Back to Journals » Cancer Management and Research » Volume 13
Prognostic Value of Preoperative Mean Platelet Volume and a Predictive Nomogram in Oral Squamous Cell Carcinoma Patients Based on Real-World Data
Authors Ning Y, Yang H, Qin S, Cao B, Zhong Z, He C, Zhu G
Received 8 June 2021
Accepted for publication 18 October 2021
Published 10 November 2021 Volume 2021:13 Pages 8495—8509
DOI https://doi.org/10.2147/CMAR.S323117
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Beicheng Sun
Yudong Ning,1 Hong Yang,2 Sheng Qin,2 Bangrong Cao,3 Zuxian Zhong,4 Chuanshi He,5 Guiquan Zhu6
1Department of Head and Neck Surgery, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China; 2Department of Pathology, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China; 3Radiation Oncology Key Laboratory of Sichuan Province, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China; 4Department of Head and Neck Radiotherapy, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China; 5Sichuan Key Laboratory of Radiation Oncology, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu, 610041, People’s Republic of China; 6Department of Head and Neck Oncology, West China Hospital of Stomatology, State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, Sichuan University, Chengdu, 610041, People’s Republic of China
Correspondence: Guiquan Zhu
Department of Head and Neck Oncology, West China Hospital of Stomatology, Sichuan University, No. 14, Section 3, Renmin South Road, Chengdu, Sichuan, 610041, People’s Republic of China
Email [email protected]
Objective: We aimed to identify new prognostic factors of oral squamous cell carcinoma (OSCC) among platelet-related parameters, establish a survival prediction model to predict the survival status of OSCC patients, and analyze the therapeutic effect of neoadjuvant chemotherapy on OSCC patients on the basis of real-world data.
Materials and Methods: The real-world data of patients with OSCC confirmed by pathologic examination at Cancer Hospital from January 2011 to January 2015 and May 2017 to January 2020 were collected. We analyzed clinicopathologic factors using a Cox regression analysis, the Kaplan-Meier method, and propensity score matching (PSM).
Results: The multivariate Cox regression analysis of not only validated the traditional prognostic factors such as tumor site, neural invasion, poor differentiation, and tumor-node-metastasis (TNM) stage but also identified a new prognostic factor, preoperative mean platelet volume (MPV) for overall survival (OS, HR, 0.47; 95% CI: 0.25– 0.89, P = 0.020). A nomogram was created to predict the probability of 3-year and 5-year OS. We found that neoadjuvant chemotherapy improved OS in patients with OSCC.
Conclusion: Preoperative MPV, being associated with female, neoadjuvant chemotherapy, and advanced stage (Stage III and IV), may be a new prognostic factor for OS of patients with OSCC. The nomograms provided useful prediction for OS in OSCC patients. Neoadjuvant chemotherapy may improve the OS of patients with OSCC.
Keywords: oral squamous cell carcinoma, overall survival, nomogram, mean platelet volume, neoadjuvant chemotherapy, induction chemotherapy
Introduction
Oral cancer is the most common form of head and neck cancer and has become a threat to people’s health, especially in developing countries.1,2 A primary surgical resection combined with postoperative radiotherapy (RT) is the main treatment of this locoregionally advanced cancer.3 In the treatment of oral cancer, chemotherapy is considered as an adjunct to surgery and RT. Postoperative concurrent chemoradiotherapy (CRT) in combination with cisplatin is the standard scheme for post-resection diseases characterized by a positive surgical margin and/or extranodal dilatation. Even with multidisciplinary therapy, the prognosis of oral cancer remains unsatisfactory; with minimal improvements observed over the past 3 decades.4 Although induction chemotherapy, also known as neoadjuvant chemotherapy, has been used in some cases in recent years and many clinical trials have been conducted, the principle except preserving the larynx remains controversial.5
The American Joint Committee on Cancer (AJCC) has published the 8th edition of the Oral Cancer Staging System.6 However, this staging system is considered as a static tool that focuses only on tumor-specific features involving tumor, lymph node, and metastasis (TNM).7 This has limited the guidance for individualized treatment of oral cancer patients in clinical practice because of the lack of consideration of individual factors; therefore, a more accurate prediction tool involving individual characteristics to assist in clinical decision-making for oral cancer patients is necessary. Since the increase of platelet counts (PLT) among cancer patients was first reported by foreign scholars in 1872,8 the changes in the platelets in the blood of patients with malignant tumors in different parts of the body have been studied. Platelets have the functions of sensing, monitoring and transmitting information, which are closely related to the development of human immunity and tumor. It has been reported that platelets are closely related to endocrine, respiratory, digestive and urinary tumors.9–14
While there have also been some studies on the link between platelets and oral cancer, most of them have reported that the platelet-lymphocyte ratio can be a prognostic factor for oral cancer.15–18 However, no studies have investigated whether platelet-related parameters can be independent prognostic factors for oral cancer.
Nonetheless, with the development of clinical research methodology and the emergence of big data in clinical treatment, real-world research has attracted increasing attention from the academic circle due to its unique advantages and this has influenced clinical practice and research. Real-world data needs to be obtained using a scientific research design, which is realized through a Real-World Study (RWS).19 There are differences between traditional clinical research and a RWS in terms of the study design, implementation, data source, statistical analysis, and clinical application of the research results. A RWS focuses more on evaluating the actual effects and clinical measures from the perspective of the patient, that is, evaluating the actual benefits of clinical measures to the patient. A RWS uses broad inclusion criteria and fewer exclusion criteria to reflect on the impact of specific interventions on clinical outcomes in a real medical environment, which is similar to the real medical environment, and the results are more relevant.
This study aimed to identify new prognostic factors of oral cancer among platelet-related parameters to establish a survival prediction model to predict the survival status of OSCC patients and analyze the therapeutic effect of neoadjuvant chemotherapy on OSCC patients based on the basis of real-world data.
Materials and Methods
Study Populations
The data of patients with OSCC confirmed by pathologic examination in Cancer Hospital from January 2011 to January 2015 and May 2017 to January 2020 were collected based on real-world data. All the cases were screened according to strict inclusion and exclusion criteria. The inclusion criteria were: Patients 1) with primary pathologically confirmed, 2) who underwent surgical treatment and neck dissections, and 3) with no distant metastases. The exclusion criteria were: patients 1) with unclear pathological stages (AJCC 8th edition stage) and 2) with incomplete information. A total of 187 patients who were diagnosed as OSCC in Sichuan Cancer Hospital from 2011 to 2020 were included in this study. Data on clinicopathologic factors were collected separately as follows: sex, age, smoking, drinking, other diseases (hypertension, inflammation, cancer), tumor sites, neoadjuvant chemotherapy, maxilla-mandible involvement, differentiation (high, middle, poor), vascular invasions, neural invasions, TNM stage (AJCC 8th edition), preoperative blood test indicators (PLT, mean platelet volume [MPV], platelet distribution width [PDW], platelet cubic measure distributing [PCT]) and postoperative radiotherapy or chemotherapy. The peripheral blood of all patients was collected within 1 week before surgery or neoadjuvant chemotherapy. After the initial surgery, all the participants were followed up by telephone every 6 months until death or the last follow-up, dated December 11, 2020. Table 1 shows the detailed derivation of the patients enrolled.
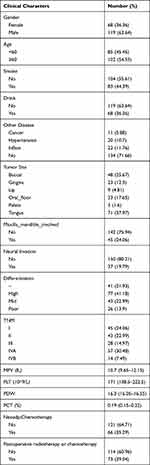 |
Table 1 Characteristics of the Cohort |
Patient Treatment
Induction chemotherapy, with mainly docetaxel, cisplatin, and 5-fluorouracil, was administered before surgery. Docetaxel and cisplatin were administered at 75 mg/m2 and 5-fluorouracil at 750 mg/m2. A course of treatment lasted for 21 days, with an interval of approximately 2 weeks. After two courses of induction chemotherapy, cancer resection was performed. An experienced surgeon was assigned to perform the standardized treatment, with emphasis on neck dissection and the radical resection of the primary lesion and properly combined with postoperative RT or chemotherapy. Postoperative defects were repaired with pedicled or free skin flaps, the safe margin of the radical resection was 1.5 cm, and an intraoperative frozen section ensured a safe margin of the operation.
Statistical Analysis
The main outcomes were composed of the predicted probability of 3-year or 5-year OS based on baseline characteristics. OS were defined as the time from the date of illness onset to death from any cause and death from oral cancer. Baseline prognostic factors of interest included sex, age, smoking, drinking, other diseases (hypertension, inflammation, malignancies other than OSCC), tumor sites, neoadjuvant chemotherapy, maxilla-mandible involvement, differentiation (high, middle, and poor), vascular invasions, neural invasions, TNM stage (AJCC 8th edition), PLT, MPV, PDW, PCT and postoperative radiotherapy or chemotherapy (Table 1).
The cut-off points for MPV referred to the reference value, while the cut-off points for other preoperative blood test indicators were identified by the medians, because they are generally distributed within the normal range. Participants were divided into two groups for further analysis according to the cut-off points of these indicators. The association of preoperative MPV levels with other clinical parameters were further analyzed (Table 2). HR and 95% CI were calculated using a Cox regression model to evaluate the potential prognostic factors (Table 3). All the variables significantly associated with OS in the analysis were included in a Cox regression model to adjust for the effects of covariates. The samples with P<0.05, evaluated by a single factor analysis, were adopted into the multivariate analysis. We included the tumor site and neoadjuvant therapy, manually (based on professional significance). Then, we obtained all the predictive variables with statistical significance, and based on the independent prognostic factors identified, dynamic histograms were created and calibration curves were generated to calibrate the assessment (Figure 5). The Harrell’s concordance index (C-index) was used to evaluate the predictive performance, and survival curves were created using the Kaplan-Meier method.
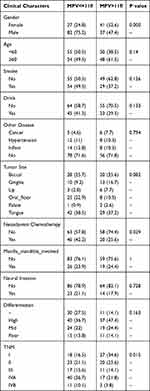 |
Table 2 The Association of Preoperative MPV Levels with Other Clinical Parameters |
 |
Table 3 Cox Regression |
Propensity score matching (PSM) was used to evaluate the therapeutic effect of neoadjuvant chemotherapy in the patients. This was not a randomized controlled trial; therefore, baseline characteristics were not balanced between the neoadjuvant and non-neoadjuvant groups. PSM was used to match the baseline levels of patients in the neoadjuvant and non-neoadjuvant groups according to baseline characteristics prior to the neoadjuvant chemotherapy (Table 4). Survival curves were used to compare the benefits of neoadjuvant therapy. We analyzed and compared MPV, PLT, PDW, and PCT between pre- and post-neoadjuvant chemotherapy with Wilcoxon test (Figure 3).
 |
Table 4 Base Line Information by Neoadjuvant Chemotherapy, Before or After PSM |
All statistical analyses were performed using the R software (https://www.r-project.org/; version 3.51). Statistical significance was set at P< 0.05.
Results
Patient Characteristics
As shown in Table 1, a total of 187 patients with OSCC were included this study. The patients were divided into two groups according to age (<60 years-old and ≥60 years-old) and into two groups according to their smoking and drinking status. Other diseases combined with OSCC included hypertension, inflammation, and malignancies other than OSCC. The tumor sites for this cohorts were: tongue (71, 37.97%), oral floor (33, 17.65%), buccal (48, 25.67%), gingiva (23, 12.3%), lip (9, 4.81%), and palate (3, 1.6%). The involvement of maxilla, mandible, and neural invasions were documented. According to 8th edition TNM staging system, patients distributed in stage I, II, III, IVA, and IVB were 45 (24.06%), 43 (22.99%), 28 (14.97%), 57 (30.48%) and 14 (7.49%) respectively. Patients had median PLT of 171×109/L (interquartile range (IQR):138.5–222.5), MPV of 10.7 fL (IQR: 9.65–12.15), PDW of 16.3% (IQR, 16.05–16.55%), and PCT of 0.19% (IQR 0.15–0.22%).
There were 66 (35.29%) patients who received neoadjuvant chemotherapy. Seventy-three (39.04%) patients received post-operative radiotherapy or chemotherapy.
Prognostic Factors for OSCC
The Cox regression analysis for patient OS is shown in Table 3. The univariable analyses showed that neural invasions (HR: 2.25, 95% CI: 1.36–3.71, P<0.05), differentiation, TNM stage, and preoperative MPV (HR: 0.51, 95% CI: 0.3–0.86, P<0.05) were significantly associated with OS. Other factors such as sex, age, smoking, drinking, other diseases, tumor site, neoadjuvant chemotherapy, PLT, PDW, and PCT were not significant correlated with patient OS. The multivariate analyses were further performed with the factors evaluated by univariable analysis with P<0.05. During this procedure, we added the tumor sites and neoadjuvant therapy manually because of their clinical significance. The multivariate analyses showed that neural invasions (HR: 2.38, 95% CI: 1.29–4.4, P=0.005), tumor sites (P<0.05), TNM stage (P<0.05), poor differentiation (HR: 2.97, 95% CI: 1.4–6.27, P=0.004), and preoperative MPV (HR: 0.47, 95% CI: 0.25–0.89, P=0.020) were independent prognostic indicators of OS.
Kaplan-Meier curves were used to confirm the prognostic value of the MPV. The OS curves of patients with MPV>11 fl were higher than those of patients with MPV≤11 fl (P=0.009) (Figure 1A). PLT, PDW, or PCT were not significantly correlated with OS (Figure 1B–D).
We further analyzed the correlation between preoperative MPV levels and other clinical parameters. We found factors with a statistical significance included gender (P<0.001), neoadjuvant chemotherapy (P=0.029), and TNM stage (P=0.015, Table 2). Interestingly, female patients with MPV > 11 fl had significant better OS than those who had MPV ≤ 11 fl (P=0.004, Figure 2A). Similarly, in patients who have advanced tumors (stage III and IV), MPV>11 fl indicated significantly increased OS compared with MPV≤11 fl (P=0.004, Figure 2D). The differences were not observed in male patients or patients with stage I and II disease (Figure 2B and C).
MPV, PLT, PDW, and PCT were compared between pre- and post- neoadjuvant chemotherapy with Wilcoxon test (Figure 3). MPV, PDW, and PCT were significantly decreased after neoadjuvant chemotherapy compared with those before neoadjuvant chemotherapy (P=0.001). Moreover, Patients with MPV >11 fl after neoadjuvant chemotherapy had better OS than those with MPV ≤ 11 fl (Figure 4). Interestingly, the difference in neoadjuvant cohort was more significant than non-neoadjuvant cohort (P=0.028 vs P=0.086).
 |
Figure 3 MPV (A) PDW (C) and PCT (D) levels were significantly decreased after neoadjuvant chemotherapy. PLT level (B) remained stable after neoadjuvant chemotherapy. |
Development and Validation of Prognostic Nomogram
Model discrimination was evaluated using the C-index, which quantified the level of agreement between the predicted and observed OS. The C-index for the final OS model was 0.79. The bias-corrected C-index generated by a bootstrap validation was 0.74. The model related to MPV, neural invasion, TNM was used to established a nomogram to estimate the predicted probability of a 3-year or 5-year OS (Figure 5A). Figure 5B shows the calibration chart for the OS model, in which the predicted probability of a 3- and 5-year OS was drew according to the observed data. The model estimates for 3-year and 5-year OS were very close to the observational estimates, but there was a slight deviation among individuals with poor survival. Kaplan-Meier curves confirmed that patients with low total scores had higher OS than patients with high total scores (P<0.01) (Figure 5C).
Effect Evaluation of Neoadjuvant Chemotherapy
Neoadjuvant chemotherapy, although added manually, showed no significant association with OS (HR: 0.66, 95% CI: 0.32–1.34, P=0.247, Table 4). Since this was not a randomized controlled trial, baseline characteristics were not balanced between the neoadjuvant and non-neoadjuvant groups. Therefore, the PSM was used to match the baseline levels of patients between the neoadjuvant and non-neoadjuvant groups according to the baseline characteristics prior to the neoadjuvant chemotherapy (Table 4). The Kaplan-Meier survival curve was used to compare the benefits of neoadjuvant therapy and showed no significant difference between the neoadjuvant and non-neoadjuvant groups in the total cohort (P=0.67, Figure 6A). However, when the baseline was matched by PSM, there was a significant difference on OS between the neoadjuvant and non-neoadjuvant groups (P=0.039, Figure 6B). These results suggest that neoadjuvant chemotherapy could benefit the OS of patients with advanced OSCC.
Discussion
With the deepening of the application of big data in the medical field, there are many types of RWSs that have developed rapidly. Compared to randomized controlled clinical trials, RWSs have merits such as high authenticity, a wide range of target populations, and strong evidence integrity. They are also an effective supplement to the traditional research form; thus, our study was performed based on a RWS.
The survival rate of oral cancer patients is strongly influenced by age, tumor stage, site, and histological grade, but is also influenced by many other factors4,20–27 including related treatment, educational levels the time between disease and perception, access to health-care services and occupation of the patients, behavioral/cultural factors exposed to risk factors involving alcohol consumption, smoking, and chewing tobacco. Despite the existence of studies on the link between platelets and oral cancer, there have been no studies on whether platelet-related parameters can be independent prognostic factors for oral cancer.
Platelet-related parameters included PLT, MPV, PDW, and PCT levels. In this study, preoperative MPV is significantly associated with OS. There are bidirectional effects between the tumor cells and the platelets.28 First, the tumor cells can damage the vascular endothelium, thereby activating the platelets, initiating the coagulation system, causing thrombosis and hemorrhage, and even severe complications such as disseminated intravascular coagulation. Second, the platelets, vascular wall, and tumor cells interact, promoting the adhesion of tumor cells to the vascular wall. The malignant tumors may cause changes in the platelet parameters; however, the reasons for this have not yet been fully elucidated and may be related to the following aspects. In patients with malignant tumors, bone marrow hyperplasia is active, tumor cells produce thrombogenic factors, and in the blood circulation, the concentration of the humoral environment that promotes the formation of megakaryocytes in bone marrow increases.27 In tumor patients, increased tumor growth promoting cytokines can also specifically stimulate an increase in platelets, including interleukin (IL)-1, IL-3, IL-6, IL-17, IL-18 and tumor necrosis factor-α.29 The malignant tumors consume a large amount of physical energy of patients, leading to chronic blood loss and malnutrition, and tissue necrosis, thereby affecting platelet morphological parameters. Platelets release transforming growth factors, which directly stimulate the growth of some tumor cells, and the proliferating tumor tissues produce more stimulating factors that promote the generation of bone marrow megakaryocytes, thus forming a vicious circle.28 Although, platelet activity is related mainly to platelet morphology, its correlation with PLT is weak. MPV and PDW are important indicators of platelet parameters, among which MPV is an alternative indicator of platelet activity.
Our results showed that MPV was associated with female and advanced tumors. Moreover, preoperative MPV, associated with female and advanced stage may be a novel prognostic factor for OS of OSCC patients. However, this significance needs to be further validated by more patient cohort in the real-world practice.
Recently, there has been a growing trend to establish prognostic histograms as an aid tool for determining outcomes that integrate multiple important prognostic factors to produce the probability of clinical events.30–32 The advantage of histograms over traditional TNM staging systems is that they can generate individualized predictions that may aid in clinical decision making and follow-up strategy development. Bobdey, et al33 established a nomogram for the 5-year OS in patients with oral cavity cancer based on clinical factors related to age, comorbidities, clinical lymph node status, stage of disease, tumor thickness, differentiation, and perineural invasions. Wang et al34 established two nomograms that have successfully predicted the long-term OS and CSS in oral SCC patients, using clinical factors.
In this study, a nomogram for predicting the probability of OS at 3- and 5-years was created based on the proven independent prognostic factors, tumor sites, neural invasions, TNM stage, and preoperative MPV. Unlike with other studies, our study added MPV as a factor to create the model, which may have been beneficial in improving the accuracy of the predictions.
Cisplatin, paclitaxel, and fluorouracil are currently the mostly accepted induction chemotherapy plan for OSCC35,36 Although there are many conducted clinical trials of induction chemotherapy, the results remain controversial.37 Large randomized studies38–43 have evaluated that various induction chemotherapy regimens were used to treat all sites of advanced oral cancer in order to improve survival in non-surgical patients or preserve the important organ. However, only some38–40 of these studies used surgery as the main treatment, resulting in the question of whether the adequate therapeutic approach was applied to oral cancer patients. Cohen’s study showed there was no difference in the OS between patients treated with induction chemotherapy before CRT and those who received CRT alone.41 In addition, this study showed that induction chemotherapy was along with more serious adverse events (47% vs 28%, P = 0.002), further induction chemotherapy was not advised to be used routinely. Meanwhile, meta-analyses have now confirmed that the use of induction chemotherapy does not confer a more benefit on OS or progression-free survival than CRT alone.43
Induction chemotherapy has been tested to improve locoregional control and preserve the organ in resectable oral cancers. A Phase III randomized study comparing induction chemotherapy followed by surgery and postoperative RT versus primary surgery and postoperative RT in patients with locally advanced and resectable oral cancer did not demonstrate improved survival with induction chemotherapy.44 The long-term results of this study reported by Bossi et al45 confirmed the same results after a median follow-up 11.5 years. Induction chemotherapy could not confer a benefit on locoregional control, distant metastasis, and OS. Chinn et al reported their Phase II experience: those who responded were treated with induction chemotherapy followed by definite simultaneous CRT, and those who did not respond were subsequently excised.46 Outcomes in this induced-selection cohort proved to be poor compared to patient cohort who underwent early surgery and appropriate adjuvant RT /CRT. Patil reported that induction chemotherapy before reassessment for surgery could be a suitable replacement of concurrent chemoradiation in borderline resectable or technically unresectable oral cancers.47 However, induction chemotherapy did not improve OS or disease-specific survival in patients with unresectable oral cancers.48
PSM is a useful method which can be used to generate a balanced cohort. Kuramatsu et al created baseline characteristics of patients with cerebellar intracerebral hemorrhage using PSM49 which has been applied by Son et al in their study published in The New England Journal of Medicine.50 In the present study, no significant difference was observed between the neoadjuvant and non-neoadjuvant groups in the whole patient cohort, because the 2 groups had unbalanced baseline. When the baseline was balanced by PSM, patients had neoadjuvant chemotherapy showed significantly increased survival compared with those who had no neoadjuvant chemotherapy. Therefore, neoadjuvant chemotherapy may benefit the OS of patients with advanced OSCC in the real-world practice.
Conclusion
Preoperative MPV, being associated with female, neoadjuvant chemotherapy, and advanced stage, could serve as a novel prognostic factor for OS of patients with OSCC; The nomogram provides a useful prediction for OS of patient with OSCC. Neoadjuvant chemotherapy may benefit the OS of OSCC patients in the real-world practice.
Abbreviations
MPV, mean platelet volume; OS, overall survival; HR, hazard ratio; RT, radiotherapy; CRT, concurrent chemoradiotherapy; AJCC, American Joint Committee on Cancer; pTNM, pathological, tumor-node-metastasis; cTNM, clinical tumor-node-metastasis; PLT, platelet count; RWS, Real-World Study; PDW, platelet distribution width; PCT, platelet cubic measure distributing; PSM, propensity score matching; IQR, interquartile range; IL, interleukin.
Data Sharing Statement
The data sets analyzed during the current study are available from the corresponding authors on reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of Sichuan Cancer Hospital. The contents were received by all the study participants. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We would thank all patients or their relatives providing the follow-up information and all authors for their help in writing and revising the article, collecting and analyzing the data.
Funding
This work was supported by National Natural Science Foundation of China (grant No. 81972541, 81872196, 81772900, and 81672690) and the Department of Science and Technology of Sichuan Province (grant No. 2020ZYD033).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Yan L, Chen F, Chen L, et al. Dynamic evaluation of conditional survival in patients with oral squamous cell carcinoma after surgical resection: a large-scale prospective study. Oral Oncol. 2020;104:104639. doi:10.1016/j.oraloncology.2020.104639
3. Patel TD, Marchiano E, Chin OY, et al. Utility of surgery/radiotherapy in distant metastatic head and neck squamous cell carcinoma: a population-based approach. Otolaryngol Head Neck Surg. 2016;154(5):868–874. doi:10.1177/0194599815627637
4. Chen F, Lin L, Liu F, et al. Three prognostic indexes as predictors of response to adjuvant chemoradiotherapy in patients with oral squamous cell carcinoma after radical surgery: a large-scale prospective study. Head Neck. 2019;41:301–308.
5. Okano S, Homma A, Kiyota N, et al. Induction chemotherapy in locally advanced squamous cell carcinoma of the head and neck. Jpn J Clin Oncol. 2021;51(2):173–179. doi:10.1093/jjco/hyaa220
6. Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi:10.3322/caac.21388
7. Sridharan S, Thompson LDR, Purgina B, et al. Early squamous cell carcinoma of the oral tongue with histologically benign lymph nodes: a model predicting local control and vetting of the eighth edition of the American joint committee on cancer pathologic T stage. Cancer. 2019;125(18):3198–3207. doi:10.1002/cncr.32199
8. Riess H, Braun G, Brehm G, Hiller E. Critical evaluation of platelet aggregation in whole human blood. Am J Clin Pathol. 1986;85(1):50–56. doi:10.1093/ajcp/85.1.50
9. Dincel O, Bayraktar C. Evaluation of platelet indices as a useful marker in papillary thyroid carcinoma. Bratisl Lek Listy. 2017;118:153–155.
10. Gu ML, Yuan CJ, Liu XM, et al. Pre-treatment elevated platelet count associates with HER2 overexpression and prognosis in patients with breast cancer. Asian Pac J Cancer Prev. 2015;16(13):5537–5540. doi:10.7314/APJCP.2015.16.13.5537
11. Oncel M, Kiyici A, Oncel M, Sunam GS, Sahin E, Adam B. Evaluation of platelet indices in lung cancer patients. Asian Pac J Cancer Prev. 2015;16(17):7599–7602. doi:10.7314/APJCP.2015.16.17.7599
12. Li JY, Li Y, Jiang Z, Wang RT, Wang XS. Elevated mean platelet volume is associated with presence of colon cancer. Asian Pac J Cancer Prev. 2014;15(23):10501–10504. doi:10.7314/APJCP.2014.15.23.10501
13. Prokopowicz G, Zyczkowski M, Nowakowski K, Bogacki R, Bryniarski P, Paradysz A. Basic parameters of blood count as prognostic factors for renal cell carcinoma. Biomed Res Int. 2016;2016:8687575. doi:10.1155/2016/8687575
14. Wang X, Cui MM, Xu Y, et al. Decreased mean platelet volume predicts poor prognosis in invasive bladder cancer. Oncotarget. 2017;8(40):68115–68122. doi:10.18632/oncotarget.19242
15. Ong HS, Gokavarapu S, Wang LZ, Tian Z, Zhang CP. Low pretreatment lymphocyte-monocyte ratio and high platelet-lymphocyte ratio indicate poor cancer outcome in early tongue cancer. J Oral Maxillofac Surg. 2017;75(8):1762–1774. doi:10.1016/j.joms.2016.12.023
16. Liu YH, Lin YS. Platelet-lymphocyte and neutrophil-lymphocyte ratios: predictive factors of response and toxicity for docetaxel-combined induction chemotherapy in advanced head and neck cancers. J Chin Med Assoc. 2019;82(11):849–855. doi:10.1097/JCMA.0000000000000178
17. Tazeen S, Prasad K, Harish K, Sagar P, Kapali AS, Chandramouli S. Assessment of pretreatment neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in prognosis of oral squamous cell carcinoma. J Oral Maxillofac Surg. 2020;78(6):949–960. doi:10.1016/j.joms.2020.01.001
18. Yamagata K, Fukuzawa S, Uchida F, Ishibashi-Kanno N, Yanagawa T, Bukawa H. Is preoperative plate-lymphocyte ratio a predictor of deep vein thrombosis in patients with oral cancer during surgery? J Oral Maxillofac Surg. 2021;79(4):914–924. doi:10.1016/j.joms.2020.10.024
19. Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence - What is it and What can it tell us? N Engl J Med. 2016;375(23):2293–2297. doi:10.1056/NEJMsb1609216
20. Sterba KR, Garrett-Mayer E, Carpenter MJ, et al. Smoking status and symptom burden in surgical head and neck cancer patients. Laryngoscope. 2017;127(1):127–133. doi:10.1002/lary.26159
21. Dantas TS, de Barros Silva PG, Sousa EF, et al. Influence of educational level, stage, and histological type on survival of oral cancer in a Brazilian population: a retrospective study of 10 years observation. Medicine. 2016;95(3):e2314. doi:10.1097/MD.0000000000002314
22. Walter V, Jansen L, Ulrich A, et al. Alcohol consumption and survival of colorectal cancer patients: a population-based study from Germany. Am J Clin Nutr. 2016;103(6):1497–1506. doi:10.3945/ajcn.115.127092
23. Mair M, Nair D, Nair S, et al. Comparison of tumor volume, thickness, and T classification as predictors of outcomes in surgically treated squamous cell carcinoma of the oral tongue. Head Neck. 2018;40:1667–1675.
24. Rajappa SK, Maheshwari U, Jaipuria J, et al. Number of positive nodes - Current relevance in determining prognosis of oral cavity cancer after the recent AJCC staging update. Oral Oncol. 2019;90:1–5. doi:10.1016/j.oraloncology.2019.01.001
25. Lee A, Givi B, Osborn VW, Schwartz D, Schreiber D. Patterns of care and survival of adjuvant radiation for major salivary adenoid cystic carcinoma. Laryngoscope. 2017;127(9):2057–2062. doi:10.1002/lary.26516
26. Liu F, Chen F, Huang J, et al. Prospective study on factors affecting the prognosis of oral cancer in a Chinese population. Oncotarget. 2017;8(3):4352–4359. doi:10.18632/oncotarget.13842
27. McDonald JT, Johnson-Obaseki S, Hwang E, Connell C, Corsten M. The relationship between survival and socio-economic status for head and neck cancer in Canada. J Otolaryngol Head Neck Surg. 2014;43(1):2. doi:10.1186/1916-0216-43-2
28. Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126(5):582–588. doi:10.1182/blood-2014-08-531582
29. Nagata S, Fukunaga R. Granulocyte colony-stimulating factor receptor and its related receptors. Growth Factors. 1993;8(2):99–107. doi:10.3109/08977199309046930
30. Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Development and validation of nomograms predictive of overall and progression-free survival in patients with oropharyngeal cancer. J Clin Oncol. 2017;35(36):4057–4065. doi:10.1200/JCO.2016.72.0748
31. Xu C, Chen YP, Liu X, et al. Establishing and applying nomograms based on the 8th edition of the UICC/AJCC staging system to select patients with nasopharyngeal carcinoma who benefit from induction chemotherapy plus concurrent chemoradiotherapy. Oral Oncol. 2017;69:99–107. doi:10.1016/j.oraloncology.2017.04.015
32. Chen F, Lin L, Yan L, et al. Nomograms and risk scores for predicting the risk of oral cancer in different sexes: a large-scale case-control study. J Cancer. 2018;9(14):2543–2548. doi:10.7150/jca.24431
33. Bobdey S, Balasubramaniam G, Mishra P. Nomogram prediction for survival of patients with oral cavity squamous cell carcinoma. Head Neck. 2016;38(12):1826–1831. doi:10.1002/hed.24507
34. Wang F, Zhang H, Wen J, et al. Nomograms forecasting long-term overall and cancer-specific survival of patients with oral squamous cell carcinoma. Cancer Med. 2018;7(4):943–952. doi:10.1002/cam4.1216
35. Pignon JP, le Maitre A, Maillard E, Bourhis J; Group M-NC. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14. doi:10.1016/j.radonc.2009.04.014
36. Blanchard P, Bourhis J, Lacas B, et al. Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol. 2013;31(23):2854–2860. doi:10.1200/JCO.2012.47.7802
37. Gau M, Karabajakian A, Reverdy T, Neidhardt EM, Fayette J. Induction chemotherapy in head and neck cancers: results and controversies. Oral Oncol. 2019;95:164–169. doi:10.1016/j.oraloncology.2019.06.015
38. Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695–1704. doi:10.1056/NEJMoa071028
39. Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705–1715. doi:10.1056/NEJMoa070956
40. Hitt R, Lopez-Pousa A, Martinez-Trufero J, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. 2005;23(34):8636–8645. doi:10.1200/JCO.2004.00.1990
41. Cohen EE, Karrison TG, Kocherginsky M, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32(25):2735–2743. doi:10.1200/JCO.2013.54.6309
42. Paccagnella A, Ghi MG, Loreggian L, et al. Concomitant chemoradiotherapy versus induction docetaxel, cisplatin and 5 fluorouracil (TPF) followed by concomitant chemoradiotherapy in locally advanced head and neck cancer: a phase II randomized study. Ann Oncol. 2010;21(7):1515–1522. doi:10.1093/annonc/mdp573
43. Budach W, Bolke E, Kammers K, et al. Induction chemotherapy followed by concurrent radio-chemotherapy versus concurrent radio-chemotherapy alone as treatment of locally advanced squamous cell carcinoma of the head and neck (HNSCC): a meta-analysis of randomized trials. Radiother Oncol. 2016;118(2):238–243. doi:10.1016/j.radonc.2015.10.014
44. Zhong LP, Zhang CP, Ren GX, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol. 2013;31:744–751.
45. Bossi P, Lo Vullo S, Guzzo M, et al. Preoperative chemotherapy in advanced resectable OCSCC: long-term results of a randomized phase III trial. Ann Oncol. 2014;25(2):462–466. doi:10.1093/annonc/mdt555
46. Chinn SB, Spector ME, Bellile EL, et al. Efficacy of induction selection chemotherapy vs primary surgery for patients with advanced oral cavity carcinoma. JAMA Otolaryngol Head Neck Surg. 2014;140(2):134–142. doi:10.1001/jamaoto.2013.5892
47. Patil VM, Prabhash K, Noronha V, et al. Neoadjuvant chemotherapy followed by surgery in very locally advanced technically unresectable oral cavity cancers. Oral Oncol. 2014;50(10):1000–1004. doi:10.1016/j.oraloncology.2014.07.015
48. Patil VM, Noronha V, Joshi A, Banavali SD, Muddu V, Prabhash K. Preoperative chemotherapy and metronomic scheduling of chemotherapy in locally advanced oral cancers. Oncology. 2016;91(Suppl 1):35–40. doi:10.1159/000447579
49. Kuramatsu JB, Biffi A, Gerner ST, et al. Association of surgical hematoma evacuation vs conservative treatment with functional outcome in patients with Cerebellar intracerebral hemorrhage. JAMA. 2019;322(14):1392–1403. doi:10.1001/jama.2019.13014
50. Son MBF, Murray N, Friedman K, et al.; Overcoming COVID-19 Investigators. Multisystem inflammatory syndrome in children - initial therapy and outcomes. N Engl J Med. 2021;385(1):23–34. doi:10.1056/NEJMoa2102605.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





