more advanced diagnostic and prognostic techniques are required to determine the survival rate and appropriate treatment modalities for patients.1,6
In recent years, examination of tumor markers using various molecular techniques, particularly gene expression panels, has been extensively used as an indicator of aggressiveness, invasiveness, and tumor extension.7 Tumor markers routinely observed in BC patients are miRNA, CEA, and CA15-3. MiRNA is an endogenous single-stranded RNA playing a prime role in regulating tumor’s cells growth, proliferation, differentiation, and death. Data from recent studies have shown its ability to control tumorigenesis by functioning as oncogenes (oncomiRNA) or tumor suppressor genes, and by influencing other mechanisms such as migration and metastasis in BC.8 MiRNA can be easily detected through biopsy specimens or serum.3 The type of miRNA that is highly susceptible to modification and multiplying in BC is miRNA-21.8 A study from Pan et al stated that miRNA-21 was more associated with Asian BC population than non-Asian.9
Carcinoembryonic antigen (CEA) and cancer antigen 15-3 (CA15-3) are also linked to BC patients survival and have been inspected for more than 30 years. CEA, one of the cell-adhered glycoproteins and CA15-3, a glycoprotein from the mucin-1 (MUC-1) group, are extensively expressed in various types of cancer. Several studies have shown that elevated CEA and CA15-3 can predict survival in BC patients.10–15 CEA and CA15-3 also have been approved by the Food and Drug Administration (FDA) as tumor markers to evaluate BC. On the contrary, The American Society of Clinical Oncology (ASCO) does not recommend them as screening, diagnosis, staging, post-therapy monitoring tool. Therefore, the function of CEA and CA15-3 as prognostic markers remains controversial.5
In our preceding study, it was concluded that miRNA-21 was detected high in BC patients and was correlated with 1-year survival.16–18 Subsequently, we proceed with the evaluation of miRNA-21 and determine its association with CEA, CA15-3, and other factors regarding the 2-year survival rate of BC patients and their clinical and pathology features.
Materials and Methods
Data Collection
From November 2019 to November 2021, we conducted a cohort-prospective analysis at the Universitas Airlangga Hospital’s Oncology-Hematology Outpatient Clinic in Surabaya. We follow through our previous research and observe 2-year survival rate of BC patients, as well as the link between CEA, CA15-3, ER/PR, and Her-2.16 The following are the requirements for inclusion: female; aged ≥18; no past chemotherapy or radiotherapy; and willing to sign an informed consent form (see Figure 1).
 |
Figure 1 Two-year follow-up on breast cancer patients. |
To assess the value of expression, we used the quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) to detect circulating plasma miRNA-21. As an endogenous control, the Hsa-miR-16-5p LNA PCR primer set was employed. The blood samples were run at the Surabaya Prodia laboratory and then transported to the Jakarta Prodia laboratory for storage and processing.
Ethical Clearance
- Ethical clearance was provided by the Research Ethics Committee of Universitas Airlangga Hospital (No: 164/KEP/2019). All patients signed informed consent before the study.
- Our research complies with the Declaration of Helsinki that each potential subject was well informed of the aims, methods, sources of funding, any possible conflicts of interest, institutional affiliations of the researcher, the anticipated benefits and potential risks, and any other relevant aspects of the study.
Statistical Analysis
The sample size for this investigation was determined using the correlative analytic sample formula. While 37 individuals were initially needed for the trial, we ended up with 49, 26 patients with early-stage breast cancer and 23 patients with advanced-stage breast cancer. We utilized SPSS version 24.0 for statistical analysis (Chicago, IL, USA). Depending on the data distribution, we expressed categorical variables as numbers (%) and continuous variables as mean (standard or median (range). For missing data, we employed list-wise deletion or univariable and multivariable analysis. We used Mann–Whitney tests, t-tests, chi-square tests, or Fischer’s exact tests based the variable type. Chi-square test was used for dichotomous variable, whereas continuous variables were analyzed with the independent sample t-test or the Mann–Whitney test.
To compare the survival rates of each group, we use the Kaplan–Meier curve and the Log rank test. We also performed univariate and multivariate Cox proportional-hazard regression models to find prognostic factors for 2-year survival rates. The researchers calculated hazard ratios (HRs) and 95% confidence intervals (CIs). The “entry” approach was used to adjust multivariate analyses for the selected covariates in order to estimate HRs of the tested tests.
Result
Of 49 patients, 26 were in the early-stage, 23 advanced-stage. Most of the patients were housewives. Twenty-five patients were menopausal. Type 2 diabetes mellitus and hypertension were the most frequent comorbid. Twenty-three patients had ER positive, while 18 had PR positive. The mean value of miRNA-21 was 6.00, CEA 5.63, and CA15-3 73.73 (see Table 1).
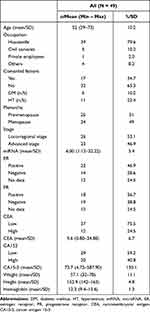 |
Table 1 Patients’ Baseline Characteristics |
Two years later, death cases reached 17 cases. Three cases occurred in the early stage, whereas 14 cases occurred in advanced-stage cases (p = 0.001). Four death cases were accompanied by DM (p=0.043). Patients with negative PR had the highest mortality rate (11 cases (p=0.022)), while patients with positive ER showed no statistical difference in mortality (p=0.120). There were significant differences among CEA, CA15-3, and miRNA-21 in those who died (p=0.028; p=0.005; p=0.003). Mean survival time in patients with advanced-stage, negative PR, and high CA 15-3 was 12.61 months, 14.47 months, and 13.00 months, respectively. Multivariate analysis showed that only cancer stage and miRNA-21 affected the 2-year survival rate (p=0.024; p=0.05) (see Tables 2 and 3).
 |
Table 2 The Comparison Among Associating Factors of BC 2-Year Survival Rate |
 |
Table 3 Survival Time Based on the BC Subtypes |
Moreover, there was a significant difference between the loco-regional and advanced stage groups (X2 (1) = 15,491 Log rank; p < 0.001) identified in the curve of survival function, as well as between high (≥4.4) and low miRNA (<4.4) groups (X2 (1) = 9571 Log rank; p = 0.002) (see Figure 2).
 |
Figure 2 (A) Two-year survival rate based on cancer stage. (B) Two-year survival rate based on miRNA-21. |
A significant difference was also identified between high and low CA15-3 (X2 (1) = 11.285 Log rank; p = 0.001). Conversely, the curve of survival function did not show any significant difference between low and high CEA (X2 (1) = 0.440 Log rank; p = 0.507) (see Figure 3). The curve also exhibited a significant difference among ER, PR, and HER2. (X2 (1) = 10.509 Log rank; p = 0.015) (see Figure 4.)
 |
Figure 3 (A) Two-year survival rate based on CA15-3. (B) Two-year survival rate based on CEA. |
 |
Figure 4 Two-year survival rate based on ER, PR, and HER2 status. |
Cox-proportional hazard showed that miRNA-21 (Adjusted HR 1.67; 95% CI = 1.179–1.632), cancer stage (Adjusted HR 12.07; 95% CI = 2.38–61.21), and CEA (Adjusted HR 1.033; 95% CI = 0.981–1.088) were correlated with 2-year mortality, whereas the other CA15-3 (Adjusted HR 1.81; 95% CI = 1.171–19.112) did not show any significant association (see Table 4).
 |
Table 4 Cox Proportional Hazard |
Discussion
MiRNA, a prognosis factor for the severity and survival of BC patients, is considerably associated with severity and mortality when detected in a higher level.2,4,8,19 There are many types of miRNAs detected in BC, one of which is miRNA-21 that mainly depicts the progression of BC.20,21 A meta-analysis study of Wang et al stated that miRNA-21 was correlated with cancer proliferation and metastasis.21 Furthermore, other studies suggested that increased miRNA-21 was related to poor survival of BC patients.9,22,23 Subgroup studies in a meta-analysis by Pan et al stated that miRNA-21 was strongly associated with BC patients from Asia.9 In our previous study, the increase in miRNA-21 values was not associated with BC mortality in the earlier stages (r= 0.25; p=0.218), otherwise, we found that the increase in miRNA-21 had a correlation with mortality in the later stage (r=0.866; p<0.05).16 However, our study revealed the different values of miRNA-21 in the early and advanced stages. Higher 2-year survival rate was identified in patients with advanced BC and high miRNA-21.
Estrogen receptor (ER), progesterone receptor (PR), and Her2 are markedly associated with the prognosis and treatment of BC. A retrospective-7-year survival study concluded that the triple-negative subtype (ER/PR-, Her2-) had the worst progression and disease-free survival. Like triple-negative subtype, ER/PR-, Her2+ subtype also showed low disease-free survival.24 A cohort study demonstrated that PR, dependent on the tumor proliferation, functioned as an important prognostic value in all BC lesions with ER+/Her2-.25 Meanwhile, PR status independently could be a prognostic indicator within 5 years after the diagnosis of BC is established.26 Positive ER status was not independently associated with BC survival rate after adjustment to other influencing factors, but quantitative ER examination might have prognostic value.27
Our present study concluded that patients with negative PR had a higher mortality rate within 2 years. Meanwhile, analysis of the ER status showed no significant difference. Our survival analysis of ER/PR Her2 showed similar results to previous studies in which ER/PR-Her2- subtype had a low 2-year survival rate. Moreover, there was a significant difference in mortality within 2 years in the advanced stage and metastases.
Other related tumor markers such as CEA and CA15-3 have been widely deliberated regarding prognosis and survival. The study of Gaglia et al in the late 1980s showed that CEA, as a single prognostic factor, was capable of predicting outcome and the need for adjuvant therapy.28 Numerous studies have also looked at the relationship among existing prognostic factors. Ebeling et al investigated CEA and CA15-3 as BC prognostic tools. Lower post-operative CEA was associated with disease-free survival and mortality in BC patients, while an increase in CA15-3 was consistent with relapse of BC.29 The progression of BC was also in line with elevated CEA and CA15-3.30 According to previous studies on preoperative, treatment outcome, and survival, elevated CEA and CA15-3 values were highly associated with poor treatment outcomes and mortality.10–12,14,15,31
In addition, our present study also declared that patients who died within 2 years had a higher mean baseline CEA and CA15-3 and lower mean survival. Comparing patients with high to low CA15-3 resulted in significantly different 2-year survival rates. In contrast, no significant difference was identified when comparing CEA. Nonetheless, this is not relevant enough since we only observed the initial CEA status; hence, we ought to examine post-treatment CEA to see how it is related to outcome and prognosis.
Following our multivariate analysis, it appears that miRNA-21 was significantly associated with BC stage but not with CEA and CA15-3. The study of Pan et al disclosed that miRNA-21 was strongly correlated with the expression of ER, PR, Her2, lymph node metastases, histological grade, and Her2/neu. Each of these parameters was associated with BC severity. The study also declared a strong association among miRNA-21, lymph node metastases, and histologic grading used to determine BC stage.9
Based on Cox proportional hazard test, the unadjusted model showed that increasing miRNA-21, cancer stage, CA15-3, and CEA were still associated with a low 2-year survival rate in BC patients. Only miRNA-21, cancer stage, and CA15-3 were significantly associated in the adjusted model. A cohort study in China showed that elevated CEA and CA 15-3 were closely in line with decreased breast cancer-specific survival (BCSS) and disease-free survival (DFS).10 In BC patients with low CEA levels, levels of CA15-3 can completely predict overall prognosis.32. However, another study observing CEA and CA15-3 proclaimed no significant difference in the clinicopathological features of BC, while the observation of elevated miRNA-21 showed otherwise.8
To the best of our knowledge, studies explaining miRNA-21 in BC patients and associating factors have not been extensively discussed and published in Indonesia, although they started to be recognized worldwide. Our study provided an overview of BC patients with similar clinical features to patients presented in other studies, especially around Asia. Our study has several limitations. Regardless of petite sample size, we managed to examine other factors associated with miRNA −21, such as comorbidities, hormonal status, menarche status, and other tumor markers. Furthermore, CEA and CA15-3 were not evaluated periodically after therapy, so that the correlation between these tumor markers and miRNA-21 could not be obtained. It is necessary to examine in a more extended period of time; eg, 3–5 years, and regularly evaluate other markers such as CEA, CA15-3, CA125 to understand the association of miRNA-21 with the survival of BC patients.
Conclusion
Significant differences among cancer stage, DM, and PR were observed while predicting the 2-year mortality rate in BC patients. The low 2-year survival rate was determined by miRNA-21, cancer stage, CA15-3, and ER/PR-Her2-. MiRNA-21, a prognostic marker, is significantly related to cancer stage and several other factors, such as BC’s clinical picture and pathology. No correlation was found among other three markers. More comprehensive studies with larger sample sizes and longer survival time are essentially needed to comprehend the association between miRNA-21 and other existing factors.
Data Sharing Statement
The dataset is available on 10.6084/m9.figshare.19583815.
Acknowledgment
We would like to thank Universitas Airlangga Hospital for supporting this research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
All of the authors declare no conflicts of interest in relation to this work.
References
1. Yang Z, Liu Z. The emerging role of MicroRNAs in breast cancer. J Oncol. 2020;2020:9160905. doi:10.1155/2020/9160905
2. Isca C, Piacentini F, Mastrolia I, et al. Circulating and intracellular miRNAs as prognostic and predictive factors in HER2-positive early breast cancer treated with neoadjuvant chemotherapy: a review of the literature. Cancers. 2021;13(19):4894. doi:10.3390/cancers13194894
3. Zografos E, Zagouri F, Kalapanida D, Zakopoulou R. Prognostic role of microRNAs in breast cancer: a systematic review. Oncotarget. 2019;10(67):7156–7178. doi:10.18632/oncotarget.27327
4. Sathipati SY, Ho S-Y, Araujo MTF. Identifying a miRNA signature for predicting the stage of breast cancer. Sci Rep. 2018;8(1):1–11. doi:10.1038/s41598-018-34604-3
5. Nam SE, Lim W, Jeong J, et al. The prognostic significance of preoperative tumor marker (CEA, CA15-3) elevation in breast cancer patients: data from the Korean Breast Cancer Society Registry. Breast Cancer Res Treat. 2019;177(3):669–678. doi:10.1007/s10549-019-05357-y
6. Schnitt SJ. Classification and prognosis of invasive breast cancer: from morphology to molecular taxonomy. Mod Pathol. 2010;23(Suppl 2):S60–S64. doi:10.1038/modpathol.2010.33
7. Taneja P, Maglic D, Kai F, Zhu S, Kendig RD, Elizabeth A. Clinical medicine insights: oncology classical and novel prognostic markers for breast cancer and their clinical significance. Clin Med Insights. 2010;4:15–34. doi:10.4137/cmo.s4773
8. Swellam M, Ramadan A, El-Hussieny EA, et al. Clinical significance of blood-based miRNAs as diagnostic and prognostic nucleic acid markers in breast cancer: comparative to conventional tumor markers. J Cell Biochem. 2019;120(8):12321–12330. doi:10.1002/jcb.28496
9. Pan F, Mao H, Deng L, Li G, Geng P. Prognostic and clinicopathological significance of microRNA-21 overexpression in breast cancer: a meta-analysis. Int J Clin Exp Pathol. 2014;7(9):5622–5633.
10. Li J, Liu L, Feng Z, et al. Tumor markers CA15-3, CA125, CEA and breast cancer survival by molecular subtype: a cohort study. Breast Cancer. 2020;27(4):621–630. doi:10.1007/s12282-020-01058-3
11. Li X, Dai D, Chen B, Tang H, Xie X, Wei W. Determination of the prognostic value of preoperative CA15-3 and CEA in predicting the prognosis of young patients with breast cancer. Oncol Lett. 2018;16(4):4679–4688. doi:10.3892/ol.2018.9160
12. Yang Y, Zhang H, Zhang M, Meng Q, Cai L, Zhang Q. Elevation of serum CEA and CA15-3 levels during antitumor therapy predicts poor therapeutic response in advanced breast cancer patients. Oncol Lett. 2017;14(6):7549–7556. doi:10.3892/ol.2017.7164
13. Shao Y, Sun X, He Y, Liu C, Liu H, Batra SK. Elevated levels of serum tumor markers CEA and CA15-3 are prognostic parameters for different molecular subtypes of breast cancer. PLoS One. 2015;10(7):e0133830. doi:10.1371/journal.pone.0133830
14. Wu SG, He ZY, Zhou J, et al. Serum levels of CEA and CA15-3 in different molecular subtypes and prognostic value in Chinese breast cancer. Breast. 2014;23(1):88–93. doi:10.1016/j.breast.2013.11.003
15. Lee JS, Park S, Park JM, Cho JH, Kim SI, Park BW. Elevated levels of preoperative CA 15-3 and CEA serum levels have independently poor prognostic significance in breast cancer. Ann Oncol. 2013;24(5):1225–1231. doi:10.1093/annonc/mds604
16. Romadhon PZ, Prayoga AA, Bintoro SUY, et al. The association between plasma miRNA-21 levels with overall 1-year Survival rate of breast cancer patients at various stages. Acta Med Indones. 2021;53(4):432–441.
17. Diansyah MN, Prayogo AA, Sedana MP, et al. Early detection breast cancer: role of circulating plasma miRNA-21 expression as a potential screening biomarker. Turk j med sci. 2021;51(2):562–569. doi:10.3906/sag-2005-138
18. Savitri M, Bintoro U, Sedana M, et al. Circulating plasma miRNA-21 as a superior biomarker compared to CA 15-3: assessment in healthy age matched subjects and different stage of breast cancer patients. Indonesian Biomed J. 2020;12(2):157–164. doi:10.18585/inabj.v12i2.1142
19. Liu X, Cao M, Palomares M, et al. Metastatic breast cancer cells overexpress and secrete miR-218 to regulate type I collagen deposition by osteoblasts. Breast Cancer Res. 2018;20(1):127. doi:10.1186/s13058-018-1059-y
20. Binabaj MM, Bahrami A, Khazaei M, et al. The prognostic value of small noncoding microRNA-21 expression in the survival of cancer patients: a meta-analysis. Crit Rev Eukaryot Gene Expr. 2020;30(3):207–221. doi:10.1615/CritRevEukaryotGeneExpr.2020028719
21. Wang Y, Zhang Y, Pan C, Ma F, Zhang S, Seagroves T. Prediction of poor prognosis in breast cancer patients based on microRNA-21 expression: a meta-analysis. PLoS One. 2015;10(2):e0118647. doi:10.1371/journal.pone.0118647
22. Jinling W, Sijing S, Jie Z, Guinian W. Prognostic value of circulating microRNA-21 for breast cancer: a systematic review and meta-analysis. Artif Cells Nanomed Biotechnol. 2017;45(6):1–6. doi:10.1080/21691401.2016.1216856
23. Tang Y, Zhou X, Ji J, et al. High expression levels of miR-21 and miR-210 predict unfavorable survival in breast cancer: a systemic review and meta-analysis. Int J Biol Markers. 2015;30(4):e347–e358. doi:10.5301/jbm.5000160
24. Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7(1–2):4–13. doi:10.3121/cmr.2009.825
25. Van Asten K, Slembrouck L, Olbrecht S, et al. Prognostic value of the progesterone receptor by subtype in patients with estrogen receptor-positive, HER-2 negative breast cancer. Oncologist. 2019;24(2):165–171. doi:10.1634/theoncologist.2018-0176
26. Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836–1846. doi:10.1056/NEJMoa1701830
27. Hill DA, Barry M, Wiggins C, et al. Estrogen receptor quantitative measures and breast cancer survival. Breast Cancer Res Treat. 2017;166(3):855–864. doi:10.1007/s10549-017-4439-6
28. Gaglia P, Caldarola B, Bussone R, et al. Prognostic value of CEA and ferritin assay in breast cancer: a multivariate analysis. Eur J Cancer Clin Oncol. 1988;24(7):1151–1155. doi:10.1016/0277-5379(88)90121-6
29. Ebeling FG, Stieber P, Untch M, et al. Serum CEA and CA 15-3 as prognostic factors in primary breast cancer. Br J Cancer. 2002;86(8):1217–1222. doi:10.1038/sj.bjc.6600248
30. Laessig D, Nagel D, Heinemann V, et al. Importance of CEA and CA 15-3 during disease progression in metastatic breast cancer patients. Anticancer Res. 2007;27(4a):1963–1968.
31. Park BW, Oh JW, Kim JH, et al. Preoperative CA 15-3 and CEA serum levels as predictor for breast cancer outcomes. Ann Oncol. 2008;19(4):675–681. doi:10.1093/annonc/mdm538
32. Li X, Dai D, Chen B, et al. Prognostic values of preoperative serum CEA and CA125 levels and nomograms for young breast cancer patients. Onco Targets Ther. 2019;12:8789–8800. doi:10.2147/ott.s221335
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
