Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Prognostic Significance of Uric Acid Levels in Intracerebral Hemorrhage Patients
Authors Wu W , Geng Z, Wu A, Chen X, Meng X, Zhang Q, Tan Z, Yue H, Wu J
Received 15 November 2023
Accepted for publication 29 January 2024
Published 1 March 2024 Volume 2024:20 Pages 449—458
DOI https://doi.org/10.2147/NDT.S447851
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Wenpei Wu,1,2,* Zhi Geng,3– 5,* Aimei Wu,2,* Xinyi Chen,6,7 Xiaoying Meng,2 Qianyun Zhang,2 Zheng Tan,2 Hong Yue,2 Juncang Wu1,2
1Department of Neurology, Hefei Second People’s Hospital Affiliated to Bengbu Medical University, Hefei, Anhui, 230011, People’s Republic of China; 2Department of Neurology, Hefei Second People’s Hospital, Hefei, Anhui, 230011, People’s Republic of China; 3Department of Neurology, The First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China; 4Anhui Province Key Laboratory of Cognition and Neuropsychiatric Disorders, Hefei, People’s Republic of China; 5Collaborative Innovation Centre of Neuropsychiatric Disorder and Mental Health, Hefei, 230000, People’s Republic of China; 6Department of Neurology, Hefei Hospital Affiliated to Anhui Medical University (Hefei Second People’s Hospital), Hefei, Anhui, 230011, People’s Republic of China; 7The Fifth Clinical School of Medicine, Anhui Medical University, Hefei, Anhui, 230011, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Juncang Wu; Hong Yue, Intersection of Guangde Road and Leshui Road, Hefei, Anhui, 230011, People’s Republic of China, Tel/Fax +86 551 13956076148 ; +86 551 18949855780, Email [email protected]; [email protected]
Background and Purpose: The role of serum uric acid (UA) level in patients suffering from stroke remains controversial. Our aim was to investigate the effect of UA level on clinical outcomes in patients with intracerebral hemorrhage (ICH).
Methods: In the retrospective cohort study, we analyzed data from 250 patients with intracerebral hemorrhage (85 women and 165 men) to investigate the difference in UA levels between patients with a good prognosis and those with a poor prognosis. Additionally, we analyzed the impact of UA levels on the risk of short-time prognosis of ICH patients.
Results: Patients with a good prognosis presented with significantly lower levels of UA (348.71 ± 84.97 μmol/L) than those with poor prognosis (393.06 ± 148.46 μmol/L). Furthermore, multivariate logistic regression model demonstrated that a high UA level was a likely risk factor for worse prognosis among patients suffering in ICH (odds ratio [95% confidence interval], 1.006 [1.0012, 1.0108]; P = 0.015). Additionally, UA has a threshold effect value of 363.9 μmol/L and was presented in levels that were in a nonlinear relationship with incidence rate of short-time prognosis outcome of ICH patients.
Conclusion: Our findings indicate that higher UA levels can increase the risk of poor clinical prognosis in patients with ICH and high UA levels are not conductive to the clinical prognosis of patients with ICH. These findings provide a new perspective on the treatment and prevention of ICH.
Keywords: intracerebral hemorrhage, uric acid, oxidative stress, follow-up, nonlinear relationship, prognosis
Introduction
Stroke has become the leading cause of death and disability in China, and in a cross-sectional study of 676,394 participants aged 40 years and older, it was estimated that in 2020, 17.8 (95% CI, 17.5–18.0) million adults in China had experienced a stroke, of whom 3.4 (95% CI, 3.3–3.6) million experienced a first stroke and another 2.3 (95% CI, 2.2–2.4) million died as a result.1 According to the China Stroke Surveillance Report 2021, ICH accounts for 10.8–4.9% of all stroke events.2 ICH is the most devastating and difficult-to-treat type of stroke and often leads to severe disability for the survivors.3 The mechanism of ICH injury includes primary and secondary brain injuries. Primary brain injury is a localized compressive injury to brain tissue caused by the increase in intracranial pressure from the initial cerebral hemorrhage and hematoma enlargement.4 Secondary brain injury is caused by edema, inflammation, and clotting associated toxicity.5 The treatment options for hemorrhagic stroke are more limited in comparison to treatments for ischemic strokes.6 Therefore, secondary prevention of critical damage from cerebral hemorrhage is particularly important, including the management of risk factors, such as hypertension, diabetes and other inducers of metabolic stress.
UA is the terminal product of purine metabolism, and it is highly soluble and is excreted by the kidneys. Abnormal UA levels are known to be closely associated with many chronic diseases, such as gout, coronary heart disease and ischemic stroke.7–9 In addition, it has been demonstrated that elevated UA is associated with increased risk of adverse functional outcomes and death in atherosclerotic disease.7,10 Uric acid has also been suggested to be neuroprotective due to its role in scavenging oxygen free radicals.11 Numerous studies indicated that elevated baseline uric acid (UA) levels during the onset of acute ischemic stroke (AIS) typically correlated with a more favorable prognosis for AIS patients.12,13 Conversely, AIS patients with low UA levels face an elevated risk of hemorrhagic transformation, irrespective of whether they have undergone revascularization therapy.14–16
While there is an extensive body of literature investigating the association between uric acid and stroke, the mechanism of uric acid’s involvement remains elusive. Furthermore, prior studies have focused on ischemic stroke, and only a small body of literature has explored the association between blood uric acid levels and cerebral hemorrhage, and the findings have not been agreed upon. As a result, it is unknown if UA is an effective neuroprotective agent that promotes or protects against unfavorable outcomes in ICH, or if it simply acts as a marker indicating an elevated risk of ICH development or adverse functional outcomes. Previous meta-analyses have found support for UA’s dual role as a pro-oxidant and antioxidant, implying a potential association between UA and the pathogenesis of ICH.6 Nevertheless, this analysis failed to uncover substantial evidence connecting UA to the risk of ICH. A study of hemorrhagic stroke in black Africans indicated that high blood uric acid levels were not an independent predictor of death and poor outcome (mRS > 2 points) in ICH.17 Liu et al found that higher levels of uric acid were a protective factor for ICH severity and in-hospital complications based on ICH data from the Chinese Stroke Centre Consortium, but the article did not mention the relationship between uric acid levels and ICH patient prognosis.18 A recent study on uric acid and early neurological deterioration (END) in ICH patients discovered that ICH patients with higher serum UA levels had a significantly increased risk of END,19 potentially leading to a poor neurological prognosis in ICH patients. Based on the above, the relationship between UA level and cerebral hemorrhage has not been well studied and remains controversial. We believe that hyperuricemia has a negative effect on the prognosis of cerebral hemorrhage. Thus, in this study, we aimed to investigate the relationship between UA levels and the prognosis of patients with ICH.
Materials and Methods
Study Population
The design of this investigation was a retrospective cohort study. This retrospective analysis was performed on data collected from ICH patients consecutively admitted to the Second People’s Hospital of Hefei City, Anhui Province, between January 2018 and March 2021. The inclusion criteria were as follows: (1) ICH confirmed by cranial CT within 24 hours, (2) age ≥ 18 years, (3) signs and symptoms of focal neurological deficits present on admission. The exclusion criteria for the study were as follows: (1) other causes such as cerebral hemorrhage such as that caused by aneurysm, cerebrovascular malformation, brain trauma or brain tumor, (2) severe abnormal blood coagulation, cardiac insufficiency, and renal and pulmonary insufficiency prior to admission, (3) hemorrhagic transformation after cerebral infarction, (4) related surgical treatment receipt within 48 hours, and (5) study visit miss.
Data Acquisition
We collected demographic information, medical history, systolic blood pressure (SBP) on admission, diastolic blood pressure (DBP) on admission and other relevant clinical information from patients at admission. In addition, we also collected Glasgow Coma Scale (GCS) Scores and National Institutes of Health Stroke Scale (NIHSS) scores.20 ABC/2 method21 (A, greatest hemorrhage diameter; B, diameter perpendicular to A; C, thickness of hematoma) was used to measure the hematoma volume of ICH patient. The UA level and other related test indices were measured early in the morning on day 2 after admission. UA levels were measured with uric acid oxidase reagent using the DAX analyzer from Bayer.
Outcome
The primary outcome of our study was the modified Rankin Scale (mRS)22 score 90 days after onset of disease. We followed each patient for at least 3 months through outpatient, telephone, and inpatient visits. We defined an mRS score of 0–2 as an outcome with a good prognosis, and that of ≥3 as an outcome with a poor prognosis. This score is a marker of neurological functional outcome and is often used as an endpoint in clinical trials of stroke.7
The study was reviewed and approved by the Clinical Trial Ethics Committee of the Second People’s Hospital of Hefei City (Grant No. 2023-Scientific Research-018) and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. And because this was a retrospective cohort study, the Ethics Committee authorized the exemption of patients from informed consent.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation, categorical variables are given as numbers or percentages. The normal distribution of each variable was tested by Kolmogorov–Smirnov test. For normally distributed data, we examined intergroup differences using a two-tailed Student’s t-test. For non-parametric data, we examined intergroup differences using the Mann–Whitney U-test. We incorporated the related variables from univariate analysis (p < 0.05) into multiple regression analysis. Binary logistic regression models were used to explore the association between UA levels and ICH outcomes. Moreover, piecewise linear regression was applied to analyze the threshold effect of UA levels and outcome of short-time prognosis of ICH patients. Results were considered statistically significant if the two-tailed P-value was <0.05. Empower (R) (www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA) and R 3.6.3 (http://www.R-project.org) were used for all the statistical analyses.
Results
From January 2018 to March 2021, a total of 347 patients were diagnosed with spontaneous ICH, and based on the inclusion exclusion criteria, a total of 250 patients with spontaneous ICH were finally included in our study analysis (Figure 1). The demographic information and related risk factors are listed in Table 1. Based on our study design, we divided our study cohort into good and poor prognosis groups. The poor prognosis group included 37 women (32.46%) and 77 men (67.54%) with a mean age of 66 years. In addition, compared with the good outcome group, the poor prognosis was comprised of patients who were older (p = 0.004), had a higher proportion of patients with intraventricular hemorrhage (IVH) (p < 0.001), and had patients with significantly higher values for baseline hemorrhage volume (p < 0.001), NIHSS scores (p < 0.001), blood urea nitrogen (BUN) level (p=0.008), and higher UA levels (p = 0.003).
 |
Table 1 The Baseline Characteristics of Good Outcome and Poor Outcome on Acute Intracerebral Hemorrhage |
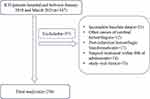 |
Figure 1 Flowchart of patient inclusion and analysis. |
Table 2 presents that the results of univariate analysis between the different variables and the poor outcome. Poor outcomes were associated with age (p = 0.004), intraventricular hemorrhage (p < 0.001), hemorrhage volume (p < 0.001), Glasgow Coma Scale (GCS) scores (p < 0.001), NIHSS scores (p < 0.001), BUN level (p = 0.011), CREA level (p<0.003), and UA level (p = 0.004).
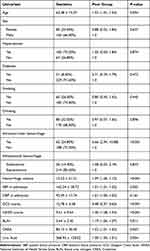 |
Table 2 Association Between Each Variable and Poor Outcome |
Table 3 lists the results of the multiple regression analysis of UA levels in association with poor outcomes in ICH patients. In the unadjusted model, multivariate regression analysis showed that high UA levels were significantly associated with poor outcomes in patients with ICH (odds ratio [OR]=1.0032, 95% confidence interval [CI] 1.001–1.0054, P = 0.004). The relationship between UA levels and poor prognostic outcomes remained significant event when adjusted for potential confounders, including age, SBP in admission, BUN, CREA, hemorrhage volume, intraventricular hemorrhage, GCS scores, NIHSS scores, the statistical association remained significant (odds ratio [OR] = 1.006, 95% confidence interval [CI] 1.0012–1.0108, P = 0.015), further confirming that a higher UA level is a hazardous factor in the short-term prognosis of patients with ICH.
 |
Table 3 Multivariate Regression for Effect of Uric Acid Level on Poor Outcome of Acute Intracerebral Hemorrhage |
As can be seen in Table 4 and Figure 2, there was a significant threshold effect noted between UA level and incidence rate of short-time prognosis outcome of ICH patients. The threshold effect value was 363.9 μmol/l. In patients with a baseline UA concentration below this value, an increase in UA level was associated with a worse prognosis (OR = 0.9949, 95% CI = 0.9852–1.0048, P = 0.311). In patients with baseline UA levels higher than this value (OR = 1.0124, 95% CI = 1.0051–1.0198, P < 0.001), the incidence rate of poor outcome was steep rise.
 |
Table 4 The Threshold Effect of Uric Acid Level on the Short-Time Prognosis Outcome of ICH Patients |
 |
Figure 2 The threshold effect of uric acid levels on short-time prognosis outcome of ICH patients. |
Discussion
In the present study, we explored the relationship between UA levels and the short-term prognosis of patients with ICH. This study demonstrated that the higher UA levels represent a significant and independent risk factor associated with a poor prognosis in patients with ICH. Furthermore, we found a threshold effect between UA levels and incidence rate of poor outcome, with an increase in UA levels significantly increasing the risk of poor prognosis until in reaching the threshold point of 363.9 µmol/L in patients with ICH. This study not only would enhance our understanding of the relationship between uric acid and ICH outcomes but highlights the importance and necessity for monitoring and management of UA as a potential part of clinical standard-of -care protocols.
UA is an important antioxidant molecule in the human body that effectively scavenges peroxynitrite, nitric oxide, and hydroxyl radicals, thus preventing protein nitrification and lipid peroxidation.8,11 UA concentration also affects the occurrence and development of stroke.23 Studies in animal models suggest that treatment with UA and its analogs may protect the brain from ischemic damage.24,25 This seems to be corroborated by some clinical observational studies on ischemic stroke. Research has shown that for every milligram increase in UA in patients with acute ischemic stroke, the likelihood of a good clinical outcome increases by 12%.26 Another study showed that higher serum uric acid levels were independently associated with lower hemorrhagic transformation after acute ischemic stroke.16 The protective effect of UA against acute ischemic stroke was based on the results of these observational studies.27 Although the clinical symptoms of ICH and ischemic stroke are similar, their specific mechanisms of occurrence and development are different. However, when uric acid exceeds the normal range, it can affect several systems in human body, which in turn lead to stroke. Previous studies on the association between uric acid and stroke have focused on ischemic stroke, while few studies have been conducted on hemorrhagic stroke.
The relationship between UA level and ICH has not been well studied and remains controversial. We found a significant difference in UA levels between the good prognosis and poor prognosis groups of ICH patients; the UA levels in the good prognosis group were significantly lower than those in the poor prognosis group. Further, further analysis revealed that UA level was an independent risk factor of poor prognosis in patients with ICH. Numerous studies have demonstrated that higher UA levels are associated with a higher incidence and poorer functional outcomes in atherosclerotic diseases such as acute stroke and cardiovascular disease.28,29 Previous studies also shown that elevated UA level is associated with carotid intima-media thickening;30,31 similar results were found in a study of proximal stenosis of the extracranial artery.32 These results indicate that UA level may play an important role in the pathophysiology of atherosclerosis and stroke. Studies have shown that elevated UA contributes to the progression of atherosclerosis by enhancing the production of free radicals and lipid peroxidation.33,34 High levels of UA can cause vascular endothelial dysfunction and abnormal proliferation of vascular smooth muscle cells, which may further contribute to cardiovascular and cerebrovascular diseases.35,36 Inflammatory responses resulting from high levels of UA can further increase the damage to the body through the cascading effects of factors such as C-reactive protein, and interleukin-6.37 Furthermore, high UA levels can magnify oxidative stress, which in turn further aggravates secondary damage after ICH through inflammation reaction, autophagy, and disruption of the blood–brain barrier.38,39
Here, with incidence rate of short-time prognosis outcome of ICH patients, we found that higher levels of UA were related to the incidence rate of short-time prognosis outcome of ICH patients with a threshold effect value of UA of 363.9μmol/l. In ICH patients, when the UA levels of ICH patients were less than 363.9 μmol/l, the effect was found to plateau; when the UA levels higher than 363.9 μmol/l, the effect was associated with a worse prognosis outcome as the UA level increased. These results suggest that an increase in UA concentration from levels higher than the threshold point may have an unfavorable effect on the prognosis of patients with ICH. A previous study reported that lower UA levels (<221μmol/l) could significantly increase the risk of poorer functional outcomes in patients with acute stroke with normoglycemic status.40 Numerous studies have suggested that UA has both pro-oxidant and antioxidant effects,11 and we presumed that pro-oxidants predominate at higher UA levels in ICH patients, further affecting their prognosis.
There are some limitations to this study. First, this was a single-center cohort study with a small sample size; thus, the statistical results may not be sufficient to represent the true population effects or detect small differences. Second, we only collected morning blood results from patients on the second day after hospitalization and did not perform multiple sample point collections. Therefore, it was not possible to assess the dynamic changes in UA levels during hospitalization and follow-up of ICH patients. Third, we adjusted for known important confounding variables in our multivariate regression analyses but did not include more comprehensive, less common risk factors, which may have influenced our results. Finally, we found a strong correlation between UA level and prognosis in patients with ICH; however, a causal relationship could not be established, and we will confirm these results by animal experiment in the future.
Conclusion
High UA levels can increase the risk of poor clinical prognosis in patients with ICH and high UA levels are not conductive to the clinical prognosis of patients with ICH. These findings provide a new perspective on the nature of UA in the context of ICH and suggest potential therapeutic and secondary prevention strategies for patients with ICH.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author, Hong Yue.
Statement of Ethics
The study was reviewed and approved by the Clinical Trial Ethics Committee of the Second People’s Hospital of Hefei City (Grant No. 2023-Scientific Research-018) and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. And because this was a retrospective cohort study, the Ethics Committee authorized the exemption of patients from informed consent. The personal information and data of all patients participating in the study are kept strictly confidential. No personal information or data that could identify specific patients were disclosed in this study.
Acknowledgments
Wenpei Wu, Zhi Geng and Aimei Wu are co-first authors for this study. The authors would like to thank all the individuals who offered help and advice on this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the Hefei Science and Technology Bureau “Borrow, Transfer and Supplement” Project (J2019Y01), “Hefei Key Common Technology R&D” Projects (GJ2022SM07).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tu WJ, Zhao Z, Yin P, et al. Estimated burden of stroke in China in 2020. JAMA Network Open. 2023;6(3):e231455. doi:10.1001/jamanetworkopen.2023.1455
2. Tu WJ, Wang LD, Yan F; On behalf of the Special Writing Group of China Stroke Surveillance Report, et al. China stroke surveillance report 2021. Military Med Res. 2023;10(1):33. doi:10.1186/s40779-023-00463-x
3. Rost NS, Smith EE, Chang Y, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke. 2008;39(8):2304–2309. doi:10.1161/STROKEAHA.107.512202
4. Eslami V, Tahsili-Fahadan P, Rivera-Lara L, et al. Influence of intracerebral hemorrhage location on outcomes in patients with severe intraventricular hemorrhage. Stroke. 2019;50(7):1688–1695. doi:10.1161/STROKEAHA.118.024187
5. Ungerer MN, Ringleb P, Reuter B, et al. Stroke unit admission is associated with better outcome and lower mortality in patients with intracerebral hemorrhage. Eur J Neurol. 2020;27(5):825–832. doi:10.1111/ene.14164
6. Zhou Z, Liang Y, Lin J, et al. Serum uric acid concentrations and risk of intracerebral hemorrhage: a systematic review and meta-analysis. Atherosclerosis. 2018;275:352–358. doi:10.1016/j.atherosclerosis.2018.07.002
7. Weir CJ, Muir SW, Walters MR, et al. Serum urate as an independent predictor of poor outcome and future vascular events after acute stroke. Stroke. 2003;34(8):1951–1956. doi:10.1161/01.STR.0000081983.34771.D2
8. Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Nat Rev Dis Prim. 2019;5(1):69. doi:10.1038/s41572-019-0115-y
9. Sakata S, Hata J, Honda T, et al. Serum uric acid levels and cardiovascular mortality in a general Japanese population: the Hisayama Study. Hypertens Res. 2020;43(6):560–568. doi:10.1038/s41440-019-0390-8
10. Holme I, Aastveit AH, Hammar N, et al. Uric acid and risk of myocardial infarction, stroke and congestive heart failure in 417 734 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). J Internal Med. 2009;266(6):558–570. doi:10.1111/j.1365-2796.2009.02133.x
11. Proctor PH. Uric Acid: neuroprotective or Neurotoxic? Stroke. 2008;39(5):e88.
12. Wang Z, Lin Y, Liu Y, et al. Serum uric acid levels and outcomes after acute ischemic stroke. Mol NeurobioL. 2016;53(3):1753–1759. doi:10.1007/s12035-015-9134-1
13. Zhang P, Wang R, Qu Y, et al. Serum uric acid levels and outcome of acute ischemic stroke: a dose–response meta-analysis. In: Molecular Neurobiology. Springer; 2023:1–10.
14. Chen Z, Chen H, Zhang Y, et al. Lower uric acid level may be associated with hemorrhagic transformation but not functional outcomes in patients with anterior circulation acute ischemic stroke undergoing endovascular thrombectomy. Metab Brain Dis. 2020;35(7):1157–1164. doi:10.1007/s11011-020-00601-7
15. Tian Y, Xie Q, You J, et al. Lower uric acid level may be associated with hemorrhagic transformation after intravenous thrombolysis. Neurol Sci. 2022;43(5):3113–3120. doi:10.1007/s10072-021-05760-8
16. Song Q, Wang Y, Cheng Y, et al. Serum uric acid and risk of hemorrhagic transformation in patients with acute ischemic stroke. J Mol Neurosci. 2020;70(1):94–101. doi:10.1007/s12031-019-01404-x
17. Mapoure YN, Ayeah CM, Ba H, et al. The prognostic value of serum uric acid in the acute phase of hemorrhagic stroke patients in black Africans. Pan Afr Med J. 2019;32. doi:10.11604/pamj.2019.32.165.15107
18. Liu X, Cao Z, Gu H, et al. Uric acid and clinical outcomes among intracerebral hemorrhage patients: results from the China Stroke Center Alliance. Front Neurol. 2020;11:609938. doi:10.3389/fneur.2020.609938
19. Gong X, Lu Z, Feng X, et al. High uric acid level predicts early neurological deterioration in intracerebral hemorrhage. Neuropsychiatr Dis Treat. 2021;17:2803–2809. doi:10.2147/NDT.S321778
20. Wityk RJ, Pessin MS, Kaplan RF, et al. Serial assessment of acute stroke using the NIH Stroke Scale. Stroke. 1994;25(2):362–365. doi:10.1161/01.STR.25.2.362
21. Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(8):1304–1305. doi:10.1161/01.STR.27.8.1304
22. van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607.
23. Qiao T, Wu H, Peng W. The relationship between elevated serum uric acid and risk of stroke in adult: an updated and dose–response meta-analysis. Front Neurol. 2021;12:674398. doi:10.3389/fneur.2021.674398
24. Aliena-Valero A, López-Morales MA, Burguete MC, et al. Emergent uric acid treatment is synergistic with mechanical recanalization in improving stroke outcomes in male and female rats. Neuroscience. 2018;388:263–273. doi:10.1016/j.neuroscience.2018.07.045
25. Cutler RG, Camandola S, Feldman NH, et al. Uric acid enhances longevity and endurance and protects the brain against ischemia. Neurobiol Aging. 2019;75:159–168. doi:10.1016/j.neurobiolaging.2018.10.031
26. Chamorro Á, Obach V, Cervera Á, et al. Prognostic significance of uric acid serum concentration in patients with acute ischemic stroke. Stroke. 2002;33(4):1048–1052. doi:10.1161/hs0402.105927
27. Chamorro Á, Amaro S, Castellanos M, et al. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): a randomised, double-blind phase 2b/3 trial. Lancet Neurol. 2014;13(5):453–460. doi:10.1016/S1474-4422(14)70054-7
28. Bos MJ, Koudstaal PJ, Hofman A, et al. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam Study. Stroke. 2006;37(6):1503–1507. doi:10.1161/01.STR.0000221716.55088.d4
29. Karagiannis A, Mikhailidis DP, Tziomalos K, et al. Serum uric acid as an independent predictor of early death after acute stroke. Circ J. 2007;71(7):1120–1127. doi:10.1253/circj.71.1120
30. Ma M, Wang L, Huang W, et al. Meta-analysis of the correlation between serum uric acid level and carotid intima-media thickness. PLoS One. 2021;16(2):e0246416. doi:10.1371/journal.pone.0246416
31. Zhang S, Wang Y, Cheng J, et al. Hyperuricemia and cardiovascular disease. Curr Pharm Des. 2019;25(6):700–709. doi:10.2174/1381612825666190408122557
32. Yang X, Lv H, Hidru TH, et al. Relation of serum uric acid to asymptomatic proximal extracranial artery stenosis in a middle-aged Chinese population: a community-based cross-sectional study. BMJ Open. 2018;8(8):e020681. doi:10.1136/bmjopen-2017-020681
33. Wong LKS. Global Burden of Intracranial Atherosclerosis. Int J Stroke. 2006;1(3):158–159. doi:10.1111/j.1747-4949.2006.00045.x
34. Song M, Li N, Yao Y, et al. Longitudinal association between serum uric acid levels and multiterritorial atherosclerosis. J Cell Mol Med. 2019;23(8):4970–4979. doi:10.1111/jcmm.14337
35. Otani N, Toyoda S, Sakuma M, et al. Effects of uric acid on vascular endothelial function from bedside to bench. Hypertens Res. 2018;41(11):923–931. doi:10.1038/s41440-018-0095-4
36. Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–1106. doi:10.1161/hy1101.092839
37. Lyngdoh T, Marques-Vidal P, Paccaud F, et al. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based colaus study. PLoS One. 2011;6(5):e19901. doi:10.1371/journal.pone.0019901
38. Kurajoh M, Fukumoto S, Yoshida S, et al. Uric acid shown to contribute to increased oxidative stress level independent of xanthine oxidoreductase activity in MedCity21 health examination registry. Sci Rep. 2021;11(1):7378. doi:10.1038/s41598-021-86962-0
39. Yao Z, Bai Q, Wang G, Zhang JH. Mechanisms of oxidative stress and therapeutic targets following intracerebral hemorrhage. Oxid Med Cell Longev. 2021;2021:1–12. doi:10.1155/2021/8815441
40. Wu S, Pan Y, Zhang N, et al.; On Behalf of the Investigators for the Survey on Abnormal Glucose Regulation in Patients With Acute Stroke Across China (ACROSS-China). Lower serum uric acid level strongly predict short-term poor functional outcome in acute stroke with normoglycaemia: a cohort study in China. BMC Neurol. 2017;17(1):21. doi:10.1186/s12883-017-0793-6
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
