Back to Journals » Journal of Inflammation Research » Volume 16
Prognostic Value of Fibrinogen-to-Albumin Ratio in Coronary Three-Vessel Disease
Authors Li X , Wang Z, Zhu Y , Lv H, Zhou X, Zhu H , Liu J, Guo L
Received 5 October 2023
Accepted for publication 28 November 2023
Published 1 December 2023 Volume 2023:16 Pages 5767—5777
DOI https://doi.org/10.2147/JIR.S443282
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Xinsheng Li,1,* Zhongzhen Wang,1,* Yifan Zhu,2 Haichen Lv,1 Xuchen Zhou,1 Hao Zhu,1 Jinqiu Liu,1 Lei Guo1
1Department of Cardiology, the First Affiliated Hospital of Dalian Medical University, Dalian City, People’s Republic of China; 2Department of Cardiology, Shengjing Hospital of China Medical University, Shenyang City, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lei Guo; Jinqiu Liu, Department of Cardiology, the First Affiliated Hospital of Dalian Medical University, 222 Zhongshan Road, Dalian City, 116011, People’s Republic of China, Tel +86 411-83635963, Email [email protected]; [email protected]
Objective: To investigate the prognostic value of fibrinogen-to-albumin ratio (FAR) in the adverse outcomes of patients with coronary three-vessel disease (TVD).
Methods: A total of 4061 patients with TVD between 2013 and 2018 were analyzed in this retrospective cohort study. The best cut‑off value of the FAR determined by receiver operating characteristic (ROC) curve analysis was 0.084. 2782 (68.5%) patients were in the low FAR group (FAR < 0.084) and 1279 (31.5%) patients were in the high FAR group (FAR ≥ 0.084), respectively. Three multivariate Cox proportional hazards models were applied to determine the associations of FAR with clinical outcomes. The concordance index (C-index), net reclassification index (NRI), and integrated discrimination improvement (IDI) were used to assess the incremental predictive value of the FAR and baseline models with respect to the additive effects of the established traditional risk factors on the discrimination of clinical outcomes. The primary endpoint was all-cause mortality. The secondary endpoint was major adverse cardiac and cerebrovascular events (MACCEs).
Results: The median follow-up duration was 2.4 years (range 1.1– 4.1 years). Multivariate Cox regression analyses showed that the incidence of all-cause mortality (4.7% vs 2.2%, adjusted hazard ratio [HR] 1.68, 95% confidence interval [CI] 1.12– 2.52, p=0.011) and MACCE (34.6% vs 27.3%, HR 1.28, 95% CI 1.13– 1.46, p< 0.001) were significantly higher in the high FAR group compared to the low FAR group. The C-index was 0.72 (p < 0.001), the value of NRI was 0.3778 (p < 0.001), and the value of IDI was 0.0098 (p < 0.001) for those with FAR. After FAR was added to the traditional model, the discrimination and risk reclassification ability can be significantly improved for all-cause mortality. The similar results were found for MACCE.
Conclusion: Higher level of FAR was associated with all-cause mortality and MACCE among patients with TVD. FAR could help to improve the prognostic performance of the traditional risk factors for TVD patients.
Keywords: three-vessel disease, fibrinogen-to-albumin ratio, mortality, major adverse cardiac and cerebrovascular events, coronary artery disease
A Letter to the Editor has been published for this article.
Introduction
Inflammation plays an important role in the development and progression of atherosclerotic coronary artery disease (CAD).1 Three-vessel disease (TVD) is a major challenge in coronary interventions, with three-vessel disease detected in 30% of patients undergoing diagnostic coronary angiography.2 The inflammatory state of patients with three-vessel disease is more prominent when compared with that of single-vessel disease.3 In addition, the risk of mortality in patients with three-vessel disease is almost twice as high as in patients with single-vessel disease and is an independent predictor of adverse clinical outcomes compared to single-vessel disease.4
Fibrinogen, a plasma protein produced by the liver, is an indicator of thrombotic status and plays a role in inflammatory processes of varying degrees of severity.5 Baseline plasma fibrinogen levels are predictive of cardiovascular events in patients with CAD.6 Albumin is a protein synthesized in the liver that influences nutrient absorption, colloid pressure and systemic inflammation. It has been suggested that serum albumin concentrations are associated with inflammatory and hemostatic processes.7 Low serum albumin levels are associated with increased cardiovascular mortality and morbidity.8
Recently, fibrinogen-to-albumin ratio (FAR), the proportion of these two indexes, has been reported as a new inflammatory marker that is strongly associated with poor prognosis in a variety of cancers, including esophageal, hepatocellular, and breast cancers.9–11 In addition, the FAR has been strongly correlated with the severity of CAD and with a poor clinical prognosis in patients with cardiovascular disease.12,13
However, there are no data regarding the prognostic value of FAR on clinical outcomes in patients with TVD. Thus, the aim of the present study is to investigate the relationship between FAR and long-term clinical outcomes in patients with TVD.
Methods
Study Population
From January 2013 to December 2018, consecutive patients underwent coronary angiography at our institution were retrospectively reviewed and those 4308 (31.0%) patients with TVD were recruited. The demographic, angiographic, and procedural characteristics were reviewed in detail, collected, and analyzed from the electronic medical record database. The exclusion criteria included patients with unavailable data on serum albumin and fibrinogen at baseline, acute infection, immune system diseases, severe hepatic injury, end-stage renal failure, and malignant tumors (Figure 1). Enrolled patients were routinely followed up through telephone interview or examination of medical records.
 |
Figure 1 The participant flow chart. Abbreviations: FAR, fibrinogen-to-albumin ratio; TVD, three-vessel disease. |
Ethics
This single-center, retrospective observational study was approved by the Ethics Committee of the First Affiliated Hospital of Dalian Medical University (Ethics No: YJ-KY-FB-2021-10). Study was conducted in full conformance with the principles of the Declaration of Helsinki. All participants provided their written informed consent.
Data Collection
All laboratory indices were measured using standard methods in the Central Laboratory of the First Affiliated Hospital of Dalian Medical University. The FAR was defined as the proportion of the plasma fibrinogen (g/L) to the plasma albumin level (g/L). All patients received venous blood samples from the antecubital veins within 24 hours after admission, which was performed prior to coronary angiography. Plasma fibrinogen levels were measured using an automatic coagulation analyzer (STAGO, FRANCE). Serum albumin concentrations were measured using an automated chemistry analyzer (HITACHI, JAPAN).
Procedures
Patients were treated with percutaneous coronary intervention (PCI), coronary artery bypass graft surgery (CABG) or medication along. Revascularization includes PCI or CABG. PCI was performed by experienced cardiologists in accordance with current guidelines and using conventional techniques.14 CABG procedures could be done with extracorporeal circulation, and use of arterial conduits was encouraged.
Clinical Endpoints
The primary endpoint was defined as all‑cause mortality during follow‑up and the secondary endpoint was a composite of major adverse cardiac and cerebrovascular events (MACCE), including all-cause mortality, myocardial infarction (MI), stroke, unscheduled repeat revascularization and rehospitalization due to angina pectoris or heart failure. Any coronary revascularization was defined as either PCI or CABG for any reason.15
Statistical Analysis
The continuous variables are presented as mean ± standard deviation or as medians and interquartile ranges. The continuous variables were compared using the Student’s t-test or Mann–Whitney U-test where appropriate, and categorical variables were compared using the chi-square test. The clinical outcomes were analyzed using the Kaplan-Meier method and compared by Log rank test. To assess the impact of potential confounders on the relationship between FAR and long-term outcomes, three multivariate Cox proportional hazards models were developed. Model 1 with the adjustment for age, sex and smoking; Model 2 with the adjustment for variables included in Model 1 plus hypertension, diabetes, dyslipidemia and chronic kidney disease (CKD); Model 3 with the adjustment for variables included in Model 2 plus left ventricular ejection fraction (LVEF), acute coronary syndrome (ACS), left main trunk (LMT) involvement, revascularization in hospital and Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) score. The receiver operating characteristic (ROC) curve analysis was also performed to determine the best cut‑off value of the FAR in predicting all‑cause mortality, based on which all patients were divided into a high FAR group and a low FAR group. The concordance index (C-index), net reclassification index (NRI), and integrated discrimination improvement (IDI) were used to assess the incremental predictive value of the FAR and baseline models with respect to the additive effects of the established traditional risk factors on the discrimination of clinical outcomes. NRI and IDI are two alternatives to the area under curve (AUC) to assess predictive accuracy of the model and improvement in risk prediction, and measure the usefulness of a new model. A P-value <0.05 was considered significant. All statistical analyses were performed by SPSS 24.0, Stata 15 and R 4.1.3.
Results
Baseline Characteristics
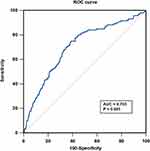
The baseline clinical, angiographic and procedural characteristics and biochemical data of the 4061 studied patients are summarized in Table 1. 3448 (84.9%) underwent PCI, and 160 (3.9%) underwent CABG, and 453 (11.2%) patients were treated with medication. The best cut‑off value of the FAR determined by ROC curve analysis in predicting all‑cause mortality was 0.084 (Figure 2). 2782 (68.5%) patients were in the low FAR group and 1279 (31.5%) patients were in the high FAR group, respectively. Patients in the high FAR group were more likely to be older and to have male patients and to have a history of hypertension, diabetes and CKD than patients in the low FAR group. ACS was more prevalent in the high FAR group. Compared with patients in the low FAR group, lipid profile, hemoglobin, estimated glomerular filtration rate (eGFR) and LVEF were lower among patients in the high FAR group. However, B-type natriuretic peptide (BNP), high-sensitivity C-reactive protein (hs-CRP) and fasting blood glucose were higher among patients in the high FAR group. There was no difference in smoking, history of MI, cerebrovascular disease and peripheral artery disease. SYNTAX score and revascularization in hospital were common between the two group.
 |
Table 1 Baseline Clinical, Angiographic and Procedural Characteristics in Patients with TVD According to FAR |
Clinical Outcomes
The median follow-up was 2.4 (1.1–4.1) years. During the follow-up period, the rate of all-cause mortality was 4.7% and 2.2% in high and low FAR group, respectively. The incidence of clinical outcomes is shown in Table 2. Kaplan-Meier survival curves for all-cause mortality and MACCE are shown in Figure 3. Table 2 shows three multivariate Cox proportional hazard models to determine the correlation between the FAR groups and clinical outcomes. Multivariate Cox regression analyses showed that the incidence of all-cause mortality (adjusted hazard ratio [HR] 1.68, 95% confidence interval [CI] 1.12–2.52, p=0.011), MACCE (HR 1.28, 95% CI 1.13–1.46, p<0.001), MI (HR 1.69, 95% CI 1.37–2.08, p<0.001) and heart failure readmission (HR 1.58, 95% CI 1.25–2.00, p<0.001) were significantly higher in the high FAR group compared to the low FAR group after adjusting for age, sex, smoking, hypertension, diabetes, dyslipidemia, CKD, LVEF, ACS, LMT involvement, revascularization in hospital and SYNTAX score according to the multivariable Model 3. Notably, these differences were also present in Model 1 and 2, which different risk factors were adjusted.
 |
Table 2 Association Between FAR and Clinical Outcomes in Patients with TVD |
Adding FAR to the Models of Established Traditional Risk Factors
Regarding all-cause mortality, compared with the traditional model (including age, gender, smoking, hypertension, diabetes mellitus and dyslipidemia) (AUC = 0.62), after FAR was added to the traditional model, the discrimination and risk reclassification ability can be significantly improved (Table 3). The C-index was 0.72 (p < 0.001), the value of NRI was 0.3778 (p < 0.001), and the value of IDI was 0.0098 (p < 0.001) for those with FAR. That means with the addition of FAR, the models had incremental prognostic value for all-cause mortality. The similar results were found for MACCE, which the discrimination and risk reclassification ability were significantly improved after FAR was added to the traditional model (all p < 0.001). Additionally, to assess inflammatory risk factors more fully, commonly available inflammatory biomarkers including hs-CRP, BNP and neutrophil-to-lymphocyte ratio were added to above traditional model. The similar results were found for all-cause mortality and MACCE, which the discrimination and risk reclassification ability were significantly improved after FAR was added to inflammatory risk model (all p < 0.05) (Table 4).
 |
Table 3 Evaluation of Predictive Models for Clinical Outcomes Using the C-Index, NRI and IDI |
 |
Table 4 Evaluation of Predictive Models for Endpoints Before and After Adding the FAR to Established Inflammatory Risk Factors |
Discussion
To our knowledge, this is the first and the largest study to report the relationship among the FAR and long-term clinical outcomes in patients with TVD, and reveal the good predictive value of FAR for adverse outcomes in TVD patients through a large cohort.
Among CAD patients, those with TVD were most susceptible to cardiovascular adverse events. It is widely acknowledged that TVD is characterized by a persistent low-grade inflammatory state. Furthermore, compared to single vessel disease, TVD patients exhibit a more prominent inflammatory state. Current evidence suggests that inflammation plays a key role in all stages of coronary atherosclerotic thrombosis, including plaque progression, rupture, and thrombosis leading to acute MI.16
Plasma fibrinogen is involved in chronic low-grade inflammatory processes by upregulating the expression of pro-inflammatory cytokines, activating platelets and upregulating the expression of adhesion molecules, inducing vascular inflammation and endothelial dysfunction, and enhancing macrophage infiltration, thereby exacerbating atherosclerotic plaque development.17,18 Furthermore, it has been reported that fibrinogen may accelerate platelet aggregation by binding to GPIIb/IIIa receptors on the platelet surface.19 Therefore, fibrinogen promotes the onset and progression of coronary atherosclerosis and contributes to the development of ACS via its interactions with other inflammatory cells, vascular endothelium and pro-thrombotic molecules.5 Previous study reported that the high value of fibrinogen was an independent risk factor for ACS.20
The rate of albumin synthesis is influenced by nutritional intake and systemic inflammation. Serum albumin possesses a variety of physiological properties such as anti-inflammatory, antioxidant, anticoagulant, antiplatelet aggregation and maintenance of capillary membrane stability.21 In addition, serum albumin is an important inhibitor of platelet activation and aggregation and an important mediator of platelet induction. There is evidence that hypoalbuminemia is associated with adverse cardiac events in patients with CAD, heart failure, atrial fibrillation and stroke.22–24
Initially, as a recognized marker of inflammation, FAR, which comprised fibrinogen and albumin, was a well-established prognostic factor in esophageal squamous cell carcinoma, breast cancer and hepatocellular carcinoma.9–11 FAR was significantly associated with angiographic no-reflow, angiographic severity and adverse outcome in patients with ST‑elevation myocardial infarction (STEMI) undergoing PCI.25–27 Karahan et al showed that FAR was significantly related to SYNTAX score in predicting the severity of CAD in patients with STEMI.28 Simultaneously, FAR was an independent predictor for major adverse cardiac events (MACE) in CAD patients after stents implantation.13
In the present research, we compared the inflammatory load revealed by FAR of patients with TVD. The rate of all-cause mortality and MACCE was significantly high in the high FAR group compared with the low FAR group in the three multivariable Cox regression analysis models after adjustment for other independent variables. C-index, NRI and IDI were calculated and showed that the discrimination and risk reclassification ability were significantly improved after FAR was added to the traditional risk model. Taken together, our study demonstrated that higher level of FAR was strongly related to increased risks of long-term adverse outcomes in patients with TVD, for the first time. Therefore, FAR, could be helpful to identify high-risk individuals in patients with TVD. Interestingly, we observed no significant differences between groups in terms of risk for some other components of MACCE, which warrants further exploration in future studies.
Both fibrinogen and albumin are important equivalent of hemorheological and inflammatory alteration. Elevated fibrinogen levels are similar to decreased albumin levels and may be associated with pro-inflammatory cytokine expression, endothelial dysfunction, and platelet aggregation. As a result of the interaction, the high ratio of fibrinogen to albumin seems to indicate the presence of a chronic inflammatory load. Those above may be potential mechanisms by which FAR is associated with poor prognosis in patients with TVD.
In the CANTOS study, IL-1β targeting Canakinumab, which was an anti-inflammatory therapy, significantly reduced the incidence of MACE in patients previous MI.29 Tranexamic acid, the inhibition of fibrinolysis, has been shown to reduce inflammatory response in patients who underwent cardiopulmonary bypass.30 Therefore, the targeted therapeutic strategies of FAR in TVD patients should be further investigated.
Limitations
This study has several limitations. First, the level of FAR was only calculated at baseline, and no further analysis of FAR during the follow-up period was performed. Second, as the nature of the observational studies, potential confounders could not be adequately adjusted. Third, cardiovascular cause-specific mortality was not obtained in this study. Fourth, since both fibrinogen and albumin both are acute-phase reactants, the exact time of fibrinogen and albumin collection was not recorded and adjusted, which may have affected the results. Fifth, inflammatory conditions like cancer, autoimmune disorders, and recent infection were not adjusted in multivariate models. Large-scale randomized controlled studies are still needed to further assess the predictive value of FAR on the prognosis of patients with TVD.
Conclusion
Higher level of FAR was associated with all-cause mortality and MACCE among patients with TVD. FAR could help to improve the prognostic performance of the traditional risk factors as well. Thus, the FAR may serve as a convenient, effective, and noninvasive biomarker to predict the prognosis, and to improve risk stratification in TVD patients. Large-scale randomized controlled studies are still needed to further assess the predictive value of FAR on the prognosis of patients with TVD.
Abbreviations
FAR, fibrinogen albumin ratio; TVD, three-vessel disease; MACCE, major adverse and cerebrovascular events; CAD, coronary artery disease; ACS, acute coronary syndrome.
Data Sharing Statement
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgment
The authors would like to graciously acknowledgement all participants of the study and the interventional cardiologists and surgeons from the First Affiliated Hospital of Dalian Medical University for their invaluable assistance.
Funding
This study was supported by the National Natural Science Foundation of China (82170252).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–327. doi:10.1161/CIRCRESAHA.118.313591
2. Head SJ, Milojevic M, Daemen J, et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet. 2018;391(10124):939–948. doi:10.1016/S0140-6736(18)30423-9
3. Liu Y, Zhu Y, Wang J, et al. Gender-based long-term outcomes after revascularization for three-vessel coronary disease: a propensity score-matched analysis of a large cohort. Clin Interv Aging. 2022;17:545–554. doi:10.2147/CIA.S362027
4. Guo L, Lv H, Wang J, et al. Predictive value of high sensitivity C-reactive protein in three-vessel disease patients with and without type 2 diabetes. Cardiovasc Diabetol. 2023;22(1):91. doi:10.1186/s12933-023-01830-7
5. Papageorgiou N, Tousoulis D, Siasos G, et al. Is fibrinogen a marker of inflammation in coronary artery disease? Hellenic J Cardiol. 2010;51(1):1–9.
6. Peng Y, Wang H, Li YM, et al. Relation between admission plasma fibrinogen levels and mortality in Chinese patients with coronary artery disease. Sci Rep. 2016;6(1):30506.
7. Roche M, Rondeau P, Singh NR, et al. The antioxidant properties of serum albumin. FEBS Lett. 2008;582(13):1783–1787. doi:10.1016/j.febslet.2008.04.057
8. Plakht Y, Gilutz H, Shiyovich A. Decreased admission serum albumin level is an independent predictor of long-term mortality in hospital survivors of acute myocardial infarction. Soroka Acute Myocardial Infarction II (SAMI-II) project. Int J Cardiol. 2016;219:20–24. doi:10.1016/j.ijcard.2016.05.067
9. Tan Z, Zhang M, Han Q, et al. A novel blood tool of cancer prognosis in esophageal squamous cell carcinoma: the Fibrinogen/Albumin Ratio. J Cancer. 2017;8(6):1025–1029. doi:10.7150/jca.16491
10. Wu W, Wang Q, Han D, et al. Prognostic value of preoperative inflammatory markers in patients with hepatocellular carcinoma who underwent curative resection. Cancer Cell Int. 2021;21(1):500. doi:10.1186/s12935-021-02204-3
11. Yang Q, Liang D, Yu Y, et al. The prognostic significance of the fibrinogen-to-albumin ratio in patients with triple-negative breast cancer: a retrospective study. Front Surg. 2022;9:916298. doi:10.3389/fsurg.2022.916298
12. Li M, Tang C, Luo E, et al. Relation of fibrinogen-to-albumin ratio to severity of coronary artery disease and long-term prognosis in patients with Non-ST elevation acute coronary syndrome. Biomed Res Int. 2020;2020:1–10.
13. Hou XG, Wu TT, Zheng YY, et al. The fibrinogen-to-albumin ratio is associated with poor prognosis in patients with coronary artery disease: findings from a large cohort. J Cardiovasc Transl Res. 2023;16(5):1177–1183. doi:10.1007/s12265-023-10402-9
14. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–e646. doi:10.1161/CIR.0000000000000678
15. Guo L, Zhong L, Chen K, et al. Long-term clinical outcomes of optimal medical therapy vs. successful percutaneous coronary intervention for patients with coronary chronic total occlusions. Hellenic J Cardiol. 2018;59(5):281–287. doi:10.1016/j.hjc.2018.03.005
16. Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):973–979. doi:10.1056/NEJM199704033361401
17. Kryczka KE, Kruk M, Demkow M, et al. Fibrinogen and a triad of thrombosis, inflammation, and the renin-angiotensin system in premature coronary artery disease in women: a new insight into sex-related differences in the pathogenesis of the disease. Biomolecules. 2021;11(7):1036. doi:10.3390/biom11071036
18. Jensen T, Kierulf P, Sandset PM, et al. Fibrinogen and fibrin induce synthesis of proinflammatory cytokines from isolated peripheral blood mononuclear cells. Thromb Haemost. 2007;97(5):822–829. doi:10.1160/TH07-01-0039
19. Induruwa I, Moroi M, Bonna A, et al. Platelet collagen receptor Glycoprotein VI-dimer recognizes fibrinogen and fibrin through their D-domains, contributing to platelet adhesion and activation during thrombus formation. J Thromb Haemost. 2018;16(2):389–404. doi:10.1111/jth.13919
20. Shi Y, Wu Y, Bian C, et al. Predictive value of plasma fibrinogen levels in patients admitted for acute coronary syndrome. Tex Heart Inst J. 2010;37(2):178–183.
21. Prajapati KD, Sharma SS, Roy N. Current perspectives on potential role of albumin in neuroprotection. Rev Neurosci. 2011;22(3):355–363. doi:10.1515/rns.2011.028
22. Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. 2018;52:8–12. doi:10.1016/j.ejim.2018.04.014
23. Batta A, Sharma YP, Hatwal J, et al. Predictors of dementia amongst newly diagnosed non-valvular atrial fibrillation patients. Indian Heart J. 2022;74(6):505–509. doi:10.1016/j.ihj.2022.11.009
24. Ozkan E, Elcik D, Barutcu S, et al. Inflammatory markers as predictors of atrial fibrillation recurrence: exploring the C-reactive protein to albumin ratio in cryoablation patients. J Clin Med. 2023;12(19):6313. doi:10.3390/jcm12196313
25. Zhao Y, Yang J, Ji Y, et al. Usefulness of fibrinogen-to-albumin ratio to predict no-reflow and short-term prognosis in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Heart Vessels. 2019;34(10):1600–1607. doi:10.1007/s00380-019-01399-w
26. Liu G, Fan C-M, Guo H, et al. Fibrinogen‑to‑albumin ratio predicts long‑term outcomes for patients with ST‑elevation myocardial infarction and multivessel disease: a prospective observational cohort study. Exp Ther Med. 2021;21(5):465. doi:10.3892/etm.2021.9896
27. Makkar K, Sharma YP, Batta A, et al. Role of fibrinogen, albumin and fibrinogen to albumin ratio in determining angiographic severity and outcomes in acute coronary syndrome. World J Cardiol. 2023;15(1):13–22. doi:10.4330/wjc.v15.i1.13
28. Karahan O, Acet H, Ertaş F, et al. The relationship between fibrinogen to albumin ratio and severity of coronary artery disease in patients with STEMI. Am J Emerg Med. 2016;34(6):1037–1042. doi:10.1016/j.ajem.2016.03.003
29. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi:10.1056/NEJMoa1707914
30. Jiménez JJ, Iribarren JL, Brouard M, et al. Safety and effectiveness of two treatment regimes with tranexamic acid to minimize inflammatory response in elective cardiopulmonary bypass patients: a randomized double-blind, dose-dependent, Phase IV clinical trial. J Cardiothorac Surg. 2011;6(1):138. doi:10.1186/1749-8090-6-138
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.