Back to Journals » Cancer Management and Research » Volume 15
Prognostic Value of the Neutrophil-to-Lymphocyte Ratio in Patients Treated with Definitive Chemoradiotherapy for Locally Advanced Oesophageal Squamous Cell Carcinoma
Authors Zhang G, Yang C, Zhao C, Xian F , Qing D, Guo Q, Song J, Liu X, Bie J
Received 28 October 2022
Accepted for publication 17 January 2023
Published 30 January 2023 Volume 2023:15 Pages 101—112
DOI https://doi.org/10.2147/CMAR.S395191
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Guojun Zhang,1 Chuan Yang,2 Caixia Zhao,1 Feng Xian,3 Dong Qing,1 Qiyu Guo,1 Junmei Song,1 Xilin Liu,1 Jun Bie1
1Department of Oncology, Nanchong Central Hospital and The Second Clinical Medical College of North Sichuan Medical College, Nanchong, People’s Republic of China; 2Department of Oncology, Guangyuan Central Hospital, Guangyuan, People’s Republic of China; 3School of Medicine, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China
Correspondence: Jun Bie, Department of Oncology, Nanchong Central Hospital and The Second Clinical Medical College of North Sichuan Medical College, Hongguang Road, Shunqing District, Nanchong, People’s Republic of China, Email [email protected]
Background: The neutrophil-to-lymphocyte ratio (NLR) has been shown to have prognostic value in several common cancers. This study aimed to investigate the prognostic value of NLR in patients with advanced oesophageal squamous cell carcinoma (ESCC) after definitive chemoradiotherapy (dCRT).
Methods: A retrospective analysis was performed on 158 patients with advanced ESCC who received dCRT from January 2012 to December 2018. The NLR for different treatment stages was calculated based on laboratory test results. The Kaplan–Meier (KM) method and Cox proportional regression model were used to analyse the relationship between NLR and overall survival (OS).
Results: The mean NLR of 158 patients with ESCC was 3.403 ± 2.479. The pre-treatment NLR cut-off was 4.839, and patients were divided into the low NLR group (NLR < 4.839) and the high NLR group (NLR ≥ 4.839). NLR in patients with ESCC was related to N stage (P < 0.05). The KM analysis showed that the median OS of all enrolled patients was 29.3 months, the median OS periods of patients in the high and low NLR groups were 15.6 and 35.8 months, respectively, and the OS of the low NLR group was better than that of the high NLR group (P < 0.001). In the multivariate analysis, NLR was an independent prognostic factor that affects the prognosis of patients with ESCC receiving dCRT. Furthermore, patients who maintained a high NLR before and after treatment showed worse clinical outcomes than the other groups.
Conclusion: Our findings suggest that NLR can effectively assess the prognosis of patients with advanced ESCC undergoing dCRT.
Keywords: oesophageal squamous cell carcinoma, radiochemotherapy, neutrophil-to-lymphocyte ratio
Graphical Abstract:
Oesophageal cancer (EC) is a malignant tumour with high morbidity and mortality, with more than 570,000 new cases annually worldwide. Approximately 70% of EC in the world occurs in China, and oesophageal squamous cell carcinoma (ESCC) is the major histological type.1 The latest epidemiological investigation and research revealed 253,000 new cases of EC in China in 2016, and 194,000 patients died of EC.2 At present, the treatment of EC is mainly focused on surgical treatment. Because EC has no special symptoms in the early stage and has a high degree of malignancy, most cases are often diagnosed in the middle and advanced stages, thereby missing the opportunity for surgical treatment.3 Therefore, concurrent chemoradiotherapy was the standard treatment for patients who are unwilling or unable to undergo surgery in the middle and advanced stages.4 Although TNM stage is currently the main method of evaluating the prognosis of patients with EC and guiding clinical treatment plans, it cannot meet the needs of individualised treatment. Finding effective, cheap, convenient and accurate biomarkers for prognosis assessment in order to screen out high-risk groups and formulate individualised treatment plans is of clinical importance.
The neutrophil-to-lymphocyte ratio (NLR) is regarded as a biomarker that can determine the level of inflammatory response and immune function in patients with tumours.5 Inflammation is a typical reaction to host–tumour interaction in patients with tumours, and it plays an important role in the occurrence, development and metastasis of malignant tumours. Some studies have shown that the prognosis of patients with malignant tumours is closely related to inflammatory markers and immune function.6,7 In recent years, an increasing number of studies have shown that a high level of NLR before treatment is a risk factor for cancer and can be used as an effective indicator to evaluate the prognosis of patients with lung cancer, liver cancer, colorectal cancer, head and neck malignancies and other tumours.8–11 However, no uniform standard has been established for the study of pre-treatment NLR in patients with advanced ESCC receiving dCRT.
Thus, the objective of this study was to determine whether the level of NLR before treatment was associated with the outcome of patients with ESCC and to further explore the effect of NLR level on the prognosis of patients with ESCC during and after treatment. In addition, we investigated the relationship between NLR and patient clinical parameters as well as the associated adverse effects of treatment.
Methods
Study Population
This study was conducted following the principles of the Declaration of Helsinki and its amendments and was approved by the Medical Ethics Committee of Nanchong Central Hospital (Approval No. 2022–079). A retrospective analysis of 158 patients with ESCC undergoing dCRT from January 2012 to December 2018 was performed. The inclusion criteria were as follows: (1) Karnofsky performance status score (KPS) ≥ 70, (2) pathological diagnosis of ESCC, (3) unwilling or unresectable by surgery, (4) intensity-modulated radiation therapy dose ≥ 50 Gy, (5) no distant metastasis and (6) seventh edition of TNM staging of AJCC was used (2012).
Treatment
All patients received dCRT, and helical computed tomography (CT) was used for localisation scanning to delineate the tumour target area and surrounding normal organs. The primary tumour and regionally positive lymph nodes were defined as the gross tumour target volume (GTV and GTVn, respectively), and the GTV was determined by combining findings of localised CT, gastrointestinal barium meal, digestive endoscopy and positron emission tomography-CT, where GTVn was defined as short-diameter >1.0 cm metastatic enlarged lymph nodes; 0.5–1.0 cm in the anterior, posterior, left and right sides of the GTV; and 3 cm at the proximal and distal ends was defined as the clinical target volume (CTV). CTV expansion by 0.5 cm was defined as the planned target volume (PTV). Three-dimensional conformal radiotherapy or intensity-modulated radiotherapy was used for radiotherapy. The prescribed dose of the PTV was 50.0–72.6 Gy, with V20 ≤ 20% in both lungs, V5 < 50% in both lungs and V30 ≤ 30% in the heart, and the maximum dose in the spinal cord was ≤45.0 Gy. Moreover, patients received concurrent chemotherapy (1–6 cycles of platinum-containing combination chemotherapy or single-agent platinum-based chemotherapy, whereas some older patients were treated with teggio, and those who could not tolerate first-line chemotherapy were treated with raltitrexed-based chemotherapy).
NLR Calculation Method
The levels and counts of neutrophils and lymphocytes in patients with ESCC were recorded 1 week before, during and after treatment. NLR = neutrophils / lymphocytes.
Observation Indicators and Follow-Up
Follow-up was performed at regular intervals of 3 months in the first year, every 6 months in the next 2 years and every year thereafter. Routine examinations include physical examination, blood work, ultrasonography, tumour marker testing, chest CT and oesophageal barium meal. All patients were followed up through outpatient examinations and telephone calls until June 2021.
Statistical Analysis
IBM SPSS Statistics for Windows version 25.0 (IBM Corp., Armonk, NY, USA.) and Rstudio 4.2.1 software (USA) were used for statistical analysis and graphing. Continuous variables conforming to a normal distribution were compared using the t-test or analysis of variance, non-normal distribution data were compared using the Mann–Whitney U-test, and count data were compared using the χ2 test. We performed a multivariate regression analysis employing a stepwise selection method. An outcome-oriented method was used to determine the optimal cut-off point for continuous variables to maximise log-rank statistics.12 Survival analysis was performed using the KM method and Log rank test. The Cox risk model was used for univariate and multivariate analyses. The test level was α = 0.05, and p < 0.05 indicated a significant difference.
Results
Patient Characteristics and Treatment Outcomes
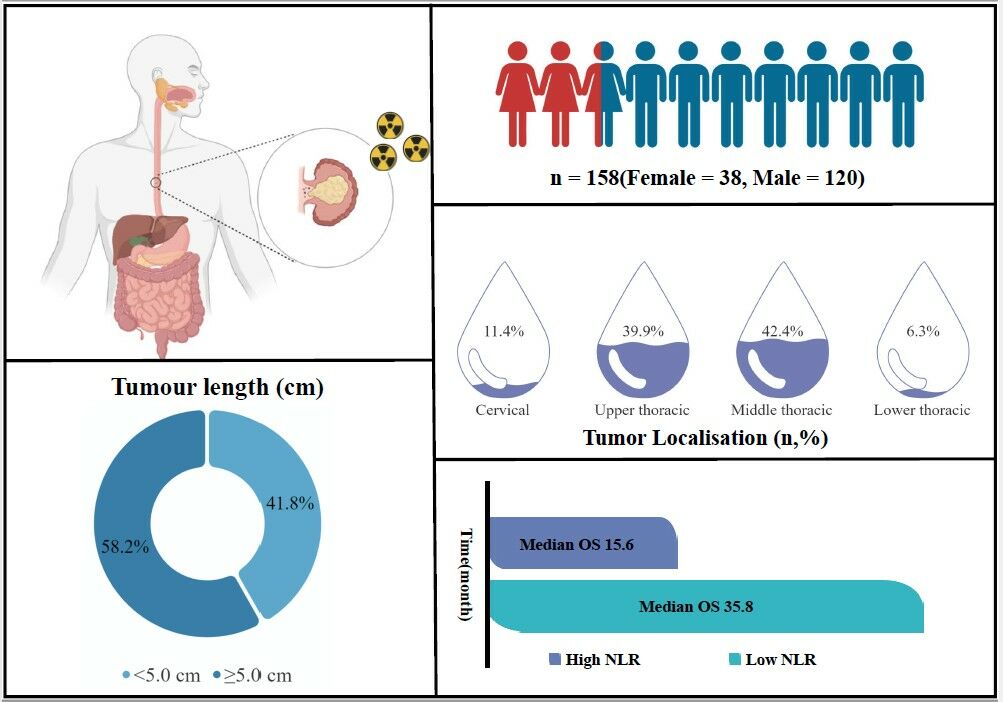
A total of 158 patients with ESCC were included in this study. The median age of the 158 patients was 63 (41–86) years, including 120 men (75.9%) and 38 women (24.1%). There were 84 (53.2%) and 74 (46.8%) with and without a smoking history, respectively. There were 88 (55.7%) and 70 (44.3%) patients with and without a drinking history, respectively. Moreover, 66 patients (41.8%) had tumour length <5 cm, and 92 (58.2%) had tumour length ≥5 cm. Six cases (3.8%) were in stage II, and 152 (96.2%) were in stage III. In terms of radiation dose, 48 (30.4%) patients received <60 Gy, and 110 (69.6%) patients received ≥60 Gy (Table 1).
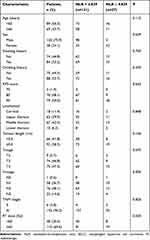 |
Table 1 Clinicopathological Features of 158 Patients with ESCC and Their Relationship with NLR |
Correlation Between Baseline NLR and Clinicopathologic Characteristics
The KM curve was used to take the best cut-off value of continuous variables, and the patients were divided into the low NLR group (NLR < 4.839) with 131 cases and the high NLR group (NLR ≥ 4.839) with 27 cases (Figure 1). The mean NLR of 158 patients with ESCC was 3.403 ± 2.479. NLR was associated with the N stage in patients with ESCC (P < 0.05) and was not associated with age, sex, smoking, drinking history, tumour location, tumour length, clinical stage and radiation dose (P > 0.05, Table 1).
 |
Figure 1 NLR before treatment using the Log rank test to calculate optimal stratification cutoffs for continuous covariates. |
Relationship Between NLR and Prognosis
The 1-, 2-, 3- and 5-year overall survival (OS) rates of the 158 patients were 74.1%, 55.0%, 45.6% and 37.0%, respectively. The 1-, 2-, 3- and 5-year OS rates were 51.9%, 29.6%, 21.2% and 16.9%, respectively, in the high NLR group. Moreover, the 1-, 2-, 3- and 5-year OS rates in the low NLR group were 78.6%, 60.2%, 49.7% and 41.2%, respectively. The KM analysis showed that the low NLR group had better OS than the high NLR group (P < 0.001, Figure 2).
 |
Figure 2 Survival curve and corresponding risk table of high NLR group and low NLR group before treatment. |
Univariate and Multivariate Analyses
The univariate analysis showed that NLR was associated with the prognosis of patients with advanced ESCC (P < 0.05, Table 2). In the multivariate analysis, NLR (hazard ratio 2.618; 95% confidence interval 1.615–4.243; P < 0.001) was an independent factor that affects the prognosis of patients with advanced ESCC (Table 2).
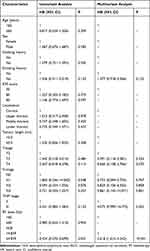 |
Table 2 Univariate and Multivariate Analysis of Prognostic Factors |
Relationship Between NLR and Survival Prognosis in Different Treatment Stages
We also observed changes in NLR values according to treatment stages. The pre-treatment NLR value was 3.403 ± 2.479, including 131 cases in low the NLR group (NLR < 4.839, 82.9%) and 27 cases in high NLR group (NLR ≥ 4.839, 17.1%). The NLR value during treatment was 7.516 ± 9.287, including 77 cases in the low NLR group (NLR < 4.839, 46.8%) and 84 cases in the high NLR group (NLR ≥ 4.839, 53.2%). The NLR value after treatment was 11.831 ± 11.119, which was analysed from 41 cases in the low NLR group (NLR < 4.839, 25.9%) and 117 cases in the high NLR group (NLR ≥ 4.839, 74.1%). A significant difference was found between the NLR values before, during and after treatment (p < 0.0001, Figure 3). According to the two prognostic models formed by different NLR levels before treatment, ie, NLR during treatment and NLR after treatment, in model 1 (different levels of NLR pre-treatment and mid-treatment), four groups were formed as follows: group A (pre-treatment high NLR + mid-treatment high NLR), group B (pre-treatment high NLR + mid-treatment low NLR), group C (pre-treatment low NLR + mid-treatment low NLR) and group D (pre-treatment low NLR + mid-treatment high NLR). There was a significant difference in OS between the groups (p < 0.001, Table 3 and Figure 4A). In model 2 (different levels of NLR pre-treatment and post-treatment), four groups were formed as follows: group E (pre-treatment high NLR + post-treatment high NLR), group F (pre-treatment high NLR + post-treatment low NLR), group G (pre-treatment low NLR + post-treatment low NLR) and group H (pre-treatment low NLR + post-treatment high NLR). There was a significant difference in OS between the groups (p = 0.005, Table 4 and Figure 4B).
 |
Table 3 Relationship Between Pre-Treatment NLR and Mid-Treatment NLR |
 |
Table 4 Relationship Between Pre-Treatment NLR and Post-Treatment NLR |
Toxic and Side Effects
Haematologic and non-haematologic-related adverse reactions observed during treatment are summarised. Overall, haematological grade 3 or 4 adverse reactions were mainly neutropenia, occurring in 50 (38.2%) and 5 (18.5%) patients in the low and high NLR groups, respectively (p = 0.074). Non-haematological grade 3 or 4 adverse reactions were mainly radiation esophagitis, which occurred in which 12 (9.9%) and 5 (18.5%) patients in the low and high NLR groups, respectively (p = 1.000). No significant differences were found in the treatment-related adverse events between the two groups (Table 5).
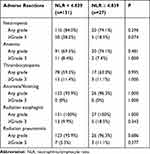 |
Table 5 Adverse Events During Definitive Chemoradiotherapy |
Discussion
Recently, multiple studies have demonstrated the role of pre-treatment NLR in the prognosis of solid malignancies. However, in this study, we demonstrated the significant predictive value of pre-treatment NLR in patients with intermediate-to-advanced ESCC undergoing dCRT. Our results showed that high NLR (<4.839 vs ≥4.839) was significantly associated with tumour lymph node metastasis. In addition, a high pre-treatment NLR was significantly associated with lower OS in patients with advanced ESCC on dCRT, the OS in the low NLR group was significantly better than that in the high NLR group (p < 0.001), and pre-treatment NLR was found to be an independent prognosis in the multivariate analysis, suggesting that pre-treatment NLR was an effective predictor of prognosis in patients with advanced ESCC undergoing dCRT.
In 1863, Rudolt et al13 first proposed the relationship between inflammation and cancer, and the internal environment of inflammatory response plays an important role in the occurrence, development, invasion and metastasis of tumours. NLR can reflect different inflammatory states of the body, and it has become one of the recognised effective indicators of systemic inflammatory response because of its simplicity and rapidity.14 In recent years, basic and clinical studies have confirmed this finding and revealed potential biological mechanisms related to the function at the level of neutrophils and lymphocytes before treatment, which was a phagocyte. In the case of an infection or inflammation, some neutrophils are chemotactic to the inflammation site and exert their function.15 When stimulated by pathogens, cytokines, etc., neutrophils form a network with DNA as the backbone and inlaid with various proteins to capture cancer cells and promote distant metastasis of cancer.16,17 Neutrophils have been confirmed to contain and secrete proangiogenic factors, which are directly involved in the formation of tumour-related blood vessels, tumour progression and metastasis.18 Lymphocytes play an important part of the immune response, and they play an important role in the immune surveillance that inhibits tumour occurrence and development. Moreover, lymphocytes can specifically recognise tumour surface antigens to activate anti-tumour immune responses.19,20 In addition, lymphopenia in patients with ESCC was strongly associated with a poor prognosis.21
At present, no unified conclusion was drawn between the cut-off value of NLR and ESCC. This study showed that the NLR of 158 patients with ESCC was 3.403 ± 2.479, and the cut-off value was 4.893, which was higher than that reported in related studies. Nakamura et al22 studied the relationship between NLR and the survival time of patients with ESCC after surgery, and the results showed that with NLR cut-off value of 2.420, this study could have included ESCC operable patients with an earlier tumour stage, a lesser tumour burden and a lesser inflammatory load compared with the present analysis, and patients were in better physical condition to cope with surgical treatment. In this study, dCRT was mainly performed on patients with advanced ESCC, who generally had poor physical status and had differences in the level of inflammatory response and immune function, which may be one of the reasons for the high NLR. At the same time, due to the individual differences in the study patients and the lack of uniform standards for the included covariates, the cutoff values will also be different.23 However, we found a trend similar to that in the present study, in which the prognosis was better in the group with a low NLR before treatment and worse in the group with a high NLR before treatment.
Our study shows that NLR was related to the prognosis of patients with advanced ESCC. The survival time in the high NLR group was significantly lower than that in the low NLR group, and the difference was significant (p < 0.001). The results of previous studies are consistent.24,25 This study shows that NLR levels are associated with lymph node metastasis in patients with advanced ESCC, which was consistent with the findings of Zhou et al24 This may be because cancer cell lines with strong lymphatic metastasis can upregulate the expression of interferon-inducible genes such as MHC-I and PD-L1, help tumour cells escape and promote the differentiation of regulatory T cells to form immunosuppressive environment, leading to tumour progression and metastasis.26 In addition, our study found that NLR level was not related to tumour T stage, and the difference was not significant (P > 0.05), which was inconsistent with related studies,22,27 which may be linked to our small sample size.
A study demonstrated the prognostic significance of NLR before and after treatment in patients with ESCC undergoing dCRT.24 The researchers observed that switching from high to low NLR was associated with improved survival. Our study showed a high NLR value before treatment over time, and the OS of patients with NLR ≥ 4.839 during treatment was shorter than that of patients with NLR < 4.839, which may reflect the treatment effect and help monitor the prognosis of ESCC. However, the trend of post-treatment NLR was the opposite, but not significant. The low pre-treatment NLR value goes on with the treatment time regardless of how the NLR level changes during and after treatment. These patients have better OS, indicating that low pre-treatment NLR was a beneficial factor for patients with advanced ESCC.
This study provides clinical evidence of NLR as a prognostic indicator in patients with advanced ESCC undergoing dCRT. However, this study has some limitations. First, this was a small sample retrospective study, which may be biased, and its accuracy and practicality should be verified in a prospective study. Second, the total number of patients was relatively small. Furthermore, there may be unknown factors affecting NLR that also influence our results.
Conclusion
In conclusion, this study shows that NLR at different treatment stages is an effective predictor of OS in patients with ESCC.
Ethical Conduct of Research
This study was approved by the Medical Ethics Committee of Nanchong Central Hospital and was conducted in accordance with the principles of the Helsinki Declaration and its amendments (Approval No. 2022-079). In addition, they have obtained written informed consent from the patients for the inclusion of their medical and treatment histories within this work. We confirm that the data has been anonymised, and we conducted a confidential analysis.
Acknowledgments
The authors would like to thank the patients, their families and all investigators involved in this study.
Author Contributions
All authors made a significant contribution to the work reported in terms of the conception, study design, execution, acquisition of data, analysis and interpretation; they took part in drafting, revising and reviewing the article; they gave final approval of the final manuscript to be published; they have agreed on the journal to which the article has been submitted; and they agreed to be accountable for all aspects of the work.
Funding
This work was supported by the Nanchong Science and Technology Bureau City-School Science and Technology Cooperation Project under award no. 22SXQT0340.
Disclosure
All authors declare that they have no competing interests for this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Center. 2022;2:1–9. doi:10.1016/j.jncc.2022.02.002
3. Short MW, Burgers KG, Fry VT. Esophageal Cancer. Am Fam Physician. 2017;95(1):22–28.
4. Lloyd S, Chang BW. Current strategies in chemoradiation for esophageal cancer. J Gastrointest Oncol. 2014;5(3):156–165. doi:10.3978/j.issn.2078-6891.2014.033
5. Tokumaru Y, Oshi M, Murthy V, et al. Low intratumoral genetic neutrophil-to-lymphocyte ratio (NLR) is associated with favorable tumor immune microenvironment and with survival in triple negative breast cancer (TNBC). Am J Cancer Res. 2021;11(11):5743–5755.
6. Jakubowska K, Koda M, Grudzińska M, et al. Monocyte-to-lymphocyte ratio as a prognostic factor in peripheral whole blood samples of colorectal cancer patients. World J Gastroenterol. 2020;26(31):4639–4655. doi:10.3748/wjg.v26.i31.4639
7. Yagi T, Baba Y, Ishimoto T, et al. PD-L1 expression, tumor-infiltrating lymphocytes, and clinical outcome in patients with surgically resected esophageal cancer. Ann Surg. 2019;269(3):471–478. doi:10.1097/SLA.0000000000002616
8. Scilla KA, Bentzen SM, Lam VK, et al. Neutrophil-lymphocyte ratio is a prognostic marker in patients with locally advanced (stage IIIA and IIIB) non-small cell lung cancer treated with combined modality therapy. Oncologist. 2017;22(6):737–742. doi:10.1634/theoncologist.2016-0443
9. Li S-H, Wang Q-X, Yang Z-Y, et al. Prognostic value of the neutrophil-to-lymphocyte ratio for hepatocellular carcinoma patients with portal/hepatic vein tumor thrombosis. World J Gastroenterol. 2017;23(17):3122–3132. doi:10.3748/wjg.v23.i17.3122
10. Ishikawa D, Nishi M, Takasu C, et al. The role of neutrophil-to-lymphocyte ratio on the effect of CRT for patients with rectal cancer. In Vivo. 2020;34(2):863–868. doi:10.21873/invivo.11850
11. Kumarasamy C, Tiwary V, Sunil K, et al. Prognostic utility of platelet-lymphocyte ratio, neutrophil-lymphocyte ratio and monocyte-lymphocyte ratio in head and neck cancers: a detailed PRISMA compliant systematic review and meta-analysis. Cancers. 2021;13(16):1. doi:10.3390/cancers13164166
12. Zhuang CL, Zhang FM, Li W, et al. Associations of low handgrip strength with cancer mortality: a multicentre observational study. J Cachexia Sarcopenia Muscle. 2020;11(6):1476–1486. doi:10.1002/jcsm.12614
13. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi:10.1016/S0140-6736(00)04046-0
14. Li B, Xiong F, Yi S, et al. Prognostic and clinicopathologic significance of neutrophil-to-lymphocyte ratio in esophageal cancer: an update meta-analysis. Technol Cancer Res Treat. 2022;21:15330338211070140. doi:10.1177/15330338211070140
15. Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189(6):2689–2695. doi:10.4049/jimmunol.1201719
16. Yang L, Liu Q, Zhang X, et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583(7814):133–138. doi:10.1038/s41586-020-2394-6
17. Milette S, Quail DF, Spicer JD. Neutrophil DNA webs untangled. Cancer Cell. 2020;38(2):164–166. doi:10.1016/j.ccell.2020.07.002
18. Ardi VC, Kupriyanova TA, Deryugina EI, et al. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci USA. 2007;104(51):20262–20267. doi:10.1073/pnas.0706438104
19. Liu D, Heij LR, Czigany Z, et al. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J Exp Clin Cancer Res. 2022;41(1):127. doi:10.1186/s13046-022-02340-2
20. Legut M, Sewell AK. Designer T-cells and T-cell receptors for customized cancer immunotherapies. Curr Opin Pharmacol. 2018;41:96–103. doi:10.1016/j.coph.2018.05.005
21. Okadome K, Baba Y, Yagi T, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. 2020;271:1. doi:10.1097/SLA.0000000000002985
22. Nakamura K, Yoshida N, Baba Y, et al. Elevated preoperative neutrophil-to-lymphocytes ratio predicts poor prognosis after esophagectomy in T1 esophageal cancer. Int J Clin Oncol. 2017;22(3):469–475. doi:10.1007/s10147-017-1090-5
23. Naszai M, Kurjan A, Maughan TS. The prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio (NLR) in colorectal cancer: a systematic review and meta-analysis. Cancer Med. 2021;10(17):5983–5997. doi:10.1002/cam4.4143
24. Zhou XL, Li YQ, Zhu WG, et al. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for patients with locally advanced esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Sci Rep. 2017;7:42581. doi:10.1038/srep42581
25. Kosumi K, Baba Y, Ishimoto T, et al. Neutrophil/lymphocyte ratio predicts the prognosis in esophageal squamous cell carcinoma patients. Surg Today. 2016;46(4):405–413. doi:10.1007/s00595-015-1197-0
26. Reticker-Flynn NE, Zhang W, Belk JA, et al. Lymph node colonization induces tumor-immune tolerance to promote distant metastasis. Cell. 2022;185(11):1924–1942.e1923. doi:10.1016/j.cell.2022.04.019
27. Hirahara N, Matsubara T, Kawahara D, et al. Prognostic significance of preoperative inflammatory response biomarkers in patients undergoing curative thoracoscopic esophagectomy for esophageal squamous cell carcinoma. Eur J Surg Oncol. 2017;43(2):493–501. doi:10.1016/j.ejso.2016.11.018
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


