Back to Journals » International Journal of General Medicine » Volume 16
Prognostic Value of Platelet Aggregation Function in Patients with laryngeal Carcinoma
Authors Li M, Gui J , Wang H, An J, Wu R, Liu X, Wu B , Xiao H
Received 23 July 2023
Accepted for publication 18 October 2023
Published 24 November 2023 Volume 2023:16 Pages 5559—5566
DOI https://doi.org/10.2147/IJGM.S428122
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Minghua Li,1,* Jiawei Gui,1,* Hao Wang,2 Jun An,3 Ruoqing Wu,1 Xiaotong Liu,1 Bo Wu,1 Hui Xiao1
1Department of Otolaryngology-Head and Neck Surgery, The Second Affiliated Hospital of Harbin Medical University, Harbin, 150081, People’s Republic of China; 2Department of Otolaryngology-Head and Neck Surgery, The Affiliated BenQ Hospital of Nanjing Medical University, Nanjing, 210019, People’s Republic of China; 3Department of Otolaryngology-Head and Neck Surgery, Xuzhou Central Hospital, Xuzhou, 221009, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hui Xiao, Email [email protected]
Background: Laryngeal cancer was one of the most common malignancies of the head in those years. It has become one of the most common causes of death due to its high recurrence rate and high metastasis rate. It was well known that platelets, especially activated platelets, promote the proliferation, division, and invasion of tumor cells. Activated platelets promote cancer progression and metastasis. However, the prognostic value of platelet aggregation function in laryngeal cancer remains poorly understood. The purpose of this study was to investigate the predictive significance of platelet aggregation function in laryngeal cancer.
Materials and Methods: Between January 2015 and December 2016, we conducted a retrospective analysis of 203 patients who were diagnosed with laryngeal cancer consecutively. The patients were stratified by platelet aggregation function into two groups: low “adenosine diphosphate induced light transmittance aggregometry (ADP-induced LTA) ≤ 15.1” and high (ADP-induced LTA > 15.1). Pathological tissues from different parts of the operation were collected and the pathologist determined the pathological type. We assessed the prognostic significance of platelet aggregation function using Kaplan-Meier curves and Cox regression.
Results: The low cohort had a significantly higher lymphocyte count than the high cohort. Compared with the high cohort, the low cohort had significantly lower levels of platelet-to-lymphocyte ratio (PLR), ADP-induced LTA, and Interleukins (IL)-6. The ADP-induced LTA (hazard ratio, 1.212; P < 0.001) was independently related with 5-year overall survival rate.
Conclusion: Patients with ADP-induced LTA > 15.1 experience poor outcomes. Platelet aggregation function, when elevated, could be a new prognostic indicator for laryngeal cancer.
Keywords: laryngeal carcinoma, platelet aggregation function, platelet-to-lymphocyte ratio, inflammation, overall survival rate
Introduction
In the mid to late 20th century, laryngeal cancer was one of the most common malignancies of the head and neck region. Due to its high recurrence and metastasis rates, laryngeal cancer can lead to high mortality if not treated in time. However, with the advancement of medical techniques, especially the application of radiotherapy, chemotherapy and biological therapy, the prognosis of laryngeal cancer has improved to some extent. But it remains one of the most prevalent cancers in the head and neck area, posing grave threats to patients’ health and quality of life.1 Although lesion and lymph node removal combined with targeted chemotherapy significantly increased the 5-year survival rate of laryngeal cancer patients, there are still some patients with recurrence, and the prognosis of recurrent patients is extremely poor. Therefore, it is of great clinical significance to predict laryngeal cancer recurrence as early as possible.
It was well known that platelets, especially activated platelets, promote the proliferation, division, and invasion of tumor cells.2 Furthermore, Results from several studies indicate that promoting angiogenesis and immune escape are the key mechanisms of platelets promoting tumor growth.3 In addition, several studies have found that increased platelet count, known as thrombocytosis, correlates with poorer prognosis in patients with various types of cancers, including pancreatic cancer, breast cancer and endometrial cancer. The potential mechanisms linking thrombocytosis and worse clinical outcomes are not yet fully understood but may be related to increased thrombosis, stimulation of tumor proliferation, metastasis and angiogenesis.4 More research is still needed to further establish platelet count as an independent prognostic factor for cancer patients and to elucidate the underlying pathophysiology between thrombocytosis and tumor progression. identifying cancer patients with elevated platelet counts could help stratify patients into different risk groups and guide more personalized treatment approaches to potentially improve clinical outcomes.5
Thrombopoiesis, the production of platelets, is tightly regulated by various factors to maintain platelet counts within a physiological range. However, in cancer patients, the dysregulation of thrombopoiesis can lead to abnormalities in platelet counts. Cancer-associated thrombocytosis, or high platelet counts, may be due to tumors overproducing thrombopoietic growth factors and cytokines that stimulate increased platelet production. Despite this overproduction, platelet counts in some tumor patients may remain within normal ranges due to a compensatory consumption of platelets at the tumor site or other factors. Therefore, the highly compensated mechanism of platelet regulation can mask underlying increases in thrombopoiesis and result in insignificant changes in platelet counts clinically in some cancer patients. Further research is needed to fully elucidate the complex interplay between tumors and platelet regulation and how this contributes to thrombocytosis in cancer. Identifying the mechanisms driving thrombocytosis may allow for development of therapeutic interventions to improve prognosis in cancer patients with elevated platelet counts.6
Additionally, Inflammatory responses have also been shown to be widely involved in tumor growth and metastasis. In particular, multiple inflammatory factors in tumor tissue and patient serum have been proven to be effective in predicting the clinical prognosis of tumor patients, including mortality and postoperative recurrence.7,8
Mechanically speaking, there is also evidence that inflammatory factors may lead to increased platelet counts. Interleukins (IL)-6, IL-10, and transforming growth factor-β (TGF-β) are several typical inflammatory factors, which have been confirmed to be closely related to the occurrence and development of laryngeal cancer. It was found that those inflammatory factors can increase platelet production and inhibit platelet destruction by promoting megakaryocyte proliferation and inhibiting immune response.9
Previous studies have demonstrated the role of platelets and associated inflammatory responses in predicting poor outcomes in patients with malignancies including endometrial, breast, and cervical cancers.10,11 However, the role and clinical significance of platelet aggregation in laryngeal carcinoma still need further elucidation. In the present study, we aimed to analyze the clinical role of platelet aggregation function in determining clinical prognosis in patients with laryngeal cancer.
Objective
Prior studies have demonstrated that activated platelets play a role in promoting the proliferation and metastasis of various cancers. Thrombocytosis, or high platelet count, has been associated with poorer prognosis in some cancer patients as an independent risk factor. However, the clinical utility of using platelet aggregation as a predictive biomarker for prognosis has not been well established in laryngeal cancer patients specifically. The objective of this present study was to analyze the potential prognostic value of platelet aggregation function in predicting clinical outcomes in patients with laryngeal cancer.
Methods
Ethics Statement
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and its later amendments. This retrospective study was conducted with the approval of the Research Ethics Committee of the 2nd Affiliated Hospital of Harbin Medical University, China. Each patient provided written informed consent for undergoing the procedures and for having their data collected and analyzed for research purposes.
Study Design and Participants
We retrospective studied 203 consecutive patients with laryngeal cancer between January 2015 and December 2016, with ethical approval numbers SYDWGZR-2020-128. Among the enrolled patients, all patients underwent laryngeal cancer resection in the Second Affiliated Hospital of Harbin Medical University according to the current treatment guidelines. In addition, pathological tissues from different parts of the operation were collected and the pathologist determined the pathological type. It should be mentioned that in order to exclude the effect of preoperative chemotherapy and radiotherapy on platelet count. None of the enrolled patients received preoperative radiotherapy or chemotherapy. Furthermore, patients with the following conditions that might affect platelet count were also ruled out: patients with hematological disorders, patients with coronary artery disease, patients with hypertension, patients with diabetes mellitus, and patients treatment with anticoagulant, statins, and acetylic salicylic acid.
Setting
The patients were stratified by platelet aggregation function into two groups: low “adenosine diphosphate induced light transmittance aggregometry (ADP-induced LTA) ≤15.1” and high (ADP-induced LTA >15.1). According to previous literature, platelet-to-lymphocyte ratio (PLR) was defined as the number of platelets divided by the number of lymphocytes at the patient’s first admission. Overall survival (OS) was calculated from the date of diagnosis to the date of death or last follow-up. All patients, excluding those who died, were followed for 5 years.
Blood Sampling and Measurement of Cytokine Levels
All patients were admitted to the hospital using venous blood collected to smooth platelet aggregation and estimate hs-CRP levels. Platelet function was analyzed using light transmittance and ADP induction according to the manufacturer’s instructions before operation (four-channel AggRAM Remote Analyzer Module System; Helena Laboratories, Beaumont, TX, USA). Platelet aggregation was induced by ADP of 20μM, and the degree of platelet aggregation was indicated by percentage. hs-CRP level is used to evaluate the inflammation level of laryngeal cancer patients. (BN ProSpec; Siemens, Tarrytown, NY, USA) using Cardiophase hs-CRP reagents. According to the manufacturer’s instructions, we used a commercially purchased ELISA kit to measure the levels of inflammatory factors in patients with laryngeal cancer. (Abcam, Cambridge, UK).
Follow-Up
After consultation with the patients and their consent, we followed up the general conditions of the patients through telephone follow-up and clinical outpatient visits without disturbing their lives. Patients were reviewed at the outpatient clinic every three months for two years after discharge. After that, outpatient visits were repeated every six months for three years.
Statistical Analysis
Quantitative data are expressed as the mean value ± standard deviation, while qualitative data are expressed as frequency (percentage). The independent two-sample t-test was used for comparisons between cohorts. The chi-square test or Fisher’s exact test was used to compare categorical variables, as applicable. A receiver operating characteristic (ROC) curve was generated to find cutoffs of residual ADP-induced platelet aggregation with optimal diagnostic sensitivity and specificity. To estimate survival status, the Kaplan-Meier (KM) technique was utilized, and the Log rank test was used to compare survival distributions. Two-sided P-values of <0.05, were deemed statistically significant. SPSS version 22.0 (SPSS Inc., Chicago, IL, USA) was used to conduct all statistical analyses.
Results
Baseline Characteristics
In the present study, 203 patients with laryngeal cancer were enrolled between January 2015 and December 2016. To analyze the predictive value of platelet aggregation in predicting survival in patients with laryngeal cancer, a ROC analysis was used to verify the optimal cut-off value of platelet aggregation function for predicting 5-year OS in 203 patients. The results demonstrated 15.1% of platelet aggregation gets the best predictive power (Figure 1). It was found that platelet aggregation function predicts cancer prognosis with a sensitivity of 62.86% and a specificity of 74.49% (AUC =0.735, 95% CI: 0.668 to 0.794, p <0.001).
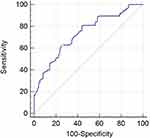 |
Figure 1 Optimized cut-off was determined for residual ADP-induced platelet aggregation using standard ROC curve analysis. |
Then, according to the value of LAT, those patients were divided into 2 groups: patients with ADP-induced LTA ≤15.1% and patients with ADP-induced LTA >15.1%. It was found that there were no significantly differences between the groups regarding baseline clinical characteristics (Table 1). However, it was found that the value of lymphocyte count was significantly higher in the low cohort compared with the high group. Patients with the low LTA exhibited considerably reduced levels of PLR, ADP-induced LTA, and IL-6 compared with those in the high cohort (Table 2).
 |
Table 1 Baseline Characteristics of Patients with Laryngeal Cancer According to Platelet Function Levels |
 |
Table 2 Baseline Laboratory Characteristics of Patients with Laryngeal Cancer According to Platelet Function Levels |
Relationship Between Platelet Aggregation Function and Clinical Outcomes
To compare the clinical outcomes of the two groups, Kaplan-Meier survival analysis was used to observe the 5-year survival of the two groups. As shown in Figure 2, we can observe clearly that patients in high platelet aggregation function level had a worse 5-year OS than those in low group (34.8% vs 72.5%, P <0.001). Moreover, we performed univariate and multivariate analysis to verified whether platelet aggregation function is an independent predictor for poor prognosis in patients with laryngeal cancer. As shown in Table 3, the Age (hazard ratio, 1.021; P =0.043) and ADP-induced LTA (hazard ratio, 1.108; P <0.001) was independently related with 5-year overall survival rate.
 |
Table 3 Univariate Logistic Regression and Cox Regression Analysis for 5-Year Overall Survival in Laryngeal Cancer Patients |
 |
Figure 2 Kaplan-Meier analysis of overall survival in patients with laryngeal cancer. |
Discussion
Until now, no study has investigated the predictive significance of platelet aggregation function for laryngeal cancer. Using a simple, inexpensive, almost universally obtainable test, we found evidence that platelets are activated in laryngeal cancer. The patients suffering poor clinical outcomes have increased platelet aggregation function level (ADP-induced LTA).
Different cancers are developed and progressed by platelets which promote cancer cell proliferation, survival, angiogenesis, and metastasis. According to several researchers, people with thrombocytosis have poorer prognoses compared to those with other cancer types. Mass of clinical reports have suggested that the parameters of platelet activation altered, including β-thromboglobulin (β-TG), soluble P-selectin and CD40 ligand, among patients suffering from cancer.12,13 Nevertheless, these indicators suggested that platelets activation were expensive, which is also not routinely evaluated during clinical studies. Testing the platelet aggregation function is easy and does not add any extra costs, so it might be appropriate for screening clinically. Platelets, whose activation is essential for promoting hypercoagulability in cancer, contribute to accelerating this state. Furthermore, activated platelets could also pave a procoagulant microenvironment for tumor cells to thrive.3 Platelets, endothelial cells, and leukocytes interact in multifactorial ways, which stimulate proinflammatory cytokines to be produced.14
Recently, accessed in view of surrogate peripheral blood-based parameters, the systemic immune-inflammatory response, including neutrophil, platelet count or lymphocyte, has been demonstrated to independently correlate with oncologic outcomes among a number of cancers.15 Medical researchers have gradually recognized the crucial role inflammation plays in tumorigenesis and development. There is an interrelationship between cancer and inflammation. Recent studies have indicated that inflammation represents an essential component of the tumor microenvironment, which act as an essential role in the progression of tumors,7,16 consisting of cellular transformation, proliferation, angiogenesis, invasion, and migration.17 As for several types of cancer, there is also a link between an elevated number of platelets and a poor prognosis.18,19 There is evidence that aggregation and degranulation of platelet are usually preceded by the release of platelet-derived pro-angiogenic factors, which accelerate the growth of tumour.20 As well, platelets that interact with malignant cells could promote the metastasis of tumor.21 Hence, it is of great worthy to access whether the markers associated with platelet-inflammation could act as predictor of the prognosis among patients suffering from laryngeal carcinoma.
Some evidence have suggested that tumor cells could mediate the activation of platelet, thereby accelerate the growth of tumor cells.22,23 On the other hand, high expressions levels of PLR in preoperative peripheral blood could induce a downregulated lymphocyte ratio, which was also demonstrated in our study. A major part of the total leukocyte population is lymphocytes, which act as a significant role in systemic inflammation.24 Lymphocytes regulate immune interactions in microenvironments, hindering the progression of malignant tumors. It is lymphocytes which contribute to the immune surveillance of malignant tumors that inhibit their proliferation and metastasis. As for cancer, elevated expressions of NLR and PLR could serve as identifiers associated with poor prognosis, indicating the inadequate immune response to the tumor. In the present study, patients with ADP-induced LTA greater than 15.1% had a higher PLR. This group with higher PLR showed lower 5-year OS. However, PLR was not independently related with 5-year OS in Cox regression. However, in the Cox regression analysis, PLR was not an independent predictor of 5-year OS.
The underlying mechanisms linking heightened platelet aggregability to reduced survival in laryngeal cancer likely involve multiple interconnected pathways. Firstly, activated platelets can stimulate angiogenesis by releasing pro-angiogenic mediators, providing the nutritive blood supply for tumor growth and spread.25 Secondly, platelets secrete mitogens that directly promote tumor cell proliferation.26 Additionally, aberrant platelet activation elicits abnormal coagulation and microthrombi formation in the tumor microenvironment, altering the niche to favor malignant progression. Platelets may also contribute to immune evasion and inflammation, blunting anti-tumor immune responses.27 Increased platelet aggregability reflects underlying coagulopathy that can heighten surgical bleeding risk, potentially impacting completeness of tumor resection. Finally, platelets may interact with and activate various cell types within the tumor stroma via paracrine signaling.28 Further research into the complex interplay between platelets and the hallmarks of cancer will provide insights into their role as prognostic biomarkers and therapeutic targets in laryngeal cancer.
Limitations
Some limitations in this study need to be pointed out. Firstly, we only included 203 patients with laryngeal cancer from the Second Affiliated Hospital of Harbin Medical University, and the number of patients in this study was small, which may lead to sample deviation. Secondly, our patients are all from northeast China, so there may be regional limitations. Secondly, in our study, due to financial reasons, we only detected the platelet aggregation function and hs-CRP and inflammatory factor levels of patients upon admission, which could not fully reflect the platelet function and inflammatory response of patient. Thirdly, the optimal critical value of ADP-induced LTA determined in the present study is 15.1%. Although the cut-off value was selected by ROC analysis, our data may still show different cut-off values compared with previous studies. Fourthly, more inflammatory factors are needed to reflect the inflammatory state of patients.
Conclusion
Patients with ADP-induced platelet aggregation (LTA) levels greater than 15.1% were found to have significantly worse clinical outcomes. Elevated preoperative platelet aggregation function was identified as an independent risk factor for 5-year mortality in laryngeal cancer patients through multivariate analysis. These findings indicate that increased platelet aggregability may potentially serve as a novel prognostic biomarker associated with poor prognosis in laryngeal cancer. Quantifying platelet aggregation levels could help stratify laryngeal cancer patients into high and low risk groups at initial diagnosis to guide more personalized treatment approaches and closer monitoring of patients with hyperaggregable platelets. Further validation through large prospective studies is still needed before platelet aggregation testing can be widely adapted as a standard prognostic assay clinically.
Data Sharing Statement
In the spirit of openness, the authors are willing to make readily accessible the raw data supporting their conclusions.
Disclosure
No conflicts of interest are declared by the authors.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi:10.3322/caac.21332
2. Sharma D, Brummel-Ziedins KE, Bouchard BA, Holmes CE. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol. 2014;229(8):1005–1015. doi:10.1002/jcp.24539
3. Mezouar S, Frère C, Darbousset R, et al. Role of platelets in cancer and cancer-associated thrombosis: experimental and clinical evidences. Thromb Res. 2016;139:65–76. doi:10.1016/j.thromres.2016.01.006
4. Suzuki K, Aiura K, Kitagou M, et al. Platelets counts closely correlate with the disease-free survival interval of pancreatic cancer patients. Hepatogastroenterology. 2004;51(57):847–853. PMID: 15143932.
5. Qiu J, Yu Y, Fu Y, Ye F, Xie X, Lu W. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J Obstet Gynaecol Res. 2012;38(4):651–657. doi:10.1111/j.1447-0756.2011.01780.x
6. Seretis C, Youssef H, Chapman M. Hypercoagulation in colorectal cancer: what can platelet indices tell us? Platelets. 2015;26(2):114–118. doi:10.3109/09537104.2014.894969
7. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/nature01322
8. Izano M, Wei EK, Tai C, et al. Chronic inflammation and risk of colorectal and other obesity-related cancers: the health, aging and body composition study. Int J Cancer. 2016;138(5):1118–1128. doi:10.1002/ijc.29868
9. Salazar-Onfray F, López MN, Mendoza-Naranjo A. Paradoxical effects of cytokines in tumor immune surveillance and tumor immune escape. Cytokine Growth Factor Rev. 2007;18(1–2):171–182. doi:10.1016/j.cytogfr.2007.01.015
10. Mao Y, Fu Y, Gao Y, Yang A, Zhang Q. Platelet-to-lymphocyte ratio predicts long-term survival in laryngeal cancer. Eur Arch Otorhinolaryngol. 2018;275(2):553–559. doi:10.1007/s00405-017-4849-4
11. Zhang H, Liu L, Fu S, et al. Higher platelet distribution width predicts poor prognosis in laryngeal cancer. Oncotarget. 2017;8(29):48138–48144. doi:10.18632/oncotarget.18306
12. Ay C, Simanek R, Vormittag R, et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). Blood. 2008;112(7):2703–2708. doi:10.1182/blood-2008-02-142422
13. Huang J, Jochems C, Talaie T, et al. Elevated serum soluble CD40 ligand in cancer patients may play an immunosuppressive role. Blood. 2012;120(15):3030–3038. doi:10.1182/blood-2012-05-427799
14. Nording HM, Seizer P, Langer HF. Platelets in inflammation and atherogenesis. Front Immunol. 2015;6:98. doi:10.3389/fimmu.2015.00098
15. Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–163. doi:10.2217/fon.09.136
16. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
17. Mantovani A. Cancer: inflammation by remote control. Nature. 2005;435(7043):752–753. doi:10.1038/435752a
18. Hisada Y, Mackman N. Cancer-associated pathways and biomarkers of venous thrombosis. Blood. 2017;130(13):1499–1506. doi:10.1182/blood-2017-03-743211
19. Takahashi Y, Bucana CD, Akagi Y, et al. Significance of platelet-derived endothelial cell growth factor in the angiogenesis of human gastric cancer. Clin Cancer Res. 1998;4(2):429–434. PMID: 9516932.
20. Iba T, Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2018;16(2):231–241. doi:10.1111/jth.13911
21. Gislason T, Nõu E. Sedimentation rate, leucocytes, platelet count and haemoglobin in bronchial carcinoma: an epidemiological study. Eur J Respir Dis. 1985;66(2):141–146. PMID: 2982631.
22. Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol. 2010;30(12):2362–2367. doi:10.1161/ATVBAHA.110.207514
23. Suzuki K, Aiura K, Ueda M, Kitajima M. The influence of platelets on the promotion of invasion by tumor cells and inhibition by antiplatelet agents. Pancreas. 2004;29(2):132–140. doi:10.1097/00006676-200408000-00008
24. Almeida VH, Rondon AMR, Gomes T, Monteiro RQ. Novel aspects of extracellular vesicles as mediators of cancer-associated thrombosis. Cells. 2019;8(7):716. doi:10.3390/cells8070716
25. Zhu Y, Si W, Sun Q, et al. Platelet-lymphocyte ratio acts as an indicator of poor prognosis in patients with breast cancer. Oncotarget. 2017;8(1):1023–1030. doi:10.18632/oncotarget
26. Cai H, Zhang ZH, Zhou YJ, Liu J, Chen HQ, Lin RY. The prognostic value of preoperative plasma fibrinogen and neutrophil-to-lymphocyte ratio in patients with laryngeal squamous cell carcinoma. Ear Nose Throat J. 2021;100(10):731–736. doi:10.1177/0145561320920746
27. Fang Y, Yang Y, Chen M, et al. Elevated peripheral inflammatory markers are related with the recurrence and canceration of vocal fold leukoplakia. Eur Arch Otorhinolaryngol. 2019;276(10):2857–2864. doi:10.1007/s00405-019-05576-5
28. Hu X, Tian T, Sun Q, et al. Prognostic value of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in laryngeal cancer: what should we expect from a meta-analysis? Front Oncol. 2022;12:945820. doi:10.3389/fonc.2022.945820
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
