Back to Journals » International Journal of General Medicine » Volume 16
Prognostic Significance of Pretreatment Plasma D-dimer Levels in EGFR-Positive Advanced Non-Small Cell Lung Cancer Patients Receiving Osimertinib: A Multicentre Retrospective Study
Authors Liu Q, Tan L, He J, Ning R, Zeng A, Chen Y
Received 1 September 2023
Accepted for publication 9 November 2023
Published 23 November 2023 Volume 2023:16 Pages 5481—5491
DOI https://doi.org/10.2147/IJGM.S437495
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Qianfei Liu,1,* Liping Tan,2 Jianbo He,2 Ruiling Ning,2 Aiping Zeng,2,* Yilin Chen1,*
1Department of Pulmonary and Critical Care Medicine, The Central Hospital of Enshi Tujia and Miao Autonomous Prefecture, Enshi City, Hu Bei, People’s Republic of China; 2Department of Respiratory Oncology, Guangxi Cancer Hospital and Guangxi Medical University Affiliated Cancer Hospital, Nanning City, Guangxi Zhuang Autonomous Region, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Aiping Zeng, No. 71 Hedi Road, Nanning City, Guangxi Zhuang Autonomous Region, 530021, People’s Republic of China, Email [email protected] Yilin Chen, No. 158 Wuyang Avenue, Enshi City, Hu Bei, 445000, People’s Republic of China, Email [email protected]
Purpose: This study examined the association between plasma D-dimer levels and overall survival (OS) in advanced non-small cell lung cancer (NSCLC) patients treated with osimertinib.
Methods: In this multicenter study, 88 patients with advanced non-small cell lung cancer (NSCLC) carrying epidermal growth factor receptor (EGFR) mutations and receiving osimertinib were enrolled. The target independent and dependent variables were the D-dimer levels before osimertinib treatment and OS time, respectively. The t-test or chi-square test was utilized to analyze the variances among various groups. The predictive significance of D-dimer for overall survival (OS) was assessed using Kaplan-Meier survival analysis and the Cox proportional hazard regression model.
Results: The selected patients had an average age of 58.01± 10.72 years, with females comprising 54.55%. Based on their D-dimer levels, the patients were categorized into three groups: low-level (< 0.6 mg/L), middle-level (0.6 ~ 2 mg/L), and high-level (≥ 2 mg/L). After accounting for possible confounding variables, the Cox proportional hazard model showed that increased D-dimer levels were linked to a greater likelihood of death (HR 2.28, 95% CI = 1.09 ~ 4.76, p = 0.029). Importantly, there was a significant trend indicating that as D-dimer levels rose, the risk of mortality also increased (p for trend, HR 1.15, 95% CI = 1.03 ~ 1.28, p = 0.012). Consistently comparable outcomes were noted in the majority of subcategories. Patients with low-middle and high D dimer levels had a median OS of 28.6 and 17 months, respectively (p =0.014).
Conclusion: In conclusion, elevated levels of D-dimer in the bloodstream were found to have a significant negative correlation with the overall survival (OS) of patients with EGFR-positive non-small cell lung cancer (NSCLC) who underwent treatment with osimertinib.
Keywords: D-dimer, EGFR, NSCLC, osimertinib, prognosis
Introduction
Activating the epidermal growth factor receptor is a common oncogenic driver in NSCLC. Approximately 80–90% of all EGFR-activating mutations are accounted for by deletions in exon 19 and point mutations in exon 21, specifically the L858R mutation.1 Currently, EGFR-tyrosine kinase inhibitors (TKIs) are regarded as the standard first-line therapy for advanced non-small cell lung cancer (NSCLC) with EGFR mutations. The TKIs, which consist of both first- and second-generation agents, have demonstrated a substantial increase in progression-free survival (PFS) and improvement in quality of life (QoL) when compared to platinum-based chemotherapy, as evidenced by studies.2–5
Oral, third-generation EGFR-TKI, osimertinib targets EGFR classical mutations as well as resistance mutations T790M.6 In clinical trials, osimertinib has demonstrated superior efficacy compared to platinum-pemetrexed chemotherapy in terms of PFS and overall response rate (ORR) for NSCLC patients who developed resistance to first-line EGFR-TKI therapy.7 Additionally, the FLAURA trial showed that osimertinib as the initial treatment for EGFR-mutant NSCLC significantly prolonged the median duration of time without disease progression and overall survival compared to initial-generation TKIs.8 As the standard of care, osimertinib is advised as the initial treatment for patients with NSCLC who have EGFR mutations, according to these findings.9 A recent study demonstrated that osimertinib offers added advantages to individuals with resectable NSCLC who have the EGFR mutation, irrespective of whether they underwent adjuvant chemotherapy.10 Due to its frequent usage in frontline and earlier-stage scenarios, osimertinib increases the chances of encountering patients who develop resistance to this medication.8,11,12 Osimertinib has demonstrated some limitations, intrinsic resistance, and an inadequate response.7,13
Several potential biomarkers that are of therapeutic significance have been identified as prognostic indicators for osimertinib therapy, including age, Eastern Cooperative Oncology Group-Performance Status (ECOG-PS), EGFR mutation, metastasis, and the pre-treatment act neutrophil-to-lymphocyte ratio.14–17 A recent meta-analysis involving 28 trials and 8452 individuals revealed a correlation between elevated plasma D-dimer levels and unfavorable prognosis among lung cancer patients.18 Specifically, elevated D-dimer levels in lung adenocarcinoma patients were associated with reduced response rates, shorter PFS, and OS to EGFR TKI.19 Therefore, this multicenter retrospective study aims to examine the prognostic significance of pretreatment plasma D-dimer levels in EGFR-positive advanced NSCLC patients receiving osimertinib.
Materials and Methods
Study Design and Patients
This study included 88 advanced NSCLC patients with EGFR mutations who were treated with osimertinib at the Guangxi Medical University Affiliated Cancer Hospital (Nanning, Guangxi, China) and The Central hospital of Enshi Tujia and Miao Autonomous Prefecture (Enshi, Hubei, China), from January 2017 to June 2022. This study aimed to investigate the relationship between plasma D-dimer levels and OS in patients with advanced NSCLC before treatment with osimertinib. The criteria for inclusion were: (1) being above the age of 18; (2) diagnosed at stage IV NSCLC by histology or cytology (Tumor Node Metastasis, TNM stage 8); (3) genetic confirmation of EGFR mutation; (4) using osimertinib (80 mg/day); (5) having at least one measurable focus as a target (as per the criteria of Response Evaluation Criteria in Solid Tumors Version 1.1); and (6) having complete follow-up data available. The exclusion criteria included: (1) a previous occurrence of other cancerous growths; (2) end-stage kidney, liver disease, cachexia; (3) a life expectancy of less than three months; (4) severe infection, recent surgery, and deep venous embolism within the past three months. (5) other contraindications for the use of osimertinib. This study received approval from the Medical Ethics Committee of the Cancer Hospital affiliated with Guangxi Medical University (Approval number: LW2022183), the Ethics Committee of the Central Hospital of Enshi Tujia and Miao Autonomous Prefecture (Approval number: 2023-048-001), and adhered to the principles stated in the Helsinki Declaration and the International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS). All research was carried out in compliance with applicable regulations and guidelines. All the data were anonymous, informed consent from patients was waived off because it was a retrospective study.
Data Collection and Variables Definition
Demographic statistics, smoking history, ECOG-PS, clinical stage, brain, bone, liver, and adrenal metastasis, mutation status at the time of initial treatment, prior treatment, the sum of treatment lines, the sum of metastasis organs, survival status, death or cutoff date, plasma D-dimer levels, etc. were obtained from the hospital’s electronic case system and follow-up system.
The therapeutic effect of the tumor was evaluated using Computed Tomography (CT) or Positron Emission Computerized Tomography (PECT) based on the Response Evaluation Criteria in Solid Tumours (RECIST) 1.1. The effectiveness and response to EGFR-TKIs were evaluated by a minimum of two doctors. The term overall survival denotes the duration from the initiation of osimertinib therapy until the occurrence of death or the conclusion of the follow-up period. If there is a difference of opinion, the team doctor talked about the findings from the collaborative assessment.
Follow-Up Procedure
The initial trio of researchers in this investigation conducted follow-ups with the patients utilizing contemporary online communication methods like patient hospitalization, outpatient revisit records, mobile phones, Wechat, and various other tools. The interval for follow-up was a minimum of once every three months, with a follow-up deadline set for September 30, 2022. The subsequent information was saved in the hospital’s follow-up database.
Statistical Analysis
Mean or quartile spacing represents continuous variables, whereas frequency or percentage expresses categorical variables. Two independent sample t-test or Mann–Whitney U-test were used to compare continuous variables, whereas chi-square test or Fisher exact test were used to compare classified variables. The cumulative overall survival was estimated using the Kaplan-Meier curve and compared using the Log rank test. To address the potential linear correlation between D-dimer levels and unfavorable prognosis, we implemented a generalized additive model and incorporated a spline smoothing function. The Cox proportional hazard model was utilized to assess the correlation between the D-dimer level and unfavorable outcome. Additionally, adjustments for confounding factors and a trend test were conducted. To assess the suitability of D-dimer in specific populations, stratified analysis was conducted. All the analyses were carried out using the statistical software packages of R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc, Boston, MA). p < 0.05 (bilateral) was considered to be statistically significant.
Results
Baseline Characteristics of the Study Population
The analysis included a total of 88 patients (in Figure 1), comprising 45.45% males and 54.55% females, with an average age of 58.01±10.72 years. We divided D-dimer levels into three groups: low-level (< 0.6 mg/L), middle-level (0.6 ~ 2 mg/L), and high-level (≥ 2 mg/L). Table 1 shows the baseline characteristics of selected patients. There were no significant differences among the three groups except for smoking history, with P values all >0.05.
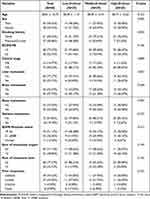 |
Table 1 Clinical Characteristics of Patients |
 |
Figure 1 Study profile. |
Univariate Analysis of Prognostic Factors
According to univariate analysis, each standard deviation (SD) increase in D-dimer level resulted in a 9% additional risk of death (HR 1.09, 95% CI = 1.02 ~ 1.17, p = 0.013). When D-dimer was divided into tertiles, the top tertile had a 2.15 times greater risk than the bottom tertile (HR 2.15, 95% CI = 1.10 ~ 4.17, p = 0.024). Additionally, ECOG-PS ≥ 2 (HR 2.18, 95% CI = 1.24 ~ 3.84, p = 0.007), liver metastasis (HR 1.83, 95% CI = 1.03 ~ 3.25, p = 0.040) were also independent predictors of OS after treatment with osimertinib in Table 2.
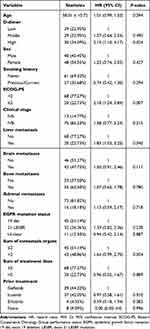 |
Table 2 Univariate Analysis of Patient Survival |
Examining the Smooth Curve Fitting of D-Dimer and Overall Survival (OS)
Enhancements were made to the smooth curve fitting technique to elucidate the linear correlation between D-dimer levels and adverse prognosis (Figure 2). The figure confirms the linear trend in univariate analysis, meaning that as the D-dimer level increases, the risk of death also increases. Based on the levels of D-dimer, the patients were categorized into three groups: low-level (< 0.6 mg/L), middle-level (0.6 ~ 2 mg/L), and high-level (≥ 2 mg/L).
Results from the Cox Proportional Hazards Model
We constructed three Cox proportional hazard models to reveal the independent effect of D-dimer on OS in Table 3. In the crude model, the risk of death increased by 9% with each increase of SD in the D-dimer level (95% CI = 1.02 ~ 1.17, p = 0.013). Based on the fully adapted model, the likelihood of mortality rose by 24% in the intermediate-level category (95% CI = 0.56 ~ 2.78, p = 0.596), and by 128% in the high-level category (95% CI = 1.09 ~ 4.76, p = 0.029), in comparison to the group with low D dimer levels. Additionally, it was noted that there was a noteworthy correlation within the tertiles group (p-value for trend, HR 1.15, 95% CI = 1.03 ~ 1.28, p = 0.012). The OS rate of NSCLC patients grouped by D-dimer level is illustrated in Figure 3 using the Kaplan-Meier curve. The median overall survival for individuals with low-middle and high levels of D dimer were 28.6 and 17 months, respectively. (HR 0.54, 95% CI = 0.29 ~ 0.87, p =0.014).
 |
Table 3 Relationship Between D-Dimer Levels and Survival in Different Models |
 |
Figure 3 Kaplan-Meier estimates of the over survival probabilities between low-middle and high D-dimer level. |
The Results of This Subgroup Analysis
A stratified analysis was conducted in a specific population to explore the relationship between D-dimer levels and adverse prognosis. The results showed a consistent positive correlation between elevated D-dimer levels and OS in most subgroups, particularly in special subgroups such as males, ECOG-PS ≥2, stage IVB, liver metastasis, brain metastases, bone metastases, and sum of metastasis organs >2. This trend was more pronounced, as shown in Table 4.
 |
Table 4 Subgroup Analysis of the Relationship Between the High D-Dimer Levels Group and Survival Outcomes |
Discussion
In this study, we discovered that elevated D-dimer levels were linked to a reduced overall survival when treated with osimertinib and served as an autonomous prognostic indicator for unfavorable outcomes in patients with positive-EGFR advanced NSCLC, even after accounting for other variables.
The cause of hypercoagulability associated with cancer is complex, encompassing multiple coagulation factors and pathways for signal transduction. Throughout the progression and spread of cancer, the cancerous cells have the ability to directly exhibit or indirectly regulate the manifestation of clotting agents, including tissue factor, cancer procoagulant, and tumor necrosis factor-α.20 Due to increased coagulability and decreased fibrinolytic activity, this process throws the coagulation, fibrinolytic, and anticoagulant systems out of balance.21 Numerous pieces of evidence indicate that a heightened tendency for blood clotting is a prevalent occurrence among individuals diagnosed with lung cancer.22 Notably, a recent meta-analysis using the largest sample sizes from 28 cohorts reported elevated plasma D-dimer levels as an independent factor of poor prognosis in lung cancer patients.18 Additional investigation uncovered that platelets derived from plasma with elevated levels of D-dimer exhibit an increased ability to adhere to cancer cells. This phenomenon plays a role in the phosphorylation of EGFR and Akt, along with the activation of SRC-induced epithelial-mesenchymal transition (EMT). This leads to the development of resistance to EGFR tyrosine kinase inhibitors such as gefitinib, erlotinib, afatinib, or osimertinib in EGFR-mutant lung cancer cells, ultimately leading to unfavorable PFS and OS outcomes. This study revealed that EGFR TKI treatment was less effective in patients with EGFR-mutant lung adenocarcinomas who exhibited elevated peripheral blood D-dimer levels. These patients were found to have an increased susceptibility to early disease progression and shorter overall survival durations.16 In this study, Cox proportional hazard model showed that high D-dimer levels were associated with an increased risk of death after adjusting for potential confounding factors; ie, the higher the D-dimer level, the greater the risk of death.
Chen et al conducted a retrospective analysis on a cohort of 233 patients diagnosed with advanced-stage NSCLC, wherein they found a significant association between elevated levels of plasma D-dimer and prognostic prediction for late-stage NSCLC.23 Similarly, Li et al retrospectively analyzed a cohort of 277 patients with advanced NSCLC who underwent immune checkpoint inhibitors (ICIS) treatment from January 2015 to March 2019, and their findings indicated that patients in the high D-dimer level group exhibited poorer PFS and overall survival.24 Further investigation revealed a significant association between elevated plasma concentrations of D-dimer and advanced age, tumor metastasis, and later TNM staging among patients diagnosed with NSCLC, leading to increased plasma D-dimer levels and decreased survival times. These findings are consistent with the results of the subgroup analysis carried out in this study.25 Chang’s study uncovered a notable correlation between genetic mutations and heightened D-dimer levels in individuals diagnosed with NSCLC. This observation implies that D-dimer could serve as a potential risk factor for NSCLC patients with genetic mutations, demonstrating substantial predictive value in identifying concurrent mutations.26
ECOG-PS is a significant clinical feature related to the incidence of lung cancer. PS status reflects the tumor burden and the comorbid conditions of patients. It represents an important clinical basis for a patient’s general body function, self-care ability, and quality of life. It is also considered one of the most significant prognostic markers for cancer patients.27 In a study conducted by Kato and his team,14 they examined 31 individuals with EGFR mutations who experienced T790M resistance mutation. These patients were treated with osimertinib as both first- and second-generation EGFR-TKI from March 2016 to January 2018. The objective was to evaluate the effectiveness of osimertinib in real-life groups and analyze how clinical characteristics influenced patient prognosis. Out of the 31 patients, 74.2% were elderly. EGFR-positive NSCLC patients with poor PS developed resistance to osimertinib. Our study, however, contradicted the findings of previous studies. Similarly, another study reported that28 younger patients were more likely to carry TP53 mutations than older patients. Additionally, under the stress of TKI treatment, some heterogeneous subclones are more likely to develop in young patients and become treatment-resistant prematurely, which may account for the poor prognosis of some young patients. Overall, further research related to predicting PS status in advanced lung cancer, particularly regarding targeted drugs, is still needed.
We also studied the importance of the total number of metastatic organs in predicting OS in patients treated with osimertinib. We found a strong correlation between the number of metastatic organs and the poor prognosis of patients treated with osimertinib, consistent with a previous real-world study in China.13 However, contrary to the findings of others, we found that for each increase in the sum of metastasis organs, the risk of death increased by 34% (p = 0.003). This showed that the prognosis of NSCLC patients treated with osimertinib became worse with the accumulation of metastatic organs, especially in patients with liver metastasis. In clinical settings, the tailored strategy of selective combination therapy of osimertinib may bring greater clinical survival benefits to patients, such as local radiotherapy combined with bone and brain metastases.
Limitations
At present, osimertinib is extensively utilized as the primary therapy for EGFR mutation-positive advanced NSCLC patients; however, the study encompasses a limited number of patients receiving osimertinib as their initial treatment. Additional investigation is necessary to ascertain the predictive significance of D-dimer as the primary treatment approach for osimertinib. Furthermore, the D-dimer level was assessed solely once upon the patient’s enrollment in the research. In our upcoming research, we will analyze the predictive importance of the alteration in D-dimer concentration while undergoing osimertinib targeted therapy. The data on driving gene mutations in NSCLC patients are not detailed (including the specific type of EGFR mutation at the time of initial treatment, whether there is a compound mutation in the EGFR pathway when receiving osimertinib treatment, and whether it is associated with other mutations outside the EGFR pathway). In some patients, EGFR mutations were detected during first-line targeted therapy, but the specific types of mutations were unclear. In some patients, the EGFR T790M loci was only detected after receiving first- or second-generation EGFR-TKI treatment. Therefore, a detailed analysis of the distribution of individuals with positive driving gene mutations among the three D-dimer groups was not possible.
Conclusions
Elevated plasma D-dimer concentrations exhibited a strong negative correlation with the overall survival (OS) of individuals with EGFR-positive non-small cell lung cancer (NSCLC) who underwent osimertinib therapy.
Ethics Approval
This study received approval from the Medical Ethics Committee of the Cancer Hospital affiliated with Guangxi Medical University (Approval number: LW2022183), the Ethics Committee of the Central Hospital of Enshi Tujia and Miao Autonomous Prefecture (Approval number: 2023-048-001), and adhered to the principles stated in the Helsinki Declaration and the International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS).
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that there are no competing interests.
References
1. Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(Suppl 1):S24–S31. doi:10.1038/onc.2009.198
2. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi:10.1056/NEJMoa0810699
3. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised Phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi:10.1016/s1470-2045(11)70393-x
4. Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–589. doi:10.1016/s1470-2045(16)30033-x
5. Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–1466. doi:10.1016/s1470-2045(17)30608-3
6. Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372(18):1689–1699. doi:10.1056/NEJMoa1411817
7. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi:10.1056/NEJMoa1612674
8. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi:10.1056/NEJMoa1713137
9. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi:10.1056/NEJMoa1913662
10. Eide IJZ, Helland Å, Ekman S, et al. Osimertinib in T790M-positive and -negative patients with EGFR-mutated advanced non-small cell lung cancer (the TREM-study). Lung Cancer. 2020;143:27–35. doi:10.1016/j.lungcan.2020.03.009
11. Ohashi K, Maruvka YE, Michor F, Pao W. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol. 2013;31(8):1070–1080. doi:10.1200/jco.2012.43.3912
12. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–2247. doi:10.1158/1078-0432.Ccr-12-2246
13. Chen Y, Wang S, Zhang B, et al. Clinical factors affecting the response to osimertinib in non-small cell lung cancer patients with an acquired epidermal growth factor receptor T790M mutation: a long-term survival analysis. Target Oncol. 2020;15(3):337–345. doi:10.1007/s11523-020-00724-y
14. Kato Y, Hosomi Y, Watanabe K, et al. Impact of clinical features on the efficacy of osimertinib therapy in patients with T790M-positive non-small cell lung cancer and acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors. J Thorac Dis. 2019;11(6):2350–2360. doi:10.21037/jtd.2019.06.03
15. Ono T, Igawa S, Ozawa T, et al. Evaluation of osimertinib efficacy according to body surface area and body mass index in patients with non-small cell lung cancer harboring an EGFR mutation: a prospective observational study. Thorac Cancer. 2019;10(4):880–889. doi:10.1111/1759-7714.13018
16. Lee MJ, Weng CM, Chao W, et al. Platelet activation in high D-dimer plasma plays a role in acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in patients with mutant lung adenocarcinoma. Front Oncol. 2022;12:876051. doi:10.3389/fonc.2022.876051
17. Peng D, Shan D, Dai C, et al. Real-world data on osimertinib in Chinese patients with pretreated, EGFR T790M mutation positive, advanced non-small cell lung cancer: a retrospective study. Cancer Manag Res. 2021;13:2033–2039. doi:10.2147/cmar.S287466
18. Ma M, Cao R, Wang W, et al. The D-dimer level predicts the prognosis in patients with lung cancer: a systematic review and meta-analysis. J Cardiothorac Surg. 2021;16(1):243. doi:10.1186/s13019-021-01618-4
19. Park JY, Jang SH, Lee CY, et al. Pretreatment neutrophil-to-lymphocyte ratio and smoking history as prognostic factors in advanced non-small cell lung cancer patients treated with osimertinib. Tuberc Respir Dis. 2022;85(2):155–164. doi:10.4046/trd.2021.0139
20. Zhang M, Wu S, Hu C. Do lung cancer patients require routine anticoagulation treatment? A meta-analysis. J Int Med Res. 2020;48(1):300060519896919. doi:10.1177/0300060519896919
21. Ma Y, Li G, Li X, et al. Clinical characteristics and prognostic analysis of Lung Cancer patients with Hypercoagulability: a single-center, retrospective, real-world study. J Cancer. 2021;12(10):2968–2974. doi:10.7150/jca.46600
22. Hammouda A, Souilah S, Ferhat-Hamida MY, Amir ZC, Aouichat-Bouguerra S, Hariti G. Activation de la coagulation chez des patients atteints du cancer du poumon [Activation of coagulation in patients with lung cancer]. Ann Biol Clin. 2019;77(3):272–280. French. doi:10.1684/abc.2019.1445
23. Chen C, Li J, Li J, et al. Application of an elevated plasma D-dimer cut-off value improves prognosis prediction of advanced non-small cell lung cancer. Ann Transl Med. 2020;8(18):1153. doi:10.21037/atm-20-5947
24. Li X, Lu D, Zhang Z, Zhang Y, Wang J, Hu Y. Prognostic value of plasma D-dimer levels in advanced non-small cell lung cancer patients treated with immune checkpoint inhibitors: a retrospective study. J Thorac Dis. 2022;14(10):4125–4135. doi:10.21037/jtd-22-1363
25. Guo J, Gao Y, Gong Z, et al. Plasma D-Dimer level correlates with age, metastasis, recurrence, Tumor-Node-Metastasis Classification (TNM), and Treatment of Non-Small-Cell Lung Cancer (NSCLC) patients. Biomed Res Int. 2021;2021:9623571. doi:10.1155/2021/9623571
26. Chang F, Zhang H, Chen C, et al. Concomitant genetic alterations are associated with plasma D-dimer level in patients with non-small-cell lung cancer. Future Oncol. 2022;18(6):679–690. doi:10.2217/fon-2021-0455
27. Sørensen JB, Klee M, Palshof T, Hansen HH. Performance status assessment in cancer patients. An inter-observer variability study. Br J Cancer. 1993;67(4):773–775. doi:10.1038/bjc.1993.140
28. Giordano M, Boldrini L, Servadio A, et al. Differential microRNA expression profiles between young and old lung adenocarcinoma patients. Am J Transl Res. 2018;10(3):892–900.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

