Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
Prognostic Value of Vimentin in Triple Negative Breast Cancer Patients Depends on Chemotherapy Regimen and p53 Mutant Expression
Authors Purwanto I , Leo B , Hutajulu SH , Kurnianda J, Taroeno-Hariadi KW , Hardianti MS, Satiti AD, Dwianingsih EK, Heriyanto DS, Widodo I, Aryandono T
Received 25 April 2023
Accepted for publication 19 July 2023
Published 27 July 2023 Volume 2023:15 Pages 515—524
DOI https://doi.org/10.2147/BCTT.S418696
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Ibnu Purwanto,1,* Benedreky Leo,2,* Susanna Hilda Hutajulu,1 Johan Kurnianda,1 Kartika Widayati Taroeno-Hariadi,1 Mardiah Suci Hardianti,1 Amanda Dania Satiti,1 Ery Kus Dwianingsih,3 Didik Setyo Heriyanto,3 Irianiwati Widodo,3 Teguh Aryandono4
1Division of Hematology and Medical Oncology, Department of Internal Medicine, Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University/Dr Sardjito Hospital, Yogyakarta, Indonesia; 2Department of Internal Medicine, Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University/Dr Sardjito Hospital, Yogyakarta, Indonesia; 3Department of Anatomical Pathology, Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University/Dr Sardjito Hospital, Yogyakarta, Indonesia; 4Division of Surgical Oncology, Department of Surgery, Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University/Dr Sardjito Hospital, Yogyakarta, Indonesia
*These authors contributed equally to this work
Correspondence: Ibnu Purwanto, Jalan Farmako, Sekip Utara, Yogyakarta, 55281, Indonesia, Tel/Fax +62 274 560300, Email [email protected]
Purpose: To determine the prognostic value of vimentin in triple negative breast cancer (TNBC) patients, specifically in relation to chemotherapy regimen and p53 mutant expression.
Patient and Methods: We retrospectively analyzed the association of pre-treatment tumor expression of vimentin with 48-month overall survival (OS) of 72 all stages TNBC patients diagnosed between 2014 and 2018 in relation to chemotherapy regimen and expression of p53 mutant. Vimentin and p53 mutant expressions were examined using immunohistochemistry. Analysis was conducted on all patients collectively, then repeated on two cohorts divided according to the chemotherapy regimen. Sub-analysis was performed to determine the effect of p53 mutant expression on the prognostic value of vimentin.
Results: Vimentin was expressed in 43.1% of patients and was not associated with clinicopathologic characteristics. Vimentin was associated with improved 48-month OS in all patients in univariate analysis but not significant in multivariate analysis. When analyzed according to chemotherapy regimen, vimentin was independently associated with improved 48-month OS in patients receiving non-platinum-based chemotherapy (80% vs 15.8%; HR: 0.17, 95% CI: 0.05– 0.58, p: 0.005). Other independent prognostic factors include T (HR: 6.18, 95% CI: 1.38– 27.7, p: 0.017) and M (HR: 5.64, 95% CI: 1.2– 26.33, p: 0.028). On subanalysis, vimentin was significantly associated with improved 48-month OS in patients expressing p53 mutant (69.2% vs 22.2%, p: 0.006) but was not significant in patients not expressing p53 mutant.
Conclusion: Vimentin expression was independently associated with improved 48-month OS in TNBC patients treated with non–platinum–based chemotherapy. Expression of p53 mutant significantly affected the prognostic value of vimentin.
Keywords: vimentin, p53 mutant, chemotherapy, platinum resistance, TNBC, prognostic factor
Introduction
Previously thought of as a singular disease, Perou et al, later expanded by Lehmann et al (Vanderbilt) and Burstein et al (Baylor), have elegantly demonstrated the genetic heterogeneity of triple negative breast cancer (TNBC), leading to the categorization of TNBC into subtypes of different characteristics and response towards systemic chemotherapy.1–3 Both Vanderbilt and Baylor classifications share overlapping subtypes, namely basal-like, mesenchymal and luminal androgen.3 Basal-like subtype is highly sensitive towards platinum-based chemotherapy due to its mechanism of action targeting homologous recombination deficiency and BRCA1/2 mutation, while treatment targeting androgen receptor in luminal androgen subtype have shown promising results.4–8 Mesenchymal subtype has been observed to have worse prognosis and poor response towards chemotherapy, with no identified potential targetable molecule.9 Very little is currently understood regarding the best treatment modality for mesenchymal TNBC.
Vimentin, an intermediate filament protein of mesenchymal cancer cell, is associated with more aggressive breast cancer, including in TNBC.10,11 Vimentin is involved in epithelial-to-mesenchymal transition (EMT) in TNBC, a process associated with disease progression and metastasis, two of the main causes of mortality in TNBC.12 Previous studies have shown conflicting results on the prognostic role of vimentin in TNBC, which among other variables, might be caused by implementation of different chemotherapy regimens, which was not analyzed in these studies.10,13–15 Furthermore, the role of vimentin in relation to EMT process might be significantly influenced by p53 loss of function (p53 mutant).16,17 This study aims to determine the prognostic value of vimentin in TNBC patients, specifically in relation to platinum-based and non-platinum-based chemotherapy, as well as p53 mutant expression.
Materials and Methods
Patient Populations
This was a retrospective observational study conducted at Dr. Sardjito Hospital, Yogyakarta, Indonesia, involving stage I–IV TNBC patients diagnosed between 2014 and 2018. We consecutively included all patients 18 years old or older, who received systemic chemotherapy, with Eastern Cooperative Oncology Group (ECOG) performance index of 0–1. We excluded patients with unretrievable formalin-fixed paraffin-embedded (FFPE) tissue for immunohistochemistry (IHC) and patients lost to follow up. Patient clinical data was extracted from medical record, while follow up status was prospectively recorded. This study has been approved by the IRB Ethics Committee Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University/Dr. Sardjito Hospital, Yogyakarta, Indonesia with approval numbers KE/0286/03/2020 and KE/FK/0789/EC/2022.
Pathology Assessment
Tumor samples were obtained FFPE tissue stored at room temperature, protected from light, at the Department of Anatomical Pathology, Dr. Sardjito General Hospital, Yogyakarta, Indonesia.
Immunohistochemistry
Block paraffin samples were cut 3 µm in thickness to analyze the expression of vimentin and p53 mutant by IHC. Anti-vimentin monoclonal antibody (PRM 312 AA, dilution 1:50, Biocare Medical) was used to detect tumor expression of vimentin. Anti-p53 mutant antibody (ab32049, dilution 1:1000, Abcam) was used to detect tumor expression of p53 mutant. Vimentin and p53 mutant were considered positive if their expressions were detected in more than 10% of the tumor cells. Immunohistochemistry examination was performed using ImageJ software by a senior pathologist, blinded to the patient’s survival status and clinical data, including which chemotherapy regimen was received.
Statistical Analysis
The association between vimentin and clinicopathologic characteristic was analyzed using chi-square test or Fisher’s exact test as appropriate. Survival analysis was performed using Kaplan–Meier curve to determine the 48-month overall survival (OS). Univariate analysis of potential prognostic factors was conducted using Log rank test. Variables significant in univariate analysis were included in multivariate analysis using Cox proportional hazard. Analysis was conducted on all patients collectively, then repeated on two cohorts divided according to the chemotherapy regimen. Subanalysis was performed to determine the effect of p53 mutant expression on the prognostic value of vimentin. Significant p-value was set at <0.05. All statistical analyses were performed using IBM SPSS version 24 software.
Results
We consecutively detected 287 patients, 173 of which were excluded due to unretrievable FFPE tissue for IHC, 42 of which were excluded due to lost to follow up, leaving 72 patients eligible for analysis. The average age of diagnosis was 50.1 ± 11 years old, with a median of 49.5 (31–82) years. Most patients were diagnosed with advanced stage, 52 patients (72.2%) had T3-4 tumor, 51 patients (70.8%) had lymph node metastasis. Distant metastasis was detected in 10 patients (13.9%). Thirty-eight patients (52.8%) received platinum–based chemotherapy, while the remaining 34 patients (47.2%) received non–platinum–based chemotherapy. Vimentin was expressed in 31 patients (43.1%), and p53 mutant was detected in 31 patients (43.1%) (Table 1). There was no significant baseline patient characteristic difference according to vimentin expression (Table 2). At the end of the 48-month follow-up, 33 patients (45.8%) were still alive (48-month OS), with a median OS of 31 months.
 |
Table 1 Patient Baseline Characteristic |
 |
Table 2 Patient Baseline Characteristic According to Vimentin Expression |
Expression of vimentin was associated with significantly improved 48-month OS, both in all patients (61.3% vs 34.1%, mean OS: 37.4 vs 29.5 months, p: 0.02), as well as in patients receiving non-platinum-based chemotherapy (80% vs 15.8%, mean OS: 42.3 vs 24.9 months, p: 0.000) (Figure 1). Larger tumor size was associated with worse 48-month OS, both in all patients (36.5% vs 70%, mean OS: 29.6 vs 41.3 months, p: 0.009), as well as in patients receiving non-platinum-based chemotherapy (30.4% vs 72.7%, mean OS: 27.7 vs 42.7 months, p: 0.018) (Figure 2). Similarly, distant metastasis was associated with worse 48-month OS, both in all patients (20% vs 50%, mean OS: 24.7 vs 34.2 months, p: 0.034), as well as in patients receiving non-platinum-based chemotherapy (0% vs 48.4%, mean OS: 22 vs 33.6 months, p: 0.04) (Figure 3). No variable was significantly associated with 48-month OS in patients receiving platinum-based chemotherapy (Table 3). When analyzed according to vimentin expression, platinum-based chemotherapy was associated with worse 48-month OS in patients expressing vimentin (80% vs 43.8%, mean OS: 32.7 vs 42.3 months, p: 0.049). On the contrary, platinum-based chemotherapy was associated with improved 48-month OS in patients not expressing vimentin (50% vs 15.8, mean OS: 33.5 vs 24.9 months, p: 0.032) (Figure 4).
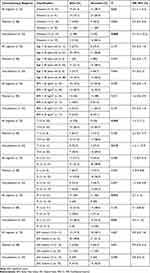 |
Table 3 Log Rank Test for 48-Month OS According to Chemotherapy Regimen |
 |
Figure 4 Kaplan – Meier curve showing 48-month OS according to chemotherapy regimen in all patients (left), in patients expressing vimentin (center), and in patients not expressing vimentin (right). |
On multivariate analysis, independent prognostic factors in all patients were T (HR: 3.24, 95% CI: 1.34–7.81, p: 0.009) and M (HR: 2.3, 95% CI: 1.03–5.12, p: 0.042) (Table 4). Whereas in patients receiving non-platinum-based chemotherapy were T (HR: 6.18, 95% CI: 1.38–27.7, p: 0.017), M (HR: 5.64, 95% CI: 1.2–26.33, p: 0.028) and vimentin (HR: 0.17, 95% CI: 0.05–0.58, p: 0.005) (Table 5). On sub-analysis, vimentin expression was associated with significantly improved 48-month OS in p53 mutant expressing patients (69.2% vs 22.2%, mean OS: 40.9 months vs 25.1 months, p: 0.006), whereas vimentin was non-prognostic in p53 non-expressing patients (55.6% vs 43.5%, mean OS: 34.8 months vs 32.9 months, p: 0.538) (Figure 5 and Table 6).
 |
Table 4 Cox Proportional Hazard Result of All Patients |
 |
Table 5 Cox Proportional Hazard Result of Patients Receiving Non-Platinum-Based Chemotherapy |
 |
Table 6 Log Rank Test for 48-Month OS According to p53 Mutant Expression |
 |
Figure 5 Kaplan – Meier curve showing 48-month OS according to vimentin regimen in all patients (left), in patients expressing p53 mutant (center), and in patients not expressing p53 mutant (right). |
Discussion
Our study has shown a significant difference in vimentin’s prognostic value in relation to chemotherapy regimen, with significantly improved prognosis in patients receiving non-platinum-based chemotherapy and non-statistically significant worse prognosis in patients receiving platinum-based chemotherapy (Figure 1). The positive prognostic value of vimentin in our study is similar to the study form Dine et al, which reported improved prognosis in patients receiving neoadjuvant chemotherapy (NAC).13 On the contrary, Yamashita et al reported a negative prognostic value of vimentin, and non-prognostic results were reported by Schmidt et al and Kusinska et al10,14,15 it was unclear whether the patients received systemic chemotherapy in studies conducted by Yamashita et al. Kusinska et al, whereas Schmidt et al did not specify the type of chemotherapy given to their patients, which might have contributed to the inconsistent results.
We demonstrated a dichotomous response of platinum-based chemotherapy, with worse prognosis observed in vimentin-expressing patients and improved prognosis in patients not expressing vimentin, suggesting platinum resistance in vimentin-expressing tumor (Figure 4). Currently, there is no clinical data on the response towards platinum-based and non-platinum-based chemotherapy in mesenchymal TNBC, especially concerning vimentin expression. A preclinical study reported mesenchymal TNBC cell line had lower sensitivity towards cisplatin compared to the epithelial TNBC cell line.18,19 Genomic analysis using the GeneWeaver database observed cisplatin resistance associated with vimentin expression, which was hypothesized due to decreased import and increased export of cisplatin to tumor cells.20
Mutation of p53 is frequently found in TNBC, with reported frequency as high as 80% of all TNBC cases.21–23 The prognostic value of p53 mutant in breast cancer remains inconsistent, depending on the cancer subtype and treatment.24 The detrimental effect of p53 mutation is closely related to EMT, resulting in the acquisition of stemness characteristic of mesenchymal cells.17 Mutation of p53 did not result in significant prognostic difference (Table 3) but affected the prognostic significance of vimentin in our study. Vimentin was associated with improved prognosis in patients expressing p53 mutant, while non-prognostic in patients without p53 mutant expression (Figure 5). The result of our study suggested that p53 mutation might not directly affect patient prognosis but was dependent on the progression of EMT.
Similar to previous studies, increased tumor size and distant metastasis were independent poor prognostic factors in our patients.25–27 Patients in our study presented with significantly more advanced stage compared to previous studies, which translated into worse OS.25,28,29 Improvement in this scenario can only be made by earlier cancer detection through improving the implementation of breast cancer screening, which is still currently ineffective in Indonesia.30 Furthermore, NAC is still very rarely implemented in Indonesia, which in combination with high proportion of T3-4 patients in our cohort, might have contributed to suboptimal tumor resection and worse survival. Our study is the first to demonstrate the different prognostic values of vimentin according to p53 mutant expression and chemotherapy regimen in TNBC patients, which at least partially explains the inconsistent prognostic value of vimentin from previous studies. We also demonstrated the dichotomy of chemotherapeutic response according to vimentin expression, suggesting the potential role of vimentin as a marker for choosing the best chemotherapy regimen in TNBC patients. The main limitations of our study are the small sample size and its retrospective design. The REMARK Checklist has been completed by the authors for this case report (Table S1).
Conclusion
In summary, the expression of vimentin was independently associated with improved 48-month OS in TNBC patients treated with non-platinum-based chemotherapy. Expression of p53 mutant significantly affected the prognostic value of vimentin.
Data Sharing Statement
The dataset analysed during the current study is available in Table S2.
Ethics Approval and Informed Consent
Written informed consent was obtained from the patients involved, including permission to use clinical data and reexamination of tissue specimen. This study was conducted in accordance with the Declaration of Helsinki. Ethical clearance was approved by the IRB Ethics Committee Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University/Dr. Sardjito Hospital, Yogyakarta, Indonesia with approval numbers KE/0286/03/2020 and KE/FK/0789/EC/2022.
Acknowledgments
The authors express gratitude to Sardjito General Hospital for providing the necessary assistance during data procurement for this publication.
Funding
This research was funded by Dana Masyarakat, Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University, Yogyakarta, Indonesia.
Disclosure
Dr Ibnu Purwanto reports grants from Faculty of Medicine, Public Health, and Nursing, Gadjah Mada University/Dr Sardjito Hospital, Yogyakarta, Indonesia, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi:10.1038/35021093
2. Lehmann BD, Jovanovic B, Chen X, et al. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS One. 2016;11(6):e0157368. doi:10.1371/journal.pone.0157368
3. Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21(7):1688–1698. doi:10.1158/1078-0432.ccr-14-0432
4. Telli ML, Timms KM, Reid J, et al. Homologous Recombination Deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res. 2016;22(15):3764–3773. doi:10.1158/1078-0432.ccr-15-2477
5. Zhang L, Chen Y, Cheng MY, et al. Homologous recombination deficiency predicts the response to platinum-based neoadjuvant chemotherapy in early-stage triple-negative breast cancer patients: a systematic review and meta-analysis. Ther Adv Med Oncol. 2022;14:17588359221096253. doi:10.1177/17588359221096253
6. Traina TA, Miller K, Yardley DA, et al. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J Clin Oncol. 2018;36(9):884–890. doi:10.1200/jco.2016.71.3495
7. Gucalp A, Tolaney S, Isakoff SJ, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res. 2013;19(19):5505–5512. doi:10.1158/1078-0432.ccr-12-3327
8. Bonnefoi H, Grellety T, Tredan O, et al. A phase II trial of Abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12-1). Ann Oncol. 2016;27(5):812–818. doi:10.1093/annonc/mdw067
9. Echavarria I, Lopez-Tarruella S, Picornell A, et al. Pathological response in a triple-negative breast cancer cohort treated with neoadjuvant carboplatin and docetaxel according to lehmann’s refined classification. Clin Cancer Res. 2018;24(8):1845–1852. doi:10.1158/1078-0432.ccr-17-1912
10. Yamashita N, Tokunaga E, Kitao H, et al. Vimentin as a poor prognostic factor for triple-negative breast cancer. J Cancer Res Clin Oncol. 2013;139(5):739–746. doi:10.1007/s00432-013-1376-6
11. Khillare CD, Sinai Khandeparkar SG, Joshi AR, et al. Immunohistochemical expression of vimentin in invasive breast carcinoma and its correlation with clinicopathological parameters. Niger Med J. 2019;60(1):17–21. doi:10.4103/nmj.nmj_7_19
12. Usman S, Waseem NH, Nguyen TKN, et al. Vimentin is at the heart of epithelial mesenchymal transition (EMT) mediated metastasis. Cancers. 2021;13(19):4985. doi:10.3390/cancers13194985
13. Dine JL, O’Sullivan CC, Voeller D, et al. The TRAIL receptor agonist drozitumab targets basal B triple-negative breast cancer cells that express vimentin and Axl. Breast Cancer Res Treat. 2016;155(2):235–251. doi:10.1007/s10549-015-3673-z
14. Schmidt G, Solomayer EF, Bohle RM, et al. Is vimentin a potential prognostic factor for patients with triple-negative breast cancer? J Cancer Res Clin Oncol. 2020;146(8):2109–2116. doi:10.1007/s00432-020-03210-0
15. Kusinska RU, Kordek R, Pluciennik E, et al. Does vimentin help to delineate the so-called ‘basal type breast cancer’? J Exp Clin Cancer Res. 2009;28(1):118. doi:10.1186/1756-9966-28-118
16. Dhar G, Banerjee S, Dhar K, et al. Gain of oncogenic function of p53 mutants induces invasive phenotypes in human breast cancer cells by silencing CCN5/WISP-2. Cancer Res. 2008;68(12):4580–4587. doi:10.1158/0008-5472.can-08-0316
17. Coradini D, Fornili M, Ambrogi F, et al. TP53 mutation, epithelial-mesenchymal transition, and stem like features in breast cancer subtypes. J Biomed Biotechnol. 2012;2012:254085. doi:10.1155/2012/254085
18. Yaourtis AM, Levina A, Lay PA. Tumour cell heterogeneity in triple-negative breast cancer cells affects response to cisplatin, but not doxorubicin. J Inorg Biochem. 2022;239:112082. doi:10.1016/j.jinorgbio.2022.112082
19. Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–2767. doi:10.1172/jci45014
20. Hill DP, Harper A, Malcolm J, et al. Cisplatin-resistant triple-negative breast cancer subtypes: multiple mechanisms of resistance. BMC Cancer. 2019;19(1):1039. doi:10.1186/s12885-019-6278-9
21. Silwal-Pandit L, Vollan HK, Chin SF, et al. TP53 mutation spectrum in breast cancer is subtype specific and has distinct prognostic relevance. Clin Cancer Res. 2014;20(13):3569–3580. doi:10.1158/1078-0432.ccr-13-2943
22. Nik-Zainal S, Davies H, Staaf J, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534(7605):47–54. doi:10.1038/nature17676
23. Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi:10.1038/nature11412
24. Shahbandi A, Nguyen HD, Jackson JG. TP53 mutations and outcomes in breast cancer: reading beyond the headlines. Trends Cancer. 2020;6(2):98–110. doi:10.1016/j.trecan.2020.01.007
25. Pogoda K, Niwinska A, Murawska M, Pienkowski T. Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med Oncol. 2013;30(1):388. doi:10.1007/s12032-012-0388-4
26. Yuan N, Meng M, Liu C, et al. Clinical characteristics and prognostic analysis of triple-negative breast cancer patients. Mol Clin Oncol. 2014;2(2):245–251. doi:10.3892/mco.2013.230
27. Tseng LM, Hsu NC, Chen SC, et al. Distant metastasis in triple-negative breast cancer. Neoplasma. 2013;60(3):290–294. doi:10.4149/neo_2013_038
28. Ma KK, Chau WW, Wong CH, et al. Triple negative status is a poor prognostic indicator in Chinese women with breast cancer: a ten year review. Asian Pac J Cancer Prev. 2012;13(5):2109–2114. doi:10.7314/apjcp.2012.13.5.2109
29. Mousavi SA, Kasaeian A, Pourkasmaee M, et al. Assessing the prognostic factors, survival, and recurrence incidence of triple negative breast cancer patients, a single center study in Iran. PLoS One. 2019;14(1):e0208701.
30. Wahidin M, Febrianti R, Susanty F, Hasanah SR. Twelve years implementation of cervical and breast cancer screening program in Indonesia. Asian Pac J Cancer Prev. 2022;23(3):829–837. doi:10.31557/apjcp.2022.23.3.829
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



