Back to Journals » Journal of Inflammation Research » Volume 16
Prognostic Value of Platelet-to-Lymphocyte Ratio Combined with CHA2DS2-VASc Score for Nonvalvular Atrial Fibrillation Induced Cardiogenic Cerebral Embolism
Authors Fan Q, Gao L, Wang Z, Ndjana Lessomo FY, Wang G
Received 18 July 2023
Accepted for publication 17 November 2023
Published 6 December 2023 Volume 2023:16 Pages 5937—5947
DOI https://doi.org/10.2147/JIR.S431149
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Qian Fan,1,* Ling Gao,1,* Zhiquan Wang,2,* Fabrice Yves Ndjana Lessomo,2 Gang Wang1
1Department of Cardiology, The Second Affiliated Hospital of Shandong First Medical University, Taian, Shandong, People’s Republic of China; 2Department of Cardiology, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Gang Wang, Email [email protected]
Aim: To determine the predictive significance of the platelet-to-lymphocyte ratio (PLR) combined with the CHA2DS2-VASc score for cardiogenic cerebral embolism (CCE) in patients with nonvalvular atrial fibrillation (NVAF).
Methods: A total of 553 patients with NVAF were included in this retrospective study. The general data, PLR, CHA2DS2-VASc score and echocardiography indicators were compared. The risk factors for CCE and the predictive value of PLR and CHA2DS2-VASc were analyzed. Stratified analysis was performed based on the cut-off value. Least absolute shrinkage and selection operator (LASSO) regression analysis was utilized to build a model. The relationship between risk score and different anticoagulants was evaluated.
Results: Multiple regression analysis showed hypertension (OR=3.95, 95% CI=2.12– 7.35, p=1.40× 10− 5), diabetes mellitus (OR=2.95, 95% CI=1.57– 5.58, p=7.65× 10− 4), PLR (OR=1.01, 95% CI=1.00– 1.01, p< 10− 6), creatinine level (OR=1.01, 95% CI=1.00– 1.02, p=7.44× 10− 3), left atrial diameter (LAD) (OR=1.90, 95% CI=1.13– 3.19, p=1.51× 10− 2), ejection fraction (EF) (OR=0.93, 95% CI=0.87– 0.98, p=8.06× 10− 3) and CHA2DS2-VASc score (OR=3.79, 95% CI=2.95– 4.85, p< 10− 6) were independent risk factors for CCE. A one-way linear analysis also showed the above seven indexes were significantly correlated with CCE (F=56.4, p< 10− 6). The area under the receiver operating characteristic (ROC) curve of PLR and CHA2DS2-VASc score was 0.760 (95% CI:0.721– 0.800), and 0.855 (95% CI: 0.824– 0.886), respectively. Pearson correlation analysis showed that PLR was correlated with CHA2DS2-VASc score (r=0.331, p< 10− 6). Stratified analysis indicated there was a positive correlation between different risk group (p< 10− 6). Using the LASSO model, a composite indicator displayed differential power for distinguishing CCE with an AUC value of 0.884 (95% CI: 0.857– 0.911). Patients with dabigatran and rivaroxaban exhibited higher risk score than those with warfarin (warfarin vs dabigatran, p=1.40× 10− 2; warfarin vs rivaroxaban p=3.00× 10− 3).
Conclusion: PLR and CHA2DS2-VASc score are independent risk factors for CCE with NVAF, and the combination of the two indices can improve the prediction of CCE.
Keywords: NVAF, CCE, PLR, CHA2DS2-VASc score, LASSO model
Introduction
Atrial fibrillation (AF) is one of the most common arrhythmias in clinical practice and is characterized by cardiac systolic dysfunction, rapid and disordered atrial electrical activity, and atrial mural thrombosis.1 Due to the prevalence of population ageing and risk factors such as hypertension, diabetes, obesity, smoking and alcohol consumption, the prevalence of AF has been increasing significantly.2–5 NVAF is one of the most common type major causes of atrial fibrillation, accounting for 65.2% of cases, and the incidence rate shows an increasing trend year by year. Thromboembolic complications are the main cause of death and disability in NVAF.6 The risk of stroke in patients suffering from NVAF is five times higher than that in the general population. NVAF causes more acute onset of cerebral infarction and higher mortality and disability than other types of cerebral infarction, and imposes a great burden on global public health.7,8 Current guidelines agree that the prevention of AF-related cerebral infarction is the primary concern of comprehensive management strategy and treatment guidance for patients. Due to the seriousness of the danger of AF, early identification of high-risk patients is needed, and in addition to traditional approaches, some new clues have been gradually discovered, such as fragmented QFR.9,10 The mechanism of the generation, maintenance and recurrence of AF is not completely clear, and an increasing number of studies have shown that external factors such as activation of the autonomic nervous system, activation of the renin angiotensinogen system, inflammatory factors, oxidative stress and genetic factors can interact to cause AF. The CHA2DS2-VASc score is an abbreviated version of the thrombus risk assessment method based on clinical risk factors and baseline characteristics of AF patients. Although widely accepted by the Society of Cardiology, it has limitations in the use of clinical data rather than biochemical and morphological data.11,12 In this context, biomarkers attempt to fill this gap by increasing dominance, expressing disease severity and duration. PLR, a new marker of the inflammatory pathway, is independently associated with adverse clinical outcomes such as cardiovascular lymphocytes. PLR also has high clinical value in evaluating the condition and prognosis of cardiovascular disease. Moreover, PLR is a comprehensive index based on the analysis of blood cells, which is more sensitive and accurate than a single complete blood count. However, there are few studies on PLR and NVAF, therefore, a case - control study was conducted to investigate the association between PLR and the CHA2DS2-VASc scoring system and the occurrence of CCE in patients with AF, and to provide help for early clinical identification of patients at high risk of thrombosis.
Methods
Patient Selection
A total of 553 patients diagnosed with NVAF in Zhongnan Hospital of Wuhan University from January to December in 2022 were selected. A total of 236 patients were diagnosed with CCE. 317 patients were diagnosed with non-CCE matched for sex and age and not suffered from an acute stroke of patients with NVAF within the same time frame. Table 1 illustrates the detailed clinical features of the patient demographics enrolled in our research. Figure 1 shows the detailed screening procedure. This study was reviewed and approved by the Medical Ethical Committee of Zhongnan Hospital of Wuhan University.
 |
Table 1 Demography of Subject |
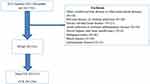 |
Figure 1 Study design flowchart. |
Inclusion Criteria
(1) NVAF was diagnosed according to the 2019 AHA/ACC/HRS guidelines for the management of patients with AF, and patients with paroxysmal or persistent AF were diagnosed with a 12-lead routine ECG or a 24-hour Holter monitor, and echocardiography confirmed NVAF.13 (2) According to the diagnostic criteria of the Chinese guidelines for the diagnosis and treatment of acute ischaemic stroke 2018, patients with CCE were diagnosed by cranial CT or magnetic resonance imaging. (3) Complete clinical data and biochemical test results during hospitalization.
Exclusion Criteria
(1) Complicated by other cerebrovascular disease or other intracranial diseases. (2) Previous history of cerebral infarction or brain imaging evidence of cerebral infarction. (3) Severe valvular heart disease. (4) Patients with acute infection or systemic inflammatory disease, severe liver and renal insufficiency, malignant tumour, blood disease, autoimmune disease and other systemic diseases.
Instruments and Methods
Statistical Analysis
SPSS 21.0 software was used. The measurement data were expressed as X±s, and the t test was used. The count data were expressed, and the chi-square test was used. The independent risk factors for acute cerebral infarction in patients with NVAF were analyzed by logistic regression analysis. The correlation between CHA2DS2 -VASc score and PLR was analyzed by Pearson correlation. Stratified analysis was performed by the chi-square test. The area under the curve (AUC) was calculated to evaluate the predictive value of the CHA2DS2 -VASc score, PLR and combined indices for CCE in NVAF patients by ROC analysis. Youden’s index was used to determine the cut-off value, sensitivity and specificity. Furthermore, to assess the potential risk of CCE for NVAF patients, LASSO regression analysis was performed using R (4.2.1) software to construct a risk score model based on PLR and CHA2DS2 -VASc score. Kruskal–Wallis test was used to evaluate the relationship between the risk score and different anticoagulants. The statistical power analysis was performed with a program that calculates the sample size and power (PASS 15.0). p<0.05 was considered statistically significant.
Results
Demographic Characteristics
The two groups of patients were compared in terms of baseline data. The proportion of hypertension and diabetes mellitus, lymphocyte count, platelet count, PLR, albumin, creatinine, D-dimer, LAD, EF% and CHA2DS2-VASc score in the CCE group were higher than those in the non-CCE group, and the difference was statistically significant (p<0.05). However, there were no significant differences in sex, age, smoking history, proportion of coronary heart disease, proportion of peripheral vascular disease, proportion of heart failure, proportion of renal failure, WBC, RBC, haemoglobin, platelet count, lymphocyte count, ALT, AST and UA (p>0.05). The sample size provided a statistical power of 98.9%. See Table 1.
Multiple Logistic Regression Analysis
To comprehensively evaluate the association between confounding factors and the occurrence of CCE in NVAF patients and determine the most valuable risk factors, a multiple regression analysis was performed, and the results showed that hypertension (OR=3.95, 95% CI=2.12–7.35, p=1.40×10−5), diabetes mellitus (OR=2.95, 95% CI =1.57–5.58, p=7.65×10−4), creatinine level (OR=1.01, 95% CI=1.00–1.02, p=7.44×10−3), PLR (OR=1.01, 95% CI=1.00–1.01, p<10−6), LAD (OR=1.90, 95% CI=1.13–3.19, p=1.51×10−2), ejection fraction (EF) (OR=0.93, 95% CI=0.87–0.98, p=8.06×10−3) and CHA2DS2-VASc score (OR=3.79, 95% CI=2.95–4.85, p<10−6) were associated with CCE. A one-way linear analysis also showed that the above seven indexes were significantly correlated with CCE (F=56.4, p<10−6). See Table 2 and Figure 2.
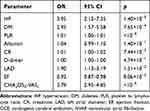 |
Table 2 Multiple Logistics Analysis Result of CCE in NVAF |
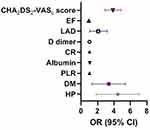 |
Figure 2 Independent risk factors for CCE in NVAF patients. |
ROC Curve Analysis
The ROC curves of PLR and CHA2DS2-VASc score were 0.760 (95% CI:0.721–0.800), and 0.855 (95% CI: 0.824–0.886) respectively. The cut-off values of PLR and CHA2DS2-VASc score for predicting CCE in NVAF patients were 150.7 and 5.0 respectively. The sensitivity was 0.712 and 0.742 and the specificity was 0.729 and 0.814 respectively. See Figure 3.
 |
Figure 3 ROC curve analysis. |
Correlation Analysis of PLR and CHA2DS2-VASc Score
Pearson correlation analysis showed that the PLR level was positively correlated with the CHA2DS2-VASc score (r = 0.331, p<10−6). See Figure 4.
 |
Figure 4 Spearman correlation analysis of PLR and CHA2DS2-VASc score. |
Stratified Analysis of CCE Risk in NVAF
Risk stratification of patients with NVAF was performed using the cut-off values of PLR and CHA2DS2-VASc score. The stratification criteria were: PLR<150.7 and CHA2DS2-VASc score<5 was low-risk group (263 cases). PLR≤150.7 and CHA2DS2-VASc score≥5 or PLR≥150.7 and CHA2DS2-VASc score≤5 were medium-risk group (N=204). PLR>150.7 and CHA2DS2-VASc score>5 was high-risk group (N=86). Chi-square test analysis showed that the risk of CCE in high-risk group was higher than that in low-risk and medium-risk group (χ2=160.7, 26.9, both p<10−6), and the medium-risk group was higher than that in low-risk group (χ2=95.2, p<10−6).
LASSO Model Analysis
To identify a comprehensive risk index to predict the occurrence of CCE in NVAF, we built a composite indicator based on the PLR and CHA2DS2-VASc score. The risk score of the LASSO model was calculated as follows: risk score = the level of PLR × 1.149289e −03 + the level of CHA2DS2-VASc score×1.40295668e−01−0.341324479 (see Figure 5). The results showed that the risk score was significant for CCE in NVAF patients and the risk score could differentiate CCE patients from patients in the NVAF group with an AUC of 0.884 (sensitivity=74.6%, specificity=87.4%, cut-off value=0.504). See Figures 3 and 6.
 |
Figure 5 LASSO model based on PLR and CHA2DS2-VASc score. (A) The coefficients of each variate in the model (1: PLR; 2: CHA2DS2-VASc score). (B) The mean-squared error of the model. |
Difference of the Risk Score Among Different Anticoagulants in CCE Patients
Furthermore, in the CCE group, patients received different anticoagulation therapy (warfarin:59 cases; dabigatran: 92 cases; rivaroxaban: 82 cases). Results showed that there were significant differences in the risk score among three groups (H=12.38, p=2.00×10−3). Patients with non-vitamin K antagonist oral anticoagulants (dabigatran and rivaroxaban) exhibited significantly higher risk score than those with warfarin (dabigatran vs warfarin, p=1.40×10−2; rivaroxaban vs warfarin p=3.00×10−3). However, there was no significant association between dabigatran and rivaroxaban in risk scores (p=2.28×10−1). See Figure 7.
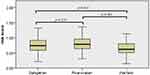 |
Figure 7 The association between the risk score and different anticoagulants in CCE patients. |
Discussion
NVAF is the unsynchronized contraction of the left atrium leading to the loss of mechanical function of the atrium, which causes blood to form eddies in the atrium, leading to thrombosis.16–18 To date, the mechanism of NVAF remains unclear, but a large number of studies have shown that inflammatory reactions are closely related to the triggering and maintenance of atrial fibrillation. Inflammation can increase the risk of atrial fibrillation in various cardiovascular diseases such as coronary heart disease, heart failure, and hypertension and the anti-inflammatory effect of glucocorticoids can reduce the risk of recurrence of atrial fibrillation within 3 days after radiofrequency ablation.19 Inflammatory conditions can also lead to a decrease in intracellular calcium currents. Inflammatory mediators cause apoptosis of atrial myocytes, which in turn leads to electrical and structural remodeling of the atria through activation of the fibrotic pathway of fibroblasts, ultimately leading to AF. AF may further aggravate the inflammatory state of the body.20,21
Previous studies have shown that many inflammatory markers are associated with AF, including CRP, IL-6, and IL-8. CRP, a marker reflecting systemic nonspecific inflammation, is now recognized as a valid marker for determining cardiovascular disease risk grading and plays a bridging role in the process of inflammation promoting the development of AF. Similar studies have been conducted on some new inflammatory markers such as red blood cell distribution width, neutrophil lymphocyte ratio (NLR) and PLR. Predictive analysis based on clinical data has been widely used in cardiovascular diseases, such as acute coronary syndrome, peripheral artery disease, and pulmonary embolism.22–24 PLR is a new inflammatory indicator that has been used to evaluate the inflammatory status of patients with acute coronary syndrome, type 2 diabetes and other diseases. At present, the relationship between PLR and the pathophysiological mechanism of AF is still controversial. Chen et al found that PLR is an independent risk factor for left atrial thrombosis in patients with NVAF, and it is associated with the severity of stroke neurological impairment, which can be used as a predictor of short-term prognosis.25 In addition, Gary et al found that high PLR levels may damage blood viscosity, thereby damaging the oxygen supply of myocardial tissue.26 However, the result from a recent study was different from those of previous studies, suggesting that PLR levels are not associated with AF in 1982 subjects.27 Our findings indicate that the PLR level in the CCE group is higher than that in the non-CCE group. The ROC curve suggests that the AUC value of PLR predicting CCE occurrence in NVAF patients is 0.760, and the cut-off value is 150.4. When the PLR of NVAF patients is greater than 150.4, it can better predict the risk of CCE occurrence. The mechanism may be that in the infarcted area, macrophages are activated by inflammatory mediators, resulting in the production of a large number of activated platelets that can release proinflammatory factors to regulate the permeability of endothelial cells, which in turn recruit monocytes for migration and aggregation, leading to the progression of the inflammatory response. This is related to the key pathogenesis of platelet-forming stroke through the activation of the GP Ib-vascular haemophilia factor-GPVI axis, and triggering of the GP Ib-vascular haemophilia factor-GPVI coagulation factor XII pathway may promote the progression of inflammatory reactions.28–30
The CHA2DS2-VAScscore system is currently one of the more reliable methods commonly used to assess the risk of stroke in patients with NVAF. Patients with a high CHA2DS2-VASc score often have a high risk of bleeding, and antithrombotic treatment based solely on the CHA2DS2-VASc score is not comprehensive. Some studies have optimized the risk stratification of acute cerebral infarction by combining other biochemical indicators, which has played an important guiding role in clinical antithrombotic treatment.31 The results of our study showed that PLR and CHA2DS2-VASc score have predictive value for the risk of CCE with NVAF, and the predictive value of the combined index is significantly higher than that of the independent index. The ROC curve suggests that the AUC value of the CHA2DS2-VASc score predicting CCE occurrence in NVAF patients is 0.855. However, the PLR combined with the CHA2DS2-VASc score index showed an AUC value of 0.884. Pearson correlation analysis showed that PLR was positively correlated with CHA2DS2-VASc score. This may be because elevated PLR levels reflect the inflammatory state of the body, increasing the risk of patients with related diseases, and thereby improving the CHA2DS2-VASc score. PLR may be more clinically relevant for patients with CCE as a complementary stratification marker for the CHA2DS2-VASc score. In addition, we further analyzed the influence of different anticoagulant drugs on the incidence of CCE, and the results showed that our conclusions were consistent with those of previous studies, indicated that non-vitamin K antagonist oral anticoagulants may have better effect for stroke prevention than warfarin in NVAF, which provided a good inspiration for our clinical drug selection.32,33
Although we have fully evaluated the potential clinical significance of NVAF related CCE risk scores, some limitations could be noted, and the retrospective nature of this investigation could have given rise to some biases. First, because the selected patients were all inpatients in the department of cardiology of our hospital, there is a selection bias. Second, the sample size of this study was small and from a single center, and the research results are one-sided, requiring greater sample size verification in the future. Third, due to the lack of data on inflammatory markers such as CRP, IL-8, and TNF, it is impossible to compare the correlation between PLR and these inflammatory markers. Finally, there is no further dynamic monitoring of the PLR of patients and evaluation of its relationship to the severity of NVAF and CCE.
Conclusion
Our study found that PLR and CHA2DS2-VASc showed greater value for identifying the occurrence of CCE in NVAF patients, which supported that inflammation and thrombosis were associated with CCE. Furthermore, PLR combined with CHA2DS2-VASc based on the LASSO model and ROC curve analysis demonstrated better performance with an accuracy of 88.4% and can be used as an indicator for predicting and evaluating CCE in NVAF patients than the single index, assessing the risk of CCE in NVAF patients and providing targeted prevention. Furthermore, non-vitamin K antagonist oral anticoagulants can lower risk scores compared to warfarin. This study has important implications for the early identification of CCE high-risk groups in NVAF patients and the selection of anticoagulants.
Patient Privacy Protection Statement
We desensitized all the data that can be used to identify patient personal information, such as their names, hospitalization ID and telephone numbers, to protect the privacy of patients.
Data Sharing Statement
The authors declare that all data supporting the findings of this study are available within the article or from the corresponding author (Gang Wang).
Ethical Approval and Consent to Participate
The studies involving human participants were reviewed and approved by the Medical Ethics Committee, Zhongnan Hospital of Wuhan University (2022157K). As this study is retrospective and presents no risk of harm to subjects, and no privacy of individuals is exposed, informed consent was waived. All procedures adhered to the ethical standards and the Helsinki Declaration (as revised in 2013).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interest in this work.
References
1. Kodani E, Atarashi H, Inoue H, et al. Impact of creatinine clearance on outcomes in patients with non-valvular atrial fibrillation: a subanalysis of the J-RHYTHM Registry. Eur Heart J Qual Care Clin Outcomes. 2018;4(1):59–68. doi:10.1093/ehjqcco/qcx032
2. Antit S, Hamraoui N, Boussabeh I, et al. Atrial fibrillation in the elderly: therapy and prognosis characteristics. Arch Cardiovasc Dis Sup. 2021;13(1):97.
3. Wang L, Feng Z, Li J, et al. Trends of global burden of atrial fibrillation/flutter from global burden of disease study 2017. Heart. 2020;107(11):881–887. doi:10.1136/heartjnl-2020-317656
4. Du X, Dong J, Ma C. Is atrial fibrillation a preventable disease? J Am Coll Cardiol. 2017;69(15):1968–1982. doi:10.1016/j.jacc.2017.02.020
5. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528. doi:10.1161/CIR.0000000000000659
6. Liu X, Li XW. Research progress of rivaroxaban in anticoagulant therapy for non-valvular atrial fibrillation. China Med. 2019;9(12):1913–1916.
7. Arboix A, Alio J. Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Curr Cardiol Rev. 2010;6(3):150–161. doi:10.2174/157340310791658730
8. Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke. 2021;16(2):217–221. doi:10.1177/1747493019897870
9. Yesin M, Kalcik M, Cagdas M, et al. Fragmented QRS may predict new onset atrial fibrillation in patients with ST-segment elevation myocardial infarction. J Electrocardiol. 2018;51(1):27–32. doi:10.1016/j.jelectrocard.2017.08.014
10. Karakayali M, Artac I, Omar T, et al. Assessment of the efficacy of the electrocardiographic P-wave peak time in predicting atrial high rate episode in patients with cardiac implantable electronic devices. J Electrocardiol. 2023;80:40–44. doi:10.1016/j.jelectrocard.2023.05.001
11. Hu WS, Lin CL. CHA2DS2-VASc score for ischaemic stroke risk stratification in patients with chronic obstructive pulmonary disease with and without atrial fibrillation: a nationwide cohort study. Europace. 2018;20(4):575–581. doi:10.1093/europace/eux065
12. Roger VL, Go AS, Lloyd-Jones DM. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi:10.1161/CIR.0b013e31823ac046
13. January CT, Wann LS, Calkins H. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16(8):e66–e93. doi:10.1016/j.hrthm.2019.01.024
14. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–2870. doi:10.1001/jama.285.22.2864
15. Jia XM, Levine GN, Birnbaum Y, et al. The CHA2DS2-VASc score: not as simple as it seems. Int J Cardiol. 2018;257:92–96. doi:10.1016/j.ijcard.2017.12.027
16. Renda G, Ricci F, Patti G, et al. CHA(2)DS(2)VASc score and adverse outcomes in middle-aged individuals without atrial fibrillation. Eur J Prev Cardiol. 2019;26(18):1987–1997. doi:10.1177/2047487319868320
17. Tateishi Y, Tsujino A, Hamabe J, et al. Cardiac diastolic dysfunction predicts in-hospital mortality in acute ischemic stroke with atrial fibrillation. J Neurol Sci. 2014;345(1–2):83–86. doi:10.1016/j.jns.2014.07.011
18. Kim T-H, Shim CY, Park JH. Left ventricular diastolic dysfunction is associated with atrial remodeling and risk or presence of stroke in patients with paroxysmal atrial fibrillation. J Cardiol. 2016;68(2):104–109. doi:10.1016/j.jjcc.2015.10.008
19. Engelmann MD, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J. 2005;26(20):2083–2092. doi:10.1093/eurheartj/ehi350
20. Hu Y-F, Chen Y-J, Lin Y-J, Chen S-A. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12(4):230–243. doi:10.1038/nrcardio.2015.2
21. Yuan Y, Zhao J, Gong Y, et al. Autophagy exacerbates electrical remodeling in atrial fibrillation by ubiquitin-dependent degradation of L-type calcium channel. Cell Death Dis. 2018;9(9):873. doi:10.1038/s41419-018-0860-y
22. Ozbeyaz NB, Gokalp G, Algul E, et al. H(2)FPEF score and contrast-induced nephropathy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Angiology. 2023;74(2):181–188. doi:10.1177/00033197221099425
23. Ozbeyaz NB, Gokalp G, Algul E, et al. Platelet-hemoglobin ratio predicts amputation in patients with below-knee peripheral arterial disease. BMC Cardiovasc Disord. 2022;22(1):337. doi:10.1186/s12872-022-02788-2
24. Ozbeyaz NB, Gokalp G, Gezer AE, et al. Novel marker for predicting the severity and prognosis of acute pulmonary embolism: platelet-to-hemoglobin ratio. Biomark Med. 2022;16(12):915–924. doi:10.2217/bmm-2022-0201
25. Chen C, Gu L, Chen L, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential predictors of prognosis in acute ischemic stroke. Front Neurol. 2020;11:525621. doi:10.3389/fneur.2020.525621
26. Gary T, Pichler M, Belaj K, et al. Platelet-to-lymphocyte ratio: a novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PLoS One. 2013;8(7):e67688. doi:10.1371/journal.pone.0067688
27. Guan Y-Z, Yin R-X, Zheng P-F, Liu C-X, Wei B-L, Deng G-X. Association of RDW, NLR, and PLR with atrial fibrillation in critical care patients: a retrospective study based on propensity score matching. Dis Markers. 2022;2022:2694499. doi:10.1155/2022/2694499
28. Dietrich-Muszalska A, Wachowicz B. Platelet haemostatic function in psychiatric disorders: effects of antidepressants and antipsychotic drugs. World J Biol Psychiatry. 2017;18(8):564–574. doi:10.3109/15622975.2016.1155748
29. Stegner D, Klaus V, Nieswandt B. Platelets as modulators of cerebral ischemia/reperfusion Injury. Front Immunol. 2019;10:2505. doi:10.3389/fimmu.2019.02505
30. Jayaraj RL, Azimullah S, Beiram R, et al. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16(1):142. doi:10.1186/s12974-019-1516-2
31. Rivera-Caravaca JM, Roldan V, Esteve-Pastor MA, et al. Prediction of long-term net clinical outcomes using the TIMI-AF score: comparison with CHA(2)DS(2)-VASc and HAS-BLED. Am Heart J. 2018;197:27–34. doi:10.1016/j.ahj.2017.11.004
32. Costello M, Murphy R, Judge C, et al. Effect of non-vitamin-K oral anticoagulants on stroke severity compared to warfarin: a meta-analysis of randomized controlled trials. Eur J Neurol. 2020;27(3):413–418. doi:10.1111/ene.14134
33. Milentijevic D, Lin JH, Connolly N, et al. Risk of stroke outcomes in atrial fibrillation patients treated with rivaroxaban and warfarin. J Stroke Cerebrovasc Dis. 2021;30(5):105715. doi:10.1016/j.jstrokecerebrovasdis.2021.105715
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

