Back to Journals » International Journal of General Medicine » Volume 17
Prognostic Value of CRP–Albumin–Lymphocyte (CALLY) Index in Patients Undergoing Surgery for Breast Cancer
Authors Zhuang J, Wang S, Wang Y, Wu Y, Hu R
Received 2 November 2023
Accepted for publication 5 March 2024
Published 15 March 2024 Volume 2024:17 Pages 997—1005
DOI https://doi.org/10.2147/IJGM.S447201
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jiaru Zhuang,1,* Shan Wang,2,* Yuan Wang,2 Yibo Wu,2 Renjing Hu1
1Department of Laboratory Medicine, Jiangnan University Medical Center, Wuxi, People’s Republic of China; 2Human Reproductive and Genetic Center, Affiliated Hospital of Jiangnan University, Wuxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Renjing Hu, Department of Laboratory Medicine, Jiangnan University Medical Center, 68 Zhongshan Road, Wuxi, Jiangsu, 214000, People’s Republic of China, Email [email protected] Yibo Wu, Human Reproductive and Genetic Center, Affiliated Hospital of Jiangnan University, 1000 Hefeng Road, Wuxi, Jiangsu, 214000, People’s Republic of China, Email [email protected]
Purpose: According to the 2023 global cancer data, breast cancer is the most common malignant tumor among women in the world. Its occurrence and development is influenced by inflammation, nutrition, and immune status. Therefore, this study combines C-reactive protein (CRP), albumin, and lymphocyte, which can reflect the above states, to form the CRP-albumin-lymphocyte (CALLY) index, an indicator to evaluate its relationship with overall survival (OS) and disease-free survival (DFS) in breast cancer patients.
Patients and Methods: We retrospectively analyzed the clinical and follow-up data of 174 patients with breast cancer. The optimal cutoff for the preoperative CALLY index was identified by considering the area under the receiver operating characteristic curve; subsequently, the discriminatory ability of the cutoff was determined. The effect of the CALLY index on overall survival (OS) and disease-free survival (DFS) was analyzed using the Kaplan–Meier method and the Cox proportional hazards model. The CALLY index was calculated as: (Albumin × Lymphocyte)/(CRP × 104).
Results: The cut-off value of the CALLY index was determined at 2.285. With a cut-off value of 2.285, patients were divided into two groups: those with CALLY < 2.285 and those with CALLY ≥ 2.285. CALLY Index ≥ 2.285 was associated with better survival outcomes. Multivariate Cox analysis showed that TNM stage and CALLY index were prognostic factors that affected OS and DFS.
Conclusion: The CALLY index is a new prognostic biomarker for breast cancer patients after surgery. This new CALLY index allows for suitable patients with a poor prognosis to receive postoperative adjuvant therapy.
Keywords: breast cancer, prognosis, CALLY index, survival
Introduction
Breast cancer (BC) is one of the most common cancers among women. This malignant tumor has a harmful impact on patients quality of life and is a significant public health issue.1 Recent data from China shows that the incidence of breast cancer is significantly increasing, especially in developed coastal cities.2 Experts predict that in the future, the incidence rate of breast cancer in China will reach a staggering 100 cases per 100,000 postmenopausal women.3 Although the current treatment methods for breast cancer include surgery, adjuvant chemotherapy, radiation therapy, targeted therapy, immunotherapy, and traditional Chinese medicine, the outcomes for patients are still unsatisfactory.4 Therefore, there is an urgent need for effective and accessible methods for BC patients to optimize risk stratification and predict postoperative survival outcomes.
The development of breast cancer is influenced by many factors, including inflammation levels, nutritional status, and immune function.5 Cancer-related systemic inflammatory response is a key indicator of tumor progression, and patients with breast cancer and higher levels of inflammation have a higher risk of death than those with lower levels of inflammation.6,7 Nutritional status also plays an important role in the prognosis of breast cancer patients, and research has shown that malnutrition is associated with poorer overall survival (OS) in breast cancer patients.8 In addition, good immune function is the main defense against the progression of breast cancer. It has been reported that the prognosis of breast cancer patients with poor immune function is much worse than that of patients with good immune function.9 Based on the above theories and research, we believe that indicators that comprehensively reflect inflammation levels, nutritional status, and immune function can better predict the prognosis of breast cancer patients.
In previous studies, hematological indicators have commonly been used to reflect inflammation levels, nutritional status, and prognosis.10 Firstly, C-reactive protein (CRP) is a common clinical indicator that can reflect the level of inflammation in breast cancer patients.11 Secondly, for decades, serum albumin has been used as an indicator of clinical nutritional status.12 Thirdly, lymphocyte count is a traditional biomarker for immune function.13 Finally, we found that the CRP-albumin-lymphocyte (CALLY) index (a parameter developed by Hiroya Iida et al) combines CRP, albumin, and lymphocytes, and is a prognostic factor for liver cancer patients.14 However, until now, no studies have shown the prognostic value of the CALLY index in breast cancer. Therefore, we retrospectively analyzed the relationship between overall survival (OS) and disease-free survival (DFS) of 174 breast cancer patients and the preoperative CALLY index to identify the best independent prognostic factors.
Materials and Methods
Study Design and Population
This study investigated 174 female breast cancer patients recently diagnosed with non-metastatic invasive breast cancer. All patients were pathologically diagnosed with primary breast cancer and received initial surgical treatment at Jiangnan University Affiliated Hospital from April 2017 to July 2018. Selection criteria: (1) All patients were diagnosed with invasive breast cancer by pathological examination. (2) Complete pre-treatment laboratory data were available. (3) with five-years follow-up information. Exclusion criteria: (1) There were other anti-cancer treatments before surgery. (2) They had an active infection, haematological or autoimmune disease. This research complies with the Declaration of Helsinki and was approved by the Medical Ethics Committee of the Affiliated Hospital of Jiangnan University (JNMS04202301073). All data are anonymous and aggregated, so the requirements for informed consent are waived.
Data Collection
Retrieving demographic, clinical, and pathological data of 174 patients from the database of the affiliated hospital of Jiangnan University. Body Mass Index (BMI) was categorized into <18.5, 18.5–23.9, and >23.9. Cancer stage (including tumor size, axillary lymph node positivity, and TNM) was evaluated for each patient, according to the eighth edition of the American Joint Committee on Cancer (AJCC) staging manual.15 Serum biochemistry, including laboratory data (lymphocytes, CRP, and albumin), was performed at baseline follow-up before surgery. Follow-up information was derived from outpatient reviews and telephone interviews. The follow-up endpoints of this study were overall survival (OS) and disease-free survival (DFS).
Follow-Up
According to the guidelines of the European Society of Oncology (ESMO) and the National Comprehensive Cancer Network (NCCN), breast cancer patients are followed up and rechecked every 3 months for the first 2 years after surgery, and every 6 months for 3–5 years. Follow-up included clinical examination with laboratory tests, breast ultrasound, mammography, and other tests as deemed appropriate. OS is calculated as the time from the date of tumor resection to the date of death or the end of follow-up. DFS is defined as the time from surgical resection to local recurrence, which was conducted until May 1, 2023, through outpatient review and telephone follow-up.
Statistical Analysis
The statistical analysis used SPSS 26.0 to process and analyze the data. We report categorical data as numbers and percentages. Continuous variables are expressed as median (interquartile range). For continuous and categorical variables, we employed the Mann–Whitney U and chi-square tests, respectively, for identifying intergroup differences in clinicopathological features. To identify the optimal cutoff values of serum biomarkers for OS, receiver operating characteristic (ROC) curves subjected to Youden’s index correction were obtained; the corresponding area under the ROC curve (AUC) values were also calculated. For the survival analysis, we estimated the OS and DFS by using Kaplan–Meier analysis and determined intergroup survival differences through the Log rank test. We used the Cox proportional hazards model to identify independent DFS and OS risk factors; the hazard ratio (HR) and 95% confidence interval (CI) for each factor are presented. Factors considered important in univariate analysis and stepwise regression were used in multivariate models to identify independent prognostic factors.
Results
Characteristics of Study Population
The demographic and clinical pathological features of the patients, and their preoperative laboratory examination results, are summarized in Table 1. In this research, 174 women who were recently diagnosed with a non-metastatic invasive breast cancer were included. All of these women were given surgery as their first treatment. The median age of all patients was 50 years (range: 29 to 75 years). Invasive ductal carcinoma was the histologic type of all primary tumors. 8 (4.6%) patients were underweight, 83 (47.7%) were of normal weight, and 83 (47.7%) were overweight. Of the total of 174 patients, 71 (40.8%) were classified as Stage I, 77 (44.3%) as Stage II, and 26 (14.9%) as Stage III, according to the AJCC classification. In terms of tumor size, 96 patients (55.2%) had T1, 73 patients (42.0%) had T2, and 5 patients (2.9%) had T3. Axillary lymph nodes were negative in 103 cases (59.2%), 1–3 positive in 47 cases (27.0%), 4–9 positive in 19 cases (10.9%), and 10 or more positive in 5 cases (2.9%). 68.4% of the patients had a positive ER status, while the remaining 31.6% had a negative ER status. Out of the 174 PR results, 59.2% were positive and 40.8% were negative. For HER-2, 27.0% of the results were positive and the remaining 73.0% were negative. The sample population was separated into two categories based on the CALLY index; those with a value above 2.285 and those with a value below. The high CALLY index group had 112 individuals (64.4%) while the low CALLY index group had 62 (35.6%). The distribution of clinicopathological and demographic features in the low CALLY index (<2.285) and high CALLY index (≥2.285) groups is detailed in Table 1.
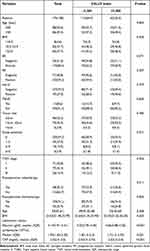 |
Table 1 Clinicopathological Characteristics of 174 Patients with Breast Cancer |
ROC Curve Analysis
The ROC curve analysis showed that the area under the curve of the CALLY index was 0.730, and the CALLY value corresponding to the maximum value of the Youden index was taken as the optimal critical point, and the optimal OS cut-off value of the CALLY index was 2.285 (sensitivity 71.7%, specificity 72.4%, p<0.001; Figure 1). To assess the prognostic distinction between the CALLY index and its constituent elements, serum albumin levels, lymphocyte count, and CRP levels, we compared their respective ROC curves and AUCs. These comparisons are listed in Table 2. The CALLY index was found to have a higher AUC (0.730, 95% CI: =0.637–0.824, p<0.001) than albumin (0.707, 95% CI: =0.599–0.816, p<0.001), lymphocyte count (0.629, 95% CI: =0.530–0.729, p<0.05) and CRP (0.722, 95% CI: =0.624–0.820, p<0.001). In addition, we also evaluated different combinations of dual parameters, such as Albumin+ Lymphocyte, Albumin+ CRP, Lymphocyte+ CRP (Table 2). The CALLY index has the highest prognostic accuracy, allowing us to further evaluate its potential to predict breast cancer OS and DFS.
 |
Table 2 Comparison of the AUC Values of CALLY Index and Its Components |
Prognosis and Survival Analysis of Patients with Breast Cancer
The results of Kaplan-Meier survival analysis and Log rank test showed that the estimated median overall survival (OS) was greater than 66 months for patients with a CALLY index of ≥ 2.285 and 52 months for patients with a CALLY index of < 2.285 (p<0.001; Figure 2A). The correlation between OS and clinicopathological variables is shown in Table 3. In univariate analysis, OS in breast cancer patients was significantly correlated with age (p=0.008), tumor size (p<0.001), positive lymph nodes (p<0.001), postoperative radiotherapy (p<0.001), TNM stage (p<0.02), and CALLY index (p<0.001). Next, stepwise regression was performed, and the results showed (Supplementary Table 1) that the OS of breast cancer patients was significantly related to Node positivity (p=0.024) and CALLY index (p=0.034). Therefore, the results of univariate analysis and stepwise regression were included in the multivariate analysis, and the results showed that TNM stage (p=0.009) and CALLY index (p=0.007) were independent predictors of OS in breast cancer patients (Table 3). The median DFS was estimated to be 64 months for patients with a CALLY index ≥ 2.285 compared with 47 months (95% CI:41.5–52.5) for patients with a CALLY < 2.285. (p<0.001, Figure 2B). The correlation between DFS and clinicopathological variables is demonstrated in Table 4. Univariate analysis revealed that DFS in breast cancer patients was linked to age (p=0.002), positive lymph nodes (p<0.001), tumor size (p<0.001), postoperative radiotherapy (p<0.001), TNM stage (p=0.001), and CALLY index (p<0.001). Next, stepwise regression was performed, and the results showed (Supplementary Table 2) that DFS of breast cancer patients was significantly related to Node positivity (p<0.001) and CALLY index (p<0.001). Therefore, the results of univariate analysis and stepwise regression were included in the multivariate analysis, and the results showed that TNM stage (p=0.015) and CALLY index (p=0.004) were independent predictors of DFS in breast cancer patients (Table 4).
 |
Table 3 Analysis of OS Prognosis Factors in 174 Breast Cancer Patients by Univariate and Multivariate Analysis |
 |
Table 4 Analysis of DFS Prognosis Factors in 174 Breast Cancer Patients by Univariate and Multivariate Analysis |
Discussion
The CALLY index is a measure of inflammation, vegetative, and immune system status, determined by analyzing serum CRP concentration, serum Alb concentration, and peripheral lymphocyte count. CRP is an acute-phase protein produced by inflammation-related cytokines such as vascular endothelial growth factor and interleukin-6.16 Serum albumin, a major protein in the blood, is a valid indicator of nutritional status, and serum albumin levels drop dramatically in patients with advanced cancer due to malnutrition and systemic inflammation.17 Pretreatment serum albumin has been used as a predictor of disease progression, severity, and prognosis.18 Low serum albumin levels have been reported to be an independent contributor to poor breast cancer survival, regardless of stage.19 CRP and serum albumin-related cytokines play an important role in cancer progression, as cytokines-mediated inflammatory responses can affect cancer cell growth and host cell-mediated immunity.5 Peripheral lymphocyte count is a surrogate marker of immune response, as lymphocytes play an important role in tumor immunity by inhibiting carcinogenesis.20 A decrease in the number of lymphocytes is thought to be responsible for the body’s poor immune response to tumors, leading to tumor progression and metastasis.21 In addition, the association between lymphopenia and reduced overall survival has been demonstrated in several prospective studies, such as metastatic breast cancer and non-Hodgkin lymphoma.22,23 Therefore, the CALLY index can be used as an indicator for a comprehensive assessment of the patient’s immunotrophic status and systemic inflammation. Cumulative studies have shown that the value of the CALLY Index correlates with early diagnosis, prognostic assessment, and recurrence prediction of tumors, which can be an independent adverse prognostic factor for ovarian, colon, and liver cancers.14,24,25 However, the prognostic value of the CALLY index in breast cancer has not yet been reported. To our knowledge, this is the first study to investigate the prognostic value of the CALLY index in breast cancer patients.
The results of this study showed that a low preoperative CALLY (CALLY<2.285) could be used as an independent indicator of poor prognosis in breast cancer patients, suggesting that a low CALLY index may be strongly associated with tumor progression and shorter survival. The CALLY index is a reproducible, widely used, and inexpensive laboratory hematology indicator that has become an important subject of study in cancer research.26 In univariate analysis, tumor size, axillary lymph node positivity, and TNM stage were correlated with OS. In multivariate analysis, tumor size and axillary lymph node positivity were not directly correlated with OS, and tumor size, lymph node status, and distant metastasis were closely related to survival in the eighth edition of the TNM classification published by AJCC.27 The authors speculate that this result is related to the small sample size and, therefore, the stratified analysis is not possible. TNM staging is a commonly used staging system that provides prognostic information and guidance for personalized management of breast cancer, and its prognostic value has also been verified in this study. To sum up, we found that the CALLY index is a reliable predictor of overall survival and disease-free survival after radical surgery in breast cancer patients. Those with a low CALLY index are more likely to have a worse outcome than those with a high CALLY index.
The study had several limitations. First, this was a single-center study, and the sample size (174 cases) may not be sufficient to fully reflect the status of breast cancer patients. Second, follow-up is short and the operating system is lacking.28 The median follow-up was 56 months, and most postoperative breast cancer patients typically face their first peak recurrence before 36 months. While the results of this study support the prognostic role of the CALLY index in breast cancer, the exact mechanism by which a low CALLY index is associated with adverse clinical and survival outcomes in breast cancer patients remains uncertain. Our results show that a low CALLY index (< 2.285) is associated with adverse pathological features, possibly low malnutrition29 and antitumor immunity,30 increased systemic inflammatory response,31 or both, and these findings may provide insights into the prognostic mechanism of the CALLY index in breast cancer.
In summary, the CALLY index, calculated using CRP values, albumin levels, and lymphocyte count, can be used as a valid predictor of postoperative prognosis in breast cancer patients. Given its cost-effectiveness and availability, we believe the CALLY index can be a viable biomarker for breast cancer management and cancer research. Further studies with larger sample sizes and longer follow-up periods are needed to confirm the prognostic value of the CALLY index in breast cancer.
Conclusion
In summary, the data from this study suggest that the CALLY index will be used as an independent prognostic indicator affecting the prognosis of breast cancer patients. Therefore, the CALLY index may be a useful biomarker in the evaluation of breast cancer to guide appropriate treatment, adjust follow-up intervals, and improve prognosis. Future studies with larger sample sizes and multicenter studies are needed to fully explore the potential of the CALLY index as a prognostic factor for breast cancer.
Highlights
- We analyzed the relationship between the CALLY Index and prognosis of patients with breast cancer.
- The results of this study show that a low CALLY index (CALLY<2.285) may be closely related to tumor progression and shortened survival. Therefore, a low preoperative CALLY index can be used as an independent indicator of poor prognosis in postoperative patients with breast cancer.
Acknowledgments
The authors express gratitude to all study participants and research staff who participated in the work.
Funding
This study was supported by a grant from the Fund of Wuxi Health Commission (M202214).
Disclosure
All authors have no conflicts of interest or financial ties to disclose for this work.
References
1. Dan J, Tan J, Huang J, et al. The dynamic change of neutrophil to lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast Cancer. 2020;27(5):982–988. doi:10.1007/s12282-020-01096-x
2. Zhou Y, Wen Y, Xiang Z, et al. Cancer survival trends in southeastern China, 2011–2021: a population-based study. Clinical Epidemiol. 2024;16:45–56. doi:10.2147/clep.S442152
3. Li N, Deng Y, Zhou L, et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: results from the Global Burden of Disease Study 2017. J Hematol Oncol. 2019;12(1):140. doi:10.1186/s13045-019-0828-0
4. Chen L, Kong X, Wang Z, Wang X, Fang Y, Wang J. Pretreatment systemic inflammation response index in patients with breast cancer treated with neoadjuvant chemotherapy as a useful prognostic indicator. Cancer Manag Res. 2020;12:1543–1567. doi:10.2147/CMAR.S235519
5. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
6. Cao X, Zhou Y, Mao F, Lin Y, Sun Q. Combination of preoperative fibrinogen concentration and neutrophil-to-lymphocyte ratio for prediction of the prognosis of patients with resectable breast cancer. Oncol Lett. 2020;20(5):200. doi:10.3892/ol.2020.12061
7. Chen F, Chen D, Jin L, Xu C, Zhao W, Hu W. Prognostic significance of neutrophil-to-lymphocyte ratio and C-reactive protein/albumin ratio in luminal breast cancers with HER2-negativity. Front Oncol. 2022;12:845935. doi:10.3389/fonc.2022.845935
8. Huang ZZ, Song CG, Huang JJ, et al. Prognostic significance of the Controlling Nutritional Status (CONUT) score in surgically treated breast cancer patients. Gland Surg. 2020;9(5):1370–1379. doi:10.21037/gs-20-294
9. Mantzorou M, Koutelidakis A, Theocharis S, Giaginis C. Clinical value of nutritional status in cancer: what is its impact and how it affects disease progression and prognosis? Nutr Cancer. 2017;69(8):1151–1176. doi:10.1080/01635581.2017.1367947
10. Tsunematsu M, Haruki K, Taniai T, et al. The impact of C-reactive protein-albumin-lymphocyte (CALLY) index on the prognosis of patients with distal cholangiocarcinoma following pancreaticoduodenectomy. Ann Gastroenterol Surg. 2023;7(3):503–511. doi:10.1002/ags3.12637
11. Sun L, Liu J, Wang D. Prognostic value of the preoperative prognostic nutritional index and systemic immuno-inflammatory index in Chinese breast cancer patients: a clinical retrospective cohort study. J Surg Oncol. 2023;127(6):921–928. doi:10.1002/jso.27210
12. Shao B, Li H, Liu X, et al. The prognostic value of neutrophil-to-lymphocyte ratio in de novo stage IV breast cancer: a retrospective cohort study. Ann Transl Med. 2023;11(2):45. doi:10.21037/atm-22-5612
13. Fan S, Xie X, Shen Y, Wang W, Gu X, Yao Z. The predictive value of preoperative serum neutrophil-to-lymphocyte ratio and tumor markers for early breast cancer patients: a retrospective study. Medicine. 2022;101(32):e30011. doi:10.1097/MD.0000000000030011
14. Muller L, Hahn F, Mahringer-Kunz A, et al. Immunonutritive scoring for patients with hepatocellular carcinoma undergoing transarterial chemoembolization: evaluation of the CALLY index. Cancers. 2021;13(19). doi:10.3390/cancers13195018
15. Yang ZJ, Yu Y, Chi JR, Guan M, Zhao Y, Cao XC. The combined pN stage and breast cancer subtypes in breast cancer: a better discriminator of outcome can be used to refine the 8th AJCC staging manual. Breast Cancer. 2018;25(3):315–324. doi:10.1007/s12282-018-0833-0
16. Rhodes B, Furnrohr BG, Vyse TJ. C-reactive protein in rheumatology: biology and genetics. Nat Rev Rheumatol. 2011;7(5):282–289. doi:10.1038/nrrheum.2011.37
17. Ma L, Zhao S. Risk factors for mortality in patients undergoing hemodialysis: a systematic review and meta-analysis. Int J Cardiol. 2017;238:151–158. doi:10.1016/j.ijcard.2017.02.095
18. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi:10.1186/1475-2891-9-69
19. Gan W, Zhang MX, Wang JX, et al. Prognostic impact of lactic dehydrogenase to albumin ratio in hepatocellular carcinoma patients with Child-Pugh I who underwent curative resection: a prognostic nomogram study. Cancer Manag Res. 2018;10:5383–5394. doi:10.2147/CMAR.S176317
20. Humphries MP, Craig SG, Kacprzyk R, et al. The adaptive immune and immune checkpoint landscape of neoadjuvant treated esophageal adenocarcinoma using digital pathology quantitation. BMC Cancer. 2020;20(1):500. doi:10.1186/s12885-020-06987-y
21. Demaria O, Vivier E. Immuno-oncology beyond TILs: unleashing TILCs. Cancer Cell. 2020;37(4):428–430. doi:10.1016/j.ccell.2020.03.021
22. Geramizadeh B, Shojazadeh A, Marzban M. Primary renal non-Hodgkin’s lymphoma: a narrative review of literature. Urologia J. 2021;89(2):185–194. doi:10.1177/0391560321990271
23. Sang Y, Kong X, Li X, et al. Langer’s axillary arch lymph node metastasis in breast cancer patients: a prospective clinical study. Surg Oncol. 2019;29:48–52. doi:10.1016/j.suronc.2019.03.003
24. Furukawa K, Tsunematsu M, Tanji Y, et al. Impact of C-reactive protein-albumin-lymphocyte (CALLY) index on prognosis after hepatectomy for colorectal liver metastasis. Surg Oncol. 2023;47:101911. doi:10.1016/j.suronc.2023.101911
25. Yang M, Lin SQ, Liu XY, et al. Association between C-reactive protein-albumin-lymphocyte (CALLY) index and overall survival in patients with colorectal cancer: from the investigation on nutrition status and clinical outcome of common cancers study. Front Immunol. 2023;14:1131496. doi:10.3389/fimmu.2023.1131496
26. Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more ”personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi:10.3322/caac.21388
27. Hyun SH, Ahn HK, Lee JH, et al. Body mass index with tumor 18F-FDG uptake improves risk stratification in patients with breast cancer. PLoS One. 2016;11(10):e0165814. doi:10.1371/journal.pone.0165814
28. He J, Tong L, Wu P, Wu Y, Shi W, Chen L. Prognostic significance of preoperative lactate dehydrogenase to albumin ratio in breast cancer: a retrospective study. Int J Gene Med. 2023;16:507–514. doi:10.2147/ijgm.S396871
29. Keller U. Nutritional laboratory markers in malnutrition. J Clin Med. 2019;8(6). doi:10.3390/jcm8060775
30. Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi:10.1093/jnci/dju124
31. Yao Z, Zhang Y, Wu H. Regulation of C-reactive protein conformation in inflammation. Inflamm Res. 2019;68(10):815–823. doi:10.1007/s00011-019-01269-1
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


