Back to Journals » Cancer Management and Research » Volume 11
Prognostic role of a new inflammatory index with neutrophil-to-lymphocyte ratio and lactate dehydrogenase (CII: Colon Inflammatory Index) in patients with metastatic colorectal cancer: results from the randomized Italian Trial in Advanced Colorectal Cancer (ITACa) study
Authors Casadei-Gardini A , Scarpi E , Ulivi P , Palladino MA, Accettura C , Bernardini I, Spallanzani A, Gelsomino F, Corbelli J, Marisi G , Ruscelli S , Valgiusti M, Frassineti GL, Passardi A
Received 18 December 2018
Accepted for publication 27 February 2019
Published 10 May 2019 Volume 2019:11 Pages 4357—4369
DOI https://doi.org/10.2147/CMAR.S198651
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Andrea Casadei-Gardini, 1 Emanuela Scarpi, 2 Paola Ulivi, 3 Maria Angela Palladino, 4 Caterina Accettura, 5 Ilaria Bernardini, 6 Andrea Spallanzani, 7 Fabio Gelsomino, 7 Jody Corbelli, 8 Giorgia Marisi, 8 Silvia Ruscelli, 1 Martina Valgiusti, 1 Giovanni Luca Frassineti, 1 Alessandro Passardi 1
1Department of Medical Oncology, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy; 2Unit of Biostatistics and Clinical Trials, IRST IRCCS, Meldola, Italy; 3Biosciences Laboratory, IRST IRCCS, Meldola, Italy; 4Medical Oncology Department, Piacenza Hospital, Piacenza, Italy; 5Medical Oncology Unit, Vito Fazzi Hospital, Lecce, Italy; 6Medical Oncology Unit, Ramazzini Hospital, Carpi, Italy; 7Department of Oncology and Hematology, Division of Oncology, University Hospital Modena, Modena, Italy; 8Oncolgy Unit, Degli Infermi Hospital, Faenza, Italy
Aim: The aim of this study was to investigate the role of a new inflammatory index (Colon Inflammatory Index [CII]) as a predictor of prognosis and treatment efficacy in patients with metastatic colorectal cancer (mCRC) enrolled in the prospective multicenter randomized ITACa (Italian Trial in Advanced Colorectal Cancer) trial to receive first-line chemotherapy (CT)+ bevacizumab or CT alone.
Patients and methods: Between November 14, 2007 and March 6, 2012, 276 patients diagnosed with CRC were available for baseline neutrophil-to-lymphocyte ratio (NLR) and lactate dehydrogenase (LDH). We divided the population into three groups on basis of the CII index.
Results: At baseline in all populations, median PFS and OS was predictive of clinical outcome (p< 0.0001). Following adjustment for clinical covariates, multivariate analysis confirmed CII index as an independent prognostic factor. The CII index was also predictive when we evaluated the two distinct arms with (p=0.0009) or without bevacizumab (p=0.0001). When we divided right side versus left side for treatment regimen (CT plus bevacizumab versus only bevacizumab), we found a benefit of bevacizumab versus only CT in the right side in patients treated with bevacizumab and not in patients treated with only chemotherapy. Conversely, we found no difference the left side, but we found a difference in the poor group of 4 months in favor to only chemotherapy.
Conclusion: Our results indicate that the CII index is a good prognostic marker for mCRC patients in first line treatment with CT with or without bevacizumab.
Trial registration: NCT01878422 ClinicalTrials.gov; date of registration: June 7, 2013.
Keywords: metastatic colorectal cancer, bevacizumab, first-line, prognosis, lactate dehydrogenase, neutrophil-to-lymphocyte ratio
Corrigendum for this paper has been published
Introduction
Colorectal cancer (CRC) is one of the major causes of comorbidity and death from cancer worldwide.1
Bevacizumab (B) is a monoclonal antibody that binds to the vascular endothelial growth factor with antiangiogenic activity. The use of B combined with fluoropyrimidine-based chemotherapy (CT) plus oxaliplatin and/or irinotecan is considered standard of care in first- and second-line treatment for patients with metastatic CRC (mCRC).2,3
There are no current validated factors that can predict sensitivity or resistance to B. Several studies have investigated this issue in recent years, but with poor results.
The literature has demonstrated the relationship between systemic chronic inflammation and various types of cancer, including CRC.4 The activation of the inflammation by the tumor determines the inhibition of apoptosis and can promote angiogenesis.5,6
Several papers in the literature have shown that neutrophil-to-lymphocyte ratio7-12 (NLR) and lactate dehydrogenase (LDH)13–17 have a predictive and prognostic role in various diseases, including CRC. We have previously demonstrated in separate studies the correlation between LDH serum levels18 and NLR19 and clinical outcome in first-line mCRC. NLR is a good peripheral inflammatory index and LDH serum levels are an indirect marker of tumor hypoxia, neo-angiogenesis, metastasis development and poor prognosis in many cancers.20
Based on these results, we have created a new inflammatory index (CII: colon inflammatory index), composed of NLR and LDH. We considered CII as a predictor of prognosis and treatment efficacy in patients with mCRC enrolled in the prospective multicenter randomized ITACa (Italian Trial in Advanced Colorectal Cancer)21 trial to receive first-line CT+B or CT alone.
Patients and methods
The ITACa trial
The study population consisted of patients with advanced CRC confirmed by pathological analysis. No patient had received previous systemic therapy.
All eligible patients were randomly assigned in a 1:1 ratio to receive CT+B or CT alone as first-line therapy. CT consisted of either FOLFOX4 or FOLFIRI at the clinician’s discretion.18 The full protocol of the ITACa trial is available in the first publication of the trial.21
Treatment continued until either progressive disease (PD) or unacceptable toxicity or withdrawal of consent. Tumor assessment was performed before the start of treatment and repeated every 8 weeks until PD. Responses were defined according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) guidelines (per investigator assessment). The National Cancer Institute Common Toxicity Criteria (NCI-CTC 3.0) were used for evaluating adverse events.
All patients provided written informed consent before enrollment in the study. The study was approved by the local ethics committee (Comitato Etico Area Vasta Romagna) on September 19, 2007, and registered in our National Clinical Trials Observatory (Osservatorio delle Sperimentazioni Cliniche) and in the European Clinical Trials Database (EudraCT no. 2007–004539-44) before patient recruitment began. Registration on ClinicalTrials.gov (NCT01878422) was not mandate and was completed at a later date after the end of the study (June 7, 2013).
The study was carried out in accordance with the Declaration of Helsinki under good clinical practice conditions and after full approval from the ethics committees of all participating centers.
Statistical analysis
The objectives of this secondary analysis were to examine the association between baseline CII and progression-free survival (PFS) and overall survival (OS) in the ITACa study. CII was developed combining NLR and LDH. Patients were divided into three risk groups depending on their CII: good (0 factor: NLR <3 and LDH ≤upper limit of normal-ULN), intermediate (1 factor: NLR ≥3 or LDH >ULN) and poor (2 factors: NLR ≥3 and LDH >ULN). The cutoff of NLR ≥3 was previously determined (Passardi A et al, Oncotarget 2016) and the ULN for LDH was defined according to the limit of each center (Passardi A et al, PloSOne 2015).
PFS was defined as the time from random assignment to the first documentation of PD (as per investigator assessment), or death from any cause. Patients undergoing curative metastasectomy were censored at the time of surgery. OS was defined as the time between random assignment and death or last follow-up visit.
Association between risk groups of CII and baseline characteristics was tested using Chi-squared or Fisher exact test. PFS, OS and their two-sided 95% CI were estimated by the Kaplan–Meier method and curves were compared by the log-rank test (at a significance level of 5%). Estimated HRs and their two-sided 95% CI were calculated using the Cox-proportional hazard model. HRs adjusted by center and baseline characteristics (gender, age, performance status, KRAS status, tumor site [rectum/colon], CT regimen [FOLFOX4/FOLFIRI] and ITACa treatment [CT+B vs CT alone]) were calculated using the Cox-proportional hazard model. Covariate selection was based on a list of suspected prognostic factors derived from the ITACa study.
The ORR was classified into partial response (PR), stable disease (SD) and PD. Either Chi-squared or Fisher's exact test was used to evaluate the association between CII and ORR. All p-values were based on two-sided testing, and statistical analyses were performed using SAS statistical software version 9.4 (SAS Inc., Cary, NC, USA).
Results
Patient characteristics
Between November 14, 2007, and March 6, 2012, 276 patients diagnosed with CRC were available for baseline NLR and LDH (Figure 1): 164 (59.4%) were males and 112 (40.6%) females with a median age at diagnosis of 66 years (range 33–83). Median follow-up was 36 months (range 1–65). Overall, median PFS was 9.1 (95% CI 8.5–9.9) and median OS was 21.4 months (95% CI 19.3–24.5).
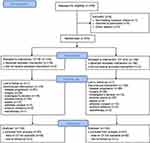 | Figure 1 Flowchart of the study. Abbreviation: CII, Colon Inflammatory Index. |
We divided the population into three groups on the basis of CII. The three groups of patients were comparable for age, gender, tumor site, KRAS status and ITACa treatment. A considerable proportion of patients with poor CII had a performance status 1–2 (Table 1). Table S1 shows the characteristics of patients treated with and without B.
 | Table 1 Baseline characteristics according to CII. Good (0 factor: NLR<3 e LDH≤UNL); intermediate (1 factor: NLR≥3 o LDH>UNL); poor (2 factors: NLR≥3 e LDH>UNL) |
CII and clinical outcome in all patients
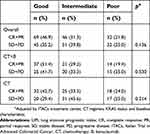
Median PFS was 10.3 months (95% CI 9.3–13.1), 8.7 months (95% CI 6.9–10.3) and 7.3 months (95% CI 5.5–8.9) for patients with good, intermediate and poor CII, respectively (p<0.0001) (Figure 2A). Median OS was 29.9 months (95%CI 24.3–37.3), 20.9 months (95%CI 16.8–25.4) and 14.4 months (95% CI 11.4–17.1) for patients with good, intermediate and poor CII, respectively (p<0.0001) (Figure 2B). The three categories were associated with different toxicities (Table 2).
 | Table 2 Association between Colon Inflammatory Index and toxicity |
 | Figure 2 Kaplan–Meier curves of progression free survival (PFS) (A) and overall survival (OS) (B) of patients for the Colon Inflammatory Index. |
In multivariate analysis, CII showed an independent prognostic factor predictive of PFS and OS after adjustment for clinical covariates (ITACa treatment, center, CT regimen, KRAS status and baseline characteristics) (Table 3).
 | Table 3 Prognostic/predictive value of the Colon Inflammatory Index in the total population (overall) and in CT plus B and CT-only treatment arms |
CII classification was not associated with response (Table 4).
 | Table 4 Association between the Colon Inflammatory Index and response |
CII and clinical outcome in patients treated with CT+B
Among patients treated with CT+B, median PFS was 12.1 months (95% CI 9.8–14.7), 10.0 months (95% CI 6.9–12.9) and 8.6 months (95% CI 3.7–9.4) for patients with good, intermediate and poor CII, respectively (p=0.0004) (Figure 3A). Median OS was 31.6 months (95% CI 22.3–41.7), 20.6 months (95% CI 13.6–27.0) and 12.7 months (95% CI 5.4–14.6) for patients with good, intermediate and poor CII, respectively (p=0.0009) (Figure 3B).
 | Figure 3 Kaplan–Meier curves of PFS (A) and OS (B) for the Colon Inflammatory Index patients treated with CT +B. Abbreviations: CT, chemotherapy; B, bevacizumab. |
Following adjustment for the same clinical covariates, multivariate analysis confirmed CII as an independent prognostic factor for predicting PFS and OS (Table 3).
CII and clinical outcome in patients treated with CT alone
Median PFS was 9.6 months (95% CI 8.6–13.0), 8.4 months (95% CI 6.2–9.1) and 7.3 months (95% CI 4.5–9.0) for patients with good, intermediate and poor CII, respectively (p=0.002) (Figure 4A). Median OS was 27.1 months (95%CI 20.8–38.7), 21.3 months (95%CI 16.8–28.0) and 17.1 months (95% CI 11.5–23.2) for patients with good, intermediate and poor CII, respectively (p=0.0001) (Figure 4B).
 | Figure 4 Kaplan–Meier curves of progression free survival (PFS) (A) and overall survival (OS) (B) for Colon Inflammatory Index in patients treated with chemotherapy alone. |
Following adjustment for the same clinical covariates, multivariate analysis confirmed CII as an independent prognostic factor for predicting PFS and OS (Table 3).
CII and tumor site
For tumors located in the right side, median PFS was 10.4 months (95% CI 8.3–13.7), 7.7 months (95% CI 5.1–10.2) and 8.9 months (95% CI 1.0–10.3) for patients with good, intermediate and poor CII, respectively (p=0.002) (Table 5). Median OS was 26.4 months (95% CI 19.2–35.7), 15.0 months (95%CI 11.5–20.9) and 15.0 months (95% CI 2.4–24.5) for patients with good, intermediate and poor CII, respectively (p=0.004). Following adjustment for clinical covariates (ITACa treatment, center, CT regimen, KRAS status and baseline characteristics), multivariate analysis confirmed CII as an independent prognostic factor for predicting PFS and OS (Table 5).
 | Table 5 Colon Inflammatory Index in relation to tumor localization in the overall/total population |
For tumors located in the left side, median PFS was 10.3 months (95% CI 9.1–13.7), 9.1 months (95% CI 6.5–11.3) and 6.5 months (95% CI 3.7–8.8) for patients with good, intermediate and poor CII, respectively (p<0.0001). Median OS was 36.6 months (95% CI 24.8–44.4), 24.8 months (95% CI 19.3–28.0) and 13.7 months (95% CI 8.2–16.8) for patients with good, intermediate and poor CII, respectively (p<0.0001). Following adjustment for clinical covariates (ITACa treatment, center, CT regimen, KRAS status and baseline characteristics), multivariate analysis confirmed CII as an independent prognostic factor for predicting PFS and OS (Table 5).
When we considered left-sided and right-sided tumors separately by treatment regimen (CT+B vs CT alone), we observed a greater benefit of CT+B than CT alone in patients with a right-sided tumor. In particular, administration of CT+B yielded a 3-month longer OS for the good-CII group of patients, whereas a decrease in OS for the poor-CII group of patients. Conversely, no difference was found in patients with left-sided tumors, although the poor-CII group experienced a 4-month longer OS with CT+B than with CT alone (Table 6).
 | Table 6 Colon Inflammatory Index in relation to tumor localization in the overall/total population |
Discussion
CII, based on NLR and LDH, allowed us to divide the patient population treated in the ITACa study into three categories: good, intermediate and poor. Good-CII patients (114 out of 276) achieved a median PFS of 30 months vs 14 months for the poor-CII patients. Interestingly, administration of CT+B resulted in a 4-month longer OS than CT alone in good-CII patients. The intermediate category of patients, however, showed no difference between the two regimens, while B+CT administration proved detrimental for the poor-CII group. When left-sided and right-sided tumors were considered separately, the good- and intermediate-CII groups with left-sided tumors had a benefit of almost 10 months compared to the right-sided tumors. No difference was seen in the poor-CII category. By assigning CT+B to right-sided tumors and CT alone to left-sided tumors we observed that B has a greater benefit in right-sided cancers, especially in CII-poor patients. This could be explained by the different tumor biology of the tumors classified according to the CII. There are no important differences with the data collected in the study. Unfortunately, we did not have the data on the BRAF that might explain this difference. The increased aggressiveness of tumors classified as poor can be seen in Table 1, where 90% of poor-CII tumors and only 66% of good-CII tumors were metastatic at diagnosis. The lower response to B in intermediate-CII and poor-CII patients can also be explained with a high LDH value, which underlies a hypoxic microenvironment. In hypoxia, Von Hippel Lindau (VHL) suppressor dissociates from its hypoxia-inducible factor-1 (HIF-1) subunit. HIF-1 once dissociated allows the transcription of several gene targets implicated in neoangiogenesis, including LDH.
The lower efficacy of B in poor-CII patients may be attributable to a lower efficacy of the drug in inflammatory and hypoxic conditions, as for high NLR and LDH levels. Inflammation is a common feature of cancer and is due to several proinflammatory cytokines such as TNF-alpha, IL-1, IL-6, reactive oxygen and nitrogen species, prostaglandins and microRNAs, which accumulate contributing to creating a pro-tumorigenic microenvironment. A strong link between inflammation and hypoxia has already been demonstrated, with a series of common activators such as HIFs and nuclear factor-κB (NF-κB).22,23 NF-kB is activated in CRC in response to inflammation, promoting tumorigenesis and cancer progression.24 A number of NF-kB target genes, such as IL-8 and VEGF, are known to be involved in the angiogenic process, and to be also target of HIF-1 alpha, highlighting the existence of an intricate crosstalk between inflammation and hypoxia in cancer cells.25 Hypoxia could be responsible for a lower efficacy of B in these tumors, as this condition has been known to induce resistance to antiangiogenic treatments.22 We observed a detrimental effect of B in poor-CII patients with a right-sided tumor. Right-sided tumors have a series of features associated with higher hypoxic and inflammation conditions. Higher expression levels of COX-2 and eNOS, both markers associated with hypoxia,27,28 have been observed in patients with these tumors,26 Moreover, high frequency of microsatellite instability (MSI), as well as elevated microsatellite alterations at selected tetranucleotide repeats (EMAST) has been observed in right-sided CRC compared to left-sided ones.29,30 Both these markers are associated with higher tumor inflammation and hypoxia.31,32
Although it is only a hypothesis, which must be evaluated in future translational studies, CII based on NLR and LDH could be an indirect index of tumor hypoxia.
In conclusion, CII appears to be a good index for identifying the prognosis of patients on first-line CT. Furthermore, the data suggest a possible role of CII in the identification of patients who may have an advantage from the use of B in first-line treatment.
Acknowledgments
This trial was partially supported by the Italian Medicines Agency (Agenzia Italiana del Farmaco [AIFA]; research grant no FARM6FJJAY). The study sponsor was involved neither in the study design nor in the collection, analysis, and interpretation of data. The study sponsor did not provide writing support for the report. All authors had full access to all the data in the study. The corresponding author had the final responsibility to submit for publication.
The authors would like to thank Veronica Zanoni for editing the manuscript, and Angela Ragazzini, Monia Dall’Agata (Meldola), Camilla Di Nunzio and Claudia Biasini (Piacenza), Elisa Pettorelli (Modena), Britt Rudnas, Barbara Venturini (Rimini), Giorgia Razzini and Antonella Pasqualini (Carpi), Alessandra Piancastelli (Faenza), Bernadette Vertogen and Federica Zumaglini (Ravenna) for participating in this study.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics. CA Cancer J Clin. 2014;64(1):9–29. doi:10.3322/caac.21208
2. Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306–1315. doi:10.1016/S1470-2045(15)00122-9
3. Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317(23):2392–2401. doi:10.1001/jama.2017.5254
4. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi:10.1016/j.cell.2010.01.025.
5. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer- related in ammation. Nature. 2008;454:436–444. doi:10.1038/nature07205
6. Ko E, Jung G. Positive association of long telomeres with the invasive capacity of hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2014;447:358–363. doi:10.1016/j.bbrc.2014.04.022
7. Casadei Gardini A, Scarpi E, Orlandi E, et al. Prognostic role of aspartate aminotransferase-lymphocyte ratio index in patients with metastatic colorectal cancer: results from the randomized ITACa trial. Onco Targets Ther. 2018;11:5261–5268. doi:10.2147/OTT.S166614
8. Casadei Gardini A, Foschi FG, Conti F, et al. Immune inflammation indicators and ALBI score to predict liver cancer in HCV-patients treated with direct-acting antivirals. Dig Liver Dis. 2018.
9. Casadei Gardini A, Conti F, Foschi FG, et al. Imbalance of neutrophils and lymphocyte counts can be predictive of hepatocellular carcinoma occurrence in hepatitis c-related cirrhosis treated with direct-acting antivirals. Gastroenterology. 2018;154(8):2281–2282. doi:10.1053/j.gastro.2017.12.051
10. Casadei Gardini A, Scarpi E, Faloppi L, et al. Immune inflammation indicators and implication for immune modulation strategies in advanced hepatocellular carcinoma patients receiving sorafenib. Oncotarget. 2016;7(41):67142–67149.
11. Bruix J
12. Lué A, Serrano MT, Bustamante FJ, et al. Neutrophil-to-lymphocyte ratio predicts survival in European patients with hepatocellular carcinoma administered sorafenib. Oncotarget. 2017;8(61):103077–103086. doi:10.18632/oncotarget.21528
13. Giampieri R, Puzzoni M, Daniele B, et al. First-line FOLFIRI and bevacizumab in patients with advanced colorectal cancer prospectively stratified according to serum LDH: final results of the GISCAD (Italian Group for the Study of Digestive Tract Cancers) CENTRAL (ColorEctalavastiNTRiAlLdh) trial. Br J Cancer. 2017;117(8):1099–1104. doi:10.1038/bjc.2017.234
14. Fanotto V, Cordio S, Pasquini G, et al. Prognostic factors in 868 advanced gastric cancer patients treated with second-line chemotherapy in the real world. Gastric Cancer. 2017;20(5):825–833. doi:10.1007/s10120-016-0681-6
15. Faloppi L, Bianconi M, Memeo R, et al. Lactate dehydrogenase in hepatocellular carcinoma: something old, something new. Biomed Res Int. 2016;2016:7196280. doi:10.1155/2016/7196280
16. Faloppi L, Del Prete M, Casadei Gardini A, et al. The correlation between LDH serum levels and clinical outcome in advanced biliary tract cancer patients treated with first line chemotherapy. Sci Rep. 2016;11(6):24136. doi:10.1038/srep24136
17. Faloppi L, Bianconi M, Giampieri R, et al. The value of lactate dehydrogenase serum levels as a prognostic and predictive factor for advanced pancreatic cancer patients receiving sorafenib. Oncotarget. 2015;6(33):35087–35094. doi:10.18632/oncotarget.5197
18. Passardi A, Scarpi E, Tamberi S, et al. Impact of pre-treatment lactate dehydrogenase levels on prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. PLoS One. 2015;10(8):e0134732. doi:10.1371/journal.pone.0134732
19. Passardi A, Scarpi E, Cavanna L, et al. Inflammatory indexes as predictors of prognosis and bevacizumab efficacy in patients with metastatic colorectal cancer. Oncotarget. 2016;7(22):33210–33219. doi:10.18632/oncotarget.8901
20. Kolev Y, Uetake H, Takagi Y, et al. Lactate dehydrogenase-5 (LDH-5) expression in human gastric cancer: association with hypoxia-inducible factor (HIF-1) pathway, angiogenic factors production and poor prognosis. Annals of Surgical Oncology. 2008;15(8):
21. Passardi A, Nanni O, Tassinari D, et al. Effectiveness of bevacizumab added to standard chemotherapy in metastatic colorectal cancer: nal results for rst-line treatment from the ITACa randomized clinical trial. Ann Oncol. 2015;26:1201–1207. doi:10.1093/annonc/mdv383
22. D’Ignazio L, Batie M, Rocha S. Hypoxia and inflammation in cancer, focus on HIF and NF-κB. Biomedicines. 2017;5(2):
23. Cuomo F, Coppola A, Botti C, et al. Pro-inflammatory cytokines activate hypoxia-inducible factor 3α via epigenetic changes in mesenchymal stromal/stem cells. Sci Rep. 2018;8(1):5842. doi:10.1038/s41598-018-24221-5
24. Wang S, Liu Z, Wang L, Zhang XN. F-kappaB signaling pathway, inflammation and colorectal cancer. Cell Mol Immunol. 2009;6(5):327–334. doi:10.1038/cmi.2009.33
25. Ulivi P, Marisi G, Passardi A. Relationship between hypoxia and response to antiangiogenic therapy in metastatic colorectal cancer. Oncotarget. 2016;7(29):46678–46691. doi:10.18632/oncotarget.v7i29
26. Ulivi P, Scarpi E, Chiadini E, et al. Right- vs. left-sided metastatic colorectal cancer: differences in tumor biology and bevacizumab efficacy. Int J Mol Sci. 2017;18(6). doi:10.3390/ijms18061240
27. Hashemi Goradel N, Najafi M, Salehi E. Cyclooxygenase-2 in cancer: a review. J Cell Physiol. 2019;234(5):5683–5699.
28. Song Y, Zhao XP, Song K, Shang Z-J, Lee JW. Ephrin-A1 is up-regulated by hypoxia in cancer cells and promotes angiogenesis of HUVECs through a coordinated cross-talk with eNOS. PLoS One. 2013;8(9):e74464. doi:10.1371/journal.pone.0074464
29. Narayanan S, Gabriel E
30. Wang Y, Vnencak-Jones CL, Cates JM, Shi C. Deciphering elevated microsatellite alterations at selected tetra/pentanucleotide repeats, microsatellite instability, and loss of heterozygosity in colorectal cancers. J Mol Diagn. 2018;20(3):366–372. doi:10.1016/j.jmoldx.2018.02.001
31. Carethers JM, Koi M, Tseng-Rogenski SS. EMAST is a form of microsatellite instability that is initiated by inflammation and modulates colorectal cancer progression. Genes (Basel). 2015;6(2):185–205.
32. Koi M, Tseng-Rogenski SS, Carethers JM, et al. Inflammation-associated microsatellite alterations: mechanisms and significance in the prognosis of patients with colorectal cancer. World J Gastrointest Oncol. 2018;10(1):1–14. doi:10.4251/wjgo.v10.i1.1
Supplementary material
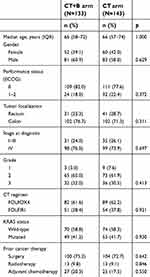 | Table S1 Patient characteristics (N=276) |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
