Back to Journals » Journal of Hepatocellular Carcinoma » Volume 7
Prognostic Nomograms for Patients with Hepatocellular Carcinoma After Curative Hepatectomy, with a Focus on Recurrence Timing and Post-Recurrence Management
Authors Xu W , Liu F, Shen X, Li R
Received 9 July 2020
Accepted for publication 10 October 2020
Published 27 October 2020 Volume 2020:7 Pages 233—256
DOI https://doi.org/10.2147/JHC.S271498
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmed Kaseb
Wei Xu,* Fei Liu,* Xianbo Shen,* Ruineng Li
Department of Hepatobiliary Surgery, Hunan Provincial People’s Hospital, The First Hospital Affiliated with Hunan Normal University, Changsha, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Xu
Department of Hepatobiliary Surgery, Hunan Provincial People’s Hospital, The First Hospital Affiliated with Hunan Normal University, Changsha, People’s Republic of China
Tel +86 13873159491
Fax +86 73182278012
Email [email protected]
Background: Prognoses of patients with hepatocellular carcinoma (HCC) after curative hepatectomy remain unsatisfactory because of the high incidence of postoperative recurrence. Published predictive systems focus on pre-resection oncological characteristics, ignoring post-recurrence factors.
Purpose: This study aimed to develop prognostic nomograms for 3- and 5-year overall survival (OS) of patients with HCC after curative hepatectomy, focusing on potentially influential post-recurrence factors.
Patients and Methods: Clinicopathological and postoperative follow-up data were extracted from 494 patients with HCC who underwent curative hepatectomy between January 2012 and June 2019. Early recurrence (ER) and late recurrence (LR) were defined as recurrence at ≤ 2 and > 2 years, respectively, after curative hepatectomy. Nomograms for the prediction of 3- and 5-year OS were established based on multivariate analysis. The areas under time-dependent receiver operating characteristic curves (AUCs) for the nomograms were calculated independently to verify predictive accuracy. The nomograms were internally validated based on 2000 bootstrap resampling of 75% of the original data.
Results: In total, 494 patients with HCC who underwent curative hepatectomy met the eligibility criteria. Cox proportional hazard regression analysis identified factors potentially influencing 3- and 5-year OS. Multivariate analysis indicated that patient age, Hong Kong Liver Cancer stage, γ-glutamyl transferase (γ-GGT) level, METAVIR inflammation activity grade, ER and post-recurrence treatment modality were influencing factors for 3-year OS (AUC, 0.891; 95% CI, 0.8364– 0.9447). γ-GGT > 60 U/L, hepatectomy extent, LR and post-recurrence treatment modality were influencing factors for 5-year OS (AUC, 0.864; 95% CI, 0.8041– 0.9237). Calibration plots showed satisfactory concordance between the predicted and actual observation cohorts.
Conclusion: We propose new prognostic nomograms for OS prediction with a focus on the differentiation of recurrence timing and post-recurrence management. These nomograms overcome the shortcomings of previous predictive nomograms and significantly improve predictive accuracy.
Keywords: hepatocellular carcinoma, hepatectomy, post-recurrence management, overall survival, nomogram
Introduction
Hepatocellular carcinoma (HCC) is the most common liver malignancy, accounting for 85–90% of all liver cancer cases. Most HCC cases are associated with chronic hepatitis B virus (HBV) infection, especially in the Asia-Pacific region.1 Newly diagnosed Chinese patients account for more than 50% of global cases.2 Despite emerging treatment modalities, the long-term prognosis of patients with HCC remains unsatisfactory. High HBV infection rates and poor prognosis of HCC lead to high HCC incidence and mortality in China, which will pose intractable national challenges in the future.3
Liver resection is the most common curative approach in the Asia-Pacific region, especially in China.2 Unfortunately, the prognosis after curative hepatectomy remains unsatisfactory because of the high incidence of postoperative recurrence.3 Many nomograms and risk score systems have been established for post-hepatectomy overall survival (OS) prediction.4–30 These systems focus on initial pre-resection oncological characteristics, but not on factors significantly affecting OS after curative hepatectomy in patients with HCC, such as the recurrence type and site, time to recurrence (TTR), and post-recurrence treatment modality.31–38 Predictive nomograms and scoring systems that ignore such influencing factors are somewhat controversial. Effective treatments for HCC recurrence based on recurrence type, recurrence site, and TTR may prolong post-recurrence survival (PRS) and OS.
The purpose of this study was to provide a satisfactory interpretation of factors that may influence OS after curative hepatectomy for HCC (eg, TTR, recurrence type and site, and post-recurrence treatment modality). Novel prognostic nomograms for 3- and 5-year OS in this context are proposed based on retrospective analyses of data from a Chinese cohort.
Patients and Methods
In total, 494 patients with HCC undergoing curative hepatectomy at Hunan Provincial People’s Hospital (The First Hospital Affiliated with Hunan Normal University; 165 cases recruited retrospectively between January 2012 and December 2014, and 329 cases recruited prospectively between January 2015 and June 2019) were included in this study.
The inclusion criteria were: a) Eastern Cooperative Oncology Group Performance Status score of 0 or 1, absence of a macroscopic portal- or hepatic-vein or bile-duct tumor thrombus, and absence of extrahepatic spread or distant metastasis; b) Child-Pugh class A, B, or C that could be improved to A or B; c) exact pathology of HCC; and d) complete liver tumor removal (R0 resection).
The exclusion criteria were: a) concomitant presence of another cancer; b) cardiovascular-pulmonary disease, or poor liver reserve preventing surgery; c) positive incisional margin; d) incomplete clinicopathological or follow-up data; and e) death within 90 days postoperatively.
Clinicopathological data and follow-up information (including postoperative recurrence time, post-recurrence treatment modality, and survival outcomes) were collected every 3–6 months. All procedures were performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was reviewed and approved by the Institutional Review Board of Hunan Provincial People’s Hospital (The First Hospital Affiliated with Hunan Normal University; no. 2015-01). For patients recruited retrospectively between January 2012 and December 2014, ethical approval was waived due to the retrospective nature of the study; patients’ privacy was ensured and the data were anonymized or maintained with confidentiality. Patients recruited prospectively between January 2015 and June 2019 provided written informed consent prior to study inclusion.
Preoperative Assessment
Patients’ demographic and clinicopathological data were collected routinely at admission. The latest preoperative laboratory and imaging examination results were used in this study. Preoperative diagnoses of HCC were confirmed by at least two types of imaging examination [eg, four-phase multidetector contrast-enhanced dynamic computed tomography (CT) and magnetic resonance imaging (MRI)], or by a single imaging examination accompanied by alpha fetoprotein (AFP) level >400 μg/L, or by histopathological assessment.39 When necessary, positron emission tomography (PET) was performed to rule out suspected distant metastasis before confirming the possibility of curative hepatectomy. Child-Turcotte-Pugh (CTP) scores were used to assess liver function and reserve. Portal hypertension (PH) was diagnosed by endoscopic findings of esophageal or gastric varices, or clinical signs of splenomegaly with platelet count < 104/mm3.39 PH was classified as mild (slight esophagogastric varices), moderate (obvious esophagogastric varices with no visible “red wale” sign), and severe (obvious esophagogastric varices with visible “red wale” signs). Values for indicators of liver fibrosis and cirrhosis were calculated using the following formulae: the aspartate aminotransferase/platelet ratio index (APRI) = [(AST (IU/L)/ULN of AST) × 100]/(platelet count × 109/L),40 fibrosis index based on four factors (FIB-4) = [age (years) × AST (IU/L)]/[platelet count (109/L) × ALT (IU/L)1/2)],41 albumin-bilirubin (ALBI) score = –0.085 × [albumin (g/L) + log10 bilirubin (mmol/L) × 0.66], ALBI grade 1 = ALBI < –2.60, ALBI grade 2 = –2.60 < ALBI = –1.39, ALBI grade 3 = ALBI > –1.39,42 and γ-glutamyl transpeptidase/platelet ratio (GPR) = {[GGT (IU/L)/ULN of GGT] × 100}/[platelet count (109/L)].43
Assessment of Tumor Staging, Characteristics and Pathological Evaluation
The American Joint Committee on Cancer’s TNM staging system (8th edition),44 the Barcelona Clinic Liver Cancer system,45 and the Hong Kong Liver Cancer (HKLC) system46 were adopted for tumor staging. Data on tumor characteristics, such as tumor size and number, differentiation, encapsulation, incisional margin status, microvascular invasion (MVI), and immunohistochemical markers, were recorded from pathological reports. Tumor size was defined as the maximum diameter of the specimen on pathological examination. Tumor number was classified as 1, 2, 3, and ≥4 (with satellite nodules). Tumor encapsulation was classified as presence/absence. Tumor cell differentiation was classified according to the Edmondson-Steiner system.47 The shortest distance from the microscopic edge of the tumor to the liver transection plane was also measured. Microvascular invasion (MVI) was defined as the presence of a cancer cell nest in vessels lined with endothelial cells on microscopy, and was classified as absent (M0), M1 (MVI < 5 and ≤1 cm from adjacent liver tissue), and M2 (MVI > 5 or >1 cm from adjacent liver tissue).48 Liver tissue inflammation and fibrosis in the non-tumor area were graded according to the METAVIR scoring system.49 Inflammatory activity was graded as absent (A0), mild (A1), moderate (A2), and severe (A3). Liver fibrosis was classified as absent (F0), portal fibrosis (F1), periportal fibrosis (F2), septal fibrosis (F3), and cirrhosis (F4). Two pathologists independently examined pathological specimens postoperatively, and consensus was reached by discussion in case of disagreement.
Surgical Therapies
The Couinaud liver segmentation criteria50 were adopted; curative open laparotomy was performed in 276 cases and curative laparoscopic hepatectomy was performed in 218 cases. The type of surgery depended on clinical guidelines and surgeons’ preference. The same oncological principle and management guidelines were applied for all open and laparoscopic liver resections. Curative hepatectomy was defined as complete resection of all tumor nodules without involvement of any major branch of the portal or hepatic vein.48 Hepatectomy modality selection was usually based on clinical guidelines combined with the remnant liver volume and hepatic functional reserve, expressed as the Child-Turcotte-Pugh (CTP) score. Intraoperative ultrasound examination was used to identify occult nodules that were not visible on preoperative radiological examination, and to further clarify relationships between tumors and main vascular structures, guaranteeing a safe and effective parenchymal transection plane. Additional nodules detected during the procedures were also removed.
The type of hepatectomy (anatomic or non-anatomic resection51) performed was selected as follows. Anatomic resection, defined as complete excision of at least one segment [ie, segmentectomy, sectoriectomy, or (tri-) hemihepatectomy] based on Couinaud’s classification, was preferred for patients with satisfactory liver function reserve. Subsegmentectomy (of <1 Couinaud segment) was performed under ultrasound guidance, with complete removal of the liver tissue supplied by the third-order portal vein branch.51 Non-anatomic resection was performed as wedge resection and tumor enucleation. In principle, the goal of hepatectomy was the achievement of an incisional margin ≥2 cm, or confirmation of a tumor-free incisional margin when this goal could not be attained. Hepatectomy was facilitated by forceps crushing or harmonic scalpel instrumentation with intermittent Pringle maneuvers; when necessary, blood flow in the liver was selectively occluded (in cycles of 15 min vascular clamping and 5 min release) to prevent massive blood loss. Patients whose preoperative HBV-DNA levels exceeded 1.00E + 02 copies/mL were given oral nucleoside/nucleotide analogs (eg, entecavir, 0.5 mg/day) before surgery. Postoperative adjuvant therapies such as transcatheter arterial chemoembolization (TACE) or targeted drug were recommended based on individual assessments of postoperative recurrence risk. Complications occurring within 90 days of surgery were graded (I–V) based on the Clavien-Dindo classification system.52
Postoperative Recurrence and Treatment
HCC recurrence or metastasis was detected by confirmative imaging (contrast-enhanced CT/MRI) findings; contrast-enhanced ultrasound and chest/bone CT were performed when necessary. Hepatic arteriography or PET/CT was performed for patients with postoperative serum AFP elevation, which raised strong suspicion of recurrence without imaging evidence. The date of recurrence, site of recurrence (intrahepatic and/or extrahepatic), and size and number of recurrent nodules were recorded. Early recurrence (ER) and late recurrence (LR) were defined as recurrence at ≤2 and >2 years after hepatectomy, respectively.
Patients with tumor recurrence were managed with various therapeutic modalities. Re-resection or metastasectomy was preferred for those with resectable tumors. Microwave ablation was offered to patients whose tumors could not be removed surgically (ie, those with recurrent intrahepatic tumors <3 cm, numerous tumors, scattered recurrence, or poor liver function reserve). Other treatment modalities, such as TACE, radiation therapy, and targeted therapy with sorafenib (Nexavar™; Bayer Health Care Pharmaceuticals, Inc. Whippany, NJ, USA), were used for those in satisfactory physical condition but who could not tolerate the above-mentioned treatments. Best supportive care was provided for patients with poor health status.
Follow-Up
All patients included in this study were followed by telephone inquiry or clinical re-examination. Follow-up assessments included routine evaluation of liver function, HBV-DNA and serum AFP levels, and imaging examination (ultrasonography, enhanced CT or MRI) every 3 months for the first 2 years and every 6 months thereafter. For patients in whom recurrence or metastasis was strongly suspected (based on abnormal AFP levels, but without radiological evidence), hepatic arteriography, PET/CT, or bone scintigraphy was performed. The clinical endpoints were 3- and 5-year OS, and follow-up was censored on 31 October 2019. The TTR was defined as the interval between the date of surgery and the date of tumor recurrence diagnosis. OS was calculated as the time from the date of surgery to the date of patient death or last follow-up. PRS was calculated from the date of tumor recurrence to the date of patient death or last follow-up. Information on deaths was obtained by notification from family members of the deceased.
Statistical Analyses
Descriptive analysis was performed for all demographic study variables. Frequencies and proportions were calculated for categorical variables, and medians and standard deviations were calculated for continuous variables. Categorical variables were compared using the Wilcoxon rank sum test. The Log rank test was used to compare PRS after the management of ER and LR. Cox proportional-hazard regression analysis was used to identify factors potentially associated with 3- and 5-year OS. Predictors that were significant (P < 0.05) in univariate analysis were selected for multivariate analysis using the backward stepwise method (threshold P < 0.05), for the construction of nomograms for the prediction of 3- and 5-year OS. Corresponding 95% confidence intervals (CIs) and hazard ratios were calculated. Areas under time-dependent receiver operating characteristic curves (AUCs) for 3- and 5-year OS on the nomograms were calculated to verify predictive accuracy. The nomograms were internally validated by 2000 bootstrap resampling of 75% of the original data. The discriminability of the nomograms was assessed by constructing AUCs and calculating Harrell’s concordance (C-) index values (range, 0.5–1). Calibration curves were plotted to assess the predictive performance of the nomograms. The calibration ability (agreement between predicted and observed frequency probabilities) was verified using the Hosmer-Lemeshow (H-L) chi-squared and goodness-of-fit tests, with P > 0.05 considered to indicate good fit. The ability to predict 3- and 5-year OS was evaluated by comparing AUCs from time-dependent ROC and C-index analyses between the proposed nomograms and the three HCC staging systems (8th AJCC-TNM, BCLC, and HKLC systems). All statistical analyses (which yielded two-tailed values) were performed using the R software (version 3.3.0) with the rms package (version 5.1-1; http://www.R-project.org) and SPSS software (version 23.0; IBM Corporation, Armonk, NY, USA). P < 0.05 was considered to indicate significance.
Results
Clinicopathological Characteristics
A flow diagram of cohort selection is shown in Figure 1. In total, 494 of 835 patients with HCC receiving hepatectomy met the eligibility criteria and were enrolled in this study. Of the patients excluded, 84 had portal-vein tumor thrombosis, 11 had hepatic-vein tumor thrombosis, 23 had biliary tumor thrombus, 9 had positive resection margins, 45 had incomplete clinical data, 7 died within 90 days postoperatively, and 162 had missing follow-up data. The characteristics of the 494 patients included in this study are summarized in Table 1. The majority (86.8%) of patients were male, the median age was 53.0 years (range, 15–85 years), and 82.6% of patients were HBsAg positive. Most (96.2%) patients were classified as Child-Pugh grade A, and 19 (3.8%) patients were classified as Child-Pugh grade B. The median follow-up period was 30.0 months (range, 3.2–113.5 months).
 |  |  |  |  |  |  |
Table 1 Patient Characteristics and Univariate Results for 3- and 5-Year Postoperative Overall Survival |
 |
Figure 1 Diagram of study flow. |
Recurrence, Survival, and Post-Recurrence Management
Postoperative recurrence was detected in 249 patients by the end of the follow-up period. The median TTR was 10.4 months (range, 1.0–153.0 months). The cumulative recurrence rates at 1–5 years were 28.1% (139/494), 41.1% (203/494), 46.8% (231/494), 48.6% (240/494), and 49.8% (246/494), respectively. The OS rates at 1–5 years were 77.5% (383/494), 51.8% (256/494), 30.4% (150/494), 16.6% (82/494), and 8.1% (40/494), respectively.
ER occurred in 203 patients. One hundred fifty-three of these patients had intrahepatic recurrence alone; 32 patients had synchronous intrahepatic and extrahepatic recurrence with metastasis to the lung (n = 18), peritoneal cavity (n = 8), lymph node (n = 3), bone (n = 1), right adrenal gland (n = 1), and brain (n = 1); and 18 patients had extrahepatic recurrence alone with metastasis to the peritoneal cavity (n = 4), lung (n = 7), lymph node (n = 3), bone (n = 3), and brain (n = 1). ER was treated by re-resection (n = 29), microwave ablation (n = 17), TACE (n = 107), radiotherapy (n = 2), targeted drugs (n = 12), and supportive care (n = 36).
LR was detected in 46 patients. Forty-one patients had intrahepatic recurrence alone; one patient had synchronous intrahepatic and extrahepatic recurrence with metastasis to the lung; and four patients had extrahepatic recurrence alone with metastasis to the peritoneal cavity (n = 1), right adrenal gland and brain (n = 1), and chest wall and mediastinal lymph nodes (n = 2). Recurrence was detected in 3 of these 46 patients at 62.3, 67.6, and 86.1 months, respectively. LR was treated by re-resection (n = 21), microwave ablation (n = 4), TACE (n = 10), and supportive care (n = 11). The recurrence site and treatment differed significantly between ER and LR cases (Wilcoxon rank sum test, P =0.029 and 0.004, respectively; Table 2).
 |
Table 2 Comparison of Recurrence Sites and Treatments Between ER and LR |
PRS of Patients with ER After Post-Recurrence Treatment
Among patients with intrahepatic ER, PRS did not differ between patients undergoing curative hepatectomy and microwave ablation (51.0 and 41.2 months, respectively). PRS was superior in these two groups of patients to that of those receiving TACE (14.2 months; χ2 = 16.630 and 13.072, respectively; both P < 0.0001) and conservative treatment (6.5 months; χ2 = 26.951 and 23.208, respectively; both P < 0.0001). The PRS of patients undergoing TACE was superior to that of those receiving conservative treatment (χ2 = 17.781, P < 0.0001). The PRS of patients receiving immunotargeted therapy (27.6 months) did not differ from that of those undergoing curative hepatectomy, microwave ablation, or TACE, but was superior to that of those receiving conservative treatment (χ2 = 8.381, P = 0.004; Tables 3 and 4). As only one patient each in the extrahepatic ER and intrahepatic and extrahepatic ER groups received microwave ablation and radiation therapy, these treatment modalities were not included in Log rank testing. Among patients with extrahepatic ER, the PRS of patients undergoing curative hepatectomy (11.8 months) did not differ from that of those receiving TACE and conservative treatment (10.7 and 8.3 months, respectively), but was superior to that of those receiving immunotargeted therapy (7.2 months; χ2 = 4.543, P = 0.033). PRS did not differ among patients receiving TACE, immunotargeted therapy, and conservative treatment (Tables 3 and 4).
 |
Table 3 Median Post-Recurrence Survival of Patients with Early Recurrence After Recurrence Management |
 |
Table 4 Comparison of Post-Recurrence Survival After Early Recurrence (Log Rank Test) |
In patients with intrahepatic and extrahepatic ER, the PRS of patients undergoing curative hepatectomy (11.933 months) did not differ from that of those receiving TACE, immunotargeted therapy, and conservative treatment (10.010, 16.033, and 5.004 months, respectively). The PRS of patients receiving TACE and immunotargeted therapy was superior to that of those receiving conservative treatment (χ2 = 4.170, P = 0.041 and χ2 = 4.021, P = 0.045, respectively; Tables 3 and 4).
PRS of Patients with LR After Post-Recurrence Treatment
Among patients with intrahepatic LR, PRS did not differ between patients undergoing curative hepatectomy and microwave ablation (18.5 and 17.3 months, respectively), but was superior in these two groups to that of those undergoing TACE (10.600 months; χ2 = 11.446, P = 0.001 and χ2 = 4.914, P = 0.027, respectively) and conservative treatment (7.350 months; χ2 = 11.983, P = 0.001 and χ2 = 4.091, P = 0.043, respectively). PRS did not differ between patients receiving TACE and conservative treatment (Tables 5 and 6). As only one patient with extrahepatic LR underwent re-resection and only one patient had intrahepatic and extrahepatic LR, these cases were not included in Log rank testing.
 |
Table 5 Median Post-Recurrence Survival of Patients with Late Recurrence After Recurrence Management |
 |
Table 6 Comparison of Post-Recurrence Survival After Late Recurrence (Log Rank Test) |
Three- and 5-Year OS
The results of multivariable analyses for the prediction of 3- and 5-year postoperative OS were shown in Tables 7 and 8, respectively. Independent influencing factors for 3-year OS were HKLC stage, γ-glutamyl transferase (γ-GGT) level, METAVIR inflammation activity grade, ER, and post-recurrence treatment modality (Table 7). Predictors associated independently with 5-year OS were γ-GGT level >60 U/L, hepatectomy extent, LR, and post-recurrence treatment modality (Table 8).
 |
Table 7 Multivariate Results for Postoperative 3-Year Overall Survival |
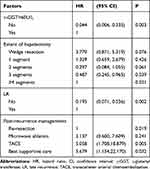 |
Table 8 Multivariate Results for Postoperative 5-Year Overall Survival |
Nomograms for 3- and 5-Year OS Prediction
The prognostic nomograms for 3- and 5-year OS are presented in Figures 2A and 3A, respectively. The AUCs of the nomograms for 3- and 5-year OS prediction were 0.891 [95% CI, 0.8364–0.9447; C-index = 0.7945 (95% CI, 0.7054–0.8541); Figure 2B] and 0.864 [95% CI, 0.8041–0.9237; C-index = 0.7033 (95% CI, 0.6879–0.7958); Figure 3B], respectively. In bootstrap analysis for internal validation, the AUCs for 3-year OS prediction in the development and validation sets were 0.886 [95% CI, 0.7986–0.9137; C-index = 0.8050 (95% CI, 0.7000–0.8970)] and 0.792 [95% CI, 0.7280–0.8605; C-index = 0.7710 (95% CI, 0.7039–0.8374)], respectively (Figure 2C); the AUCs for 5-year OS prediction in the development and validation sets were 0.815 [95% CI, 0.7235–0.9011; C-index = 0.7870 (95% CI, 0.6420–0.9322)] and 0.774 [95% CI, 0.6553–0.8329; C-index = 0.7227 (95% CI, 0.6460–0.8830)], respectively (Figure 3C). The bootstrap-corrected calibration plots showed good concordance between the predicted and actual observation cohorts, indicating the reliability of the nomograms (Figures 2D and 3D, respectively). The H-L chi-squared calibration values of 3- and 5-year OS were 8.678 (P = 0.370) and 5.453 (P = 0.708), respectively.
 |
Figure 3 (A) Nomogram for the assessment of 5-year overall survival (OS) of patients with hepatocellular carcinoma who have undergone curative hepatectomy. The nomogram is used as described for Figure 2. (a). γ-GGT, γ-glutamyl transferase; LR, late recurrence. Extent of hepatectomy: 0 = wedge resection, 1 = one Couinaud segment, 2 = two Couinaud segments, 3 = 3 Couinaud segments, 4 = ≥4 Couinaud segments. Recurrence management: 0 = no recurrence, 1 = re-resection; 2 = microwave ablation, 3 = transarterial chemoembolization, 4 = supportive care. (B) The area under the time-dependent receiver operating characteristic curve (AUC) of the nomogram for 5-year OS. (C) Internal validation by bootstrap analysis. ROC, receiver operating characteristic; D, development; V, validation. (D) Calibration plot showing the relationships between predicted and actual probabilities based on the nomogram. The x and y axes represent the nomogram-predicted and actual probabilities, respectively, of 5-year death. Red solid circles represent the curve fitting line, and blue hollow circles represent nomogram-predicted probabilities with 95% confidence intervals. The green reference line indicates the ideal nomogram, in which actual and predicted probabilities are perfectly identical. The calibration curve shows good prognostic performance. The Hosmer-Lemeshow chi-squared value for calibration was 5.453 (P = 0.708). |
Comparison of 3-Year OS Prediction Using the Nomogram and Representative HCC Staging Systems
The AUC from the time-dependent ROC analysis for the proposed nomogram for 3-year OS prediction was 0.891 (95% CI, 0.8364–0.9447; Figure 2A), which was greater than the AUCs obtained for the three representative HCC staging systems (8th AJCC-TNM system, 0.5370; BCLC system, 0.4901; HKLC system, 0.5396; Figure 4A–C). The C-index for the proposed 3-year OS predictive nomogram [0.7945 (95% CI, 0.7054–0.8541)] was much higher than those obtained with the three staging systems [8th AJCC-TNM system, 0.5316 (95% CI, 0.4815–0.5817); BCLC system, 0.4995 (95% CI, 0.4518–0.5472); HKLC system, 0.5440 (95% CI, 0.5031–0.5848)].
Comparison of 5-Year OS Prediction Using the Nomogram and Representative HCC Staging Systems
The AUC from the time-dependent ROC analysis for the proposed nomogram for 5-year OS prediction was 0.864 (95% CI, 0.8041–0.9237; Figure 3A), which was greater than the AUCs obtained for the three representative HCC stage systems (8th AJCC-TNM system, 0.5459; BCLC system, 0.5269; HKLC system, 0.5944; Figure 5A–C). The C-index for the proposed 5-year OS predictive nomogram [0.7033 (95% CI, 0.6879–0.7958)] was much higher than those obtained with the three staging systems [8th AJCC-TNM system, 0.5552 (95% CI, 0.4389–0.6255); BCLC system, 0.4961 (95% CI, 0.3969–0.5953); HKLC system, 0.5656 (95% CI, 0.4714–0.6199)].
Discussion
In this study, factors potentially influencing OS among Chinese patients with HCC undergoing curative hepatectomy were explored. Using accessible clinical parameters (preoperative and pathological characteristics), clear classifications of ER and LR, and consideration of post-recurrence treatment modality, novel prognostic nomograms for postoperative 3- and 5-year OS were proposed. These nomograms overcome the shortcomings of previous prediction systems and nomograms, which were constructed without consideration of the timing of postoperative recurrence or the post-recurrence treatment modality. The prognostic performance of the proposed nomograms was verified by internal validation, and C-index values were superior to those reported for other nomograms and scoring systems (Table 9).4–30 The use of these nomograms is expected to improve the survival of patients with HCC who are eligible for curative therapy, allowing clinicians to make appropriate predictions and perform effective surveillance as early as possible. The inclusion of variables reflecting recurrence status and post-recurrence treatment effectiveness significantly improves predictive accuracy.
 |
Table 9 Published Nomograms for the Assessment of Overall Survival After Curative Hepatectomy |
Recurrence after curative hepatectomy leads to the poor OS of patients with HCC. However, multivariate analyses performed with the simple dichotomization of recurrence as present or absent showed no effect on postoperative 3- or 5-year OS. We examined the effects of other variables (ie, TTR ≤ 1 year, ER, TTR ≤ 3 years, and LR) to clarify the effects of recurrence timing on postoperative OS. ER and LR have distinct underlying mechanisms53 and seem to have different effects on patients’ postoperative survival. ER may represent “true recurrence,” reflecting the latent aggressive biological behavior of HCC at the time of initial surgical resection. LR is assumed to be caused by the multicentric metastasis of de-novo tumors in the background of liver fibrosis or cirrhosis.53 In this study, the re-resection rate was significantly higher in the LR group, indicating that re-resection of recurrent HCC is suitable for and would prolong PRS in only a small proportion of patients with ER. Thus, the timepoint distinguishing ER and LR should be included in prognostic nomograms.
Chronic liver disease staging affected postoperative 3-year OS, with significant differences between grades A0–A2 and the indicator category of grade A3 (P = 0.030, 0.015, and 0.024, respectively). Viral replicative activity in the non-neoplastic liver has been reported to be associated with poor OS, independent of fibrosis stage. Moreover, viral infection and inflammatory response increase the potential for malignant transformation.54 We found that neither initial tumor characteristics nor HCC staging influenced postoperative 5-year OS. Moreover, some indicators used in the evaluation of liver fibrosis and cirrhosis, such as the APRI, FIB-4, ALBI, CTP grade, GPR, and METAVIR score for the postoperative pathological evaluation of non-neoplastic liver tissue, did not affect 5-year OS. In view of these results, we speculate that a) obvious inflammation and/or fibrosis heterogeneities may exist among different regions of liver tissue, meaning that inflammation and fibrosis staging of non-tumor hepatic segments after curative hepatectomy would not represent the whole liver status; and b) the correlation between HCC development and chronic liver inflammation may be more complex than recognized, and monitoring systems/models and follow-up strategies based on the management of chronic liver disease may not be suitable for patients with HCC after curative hepatectomy.
The extent of hepatectomy was found to influence the 5-year OS of patients with HCC after curative hepatectomy. Although the extent of liver resection reflects the surgical technique used, it is likely also a surrogate indicator of tumor aggressiveness. Patients with HCC who have good liver function reserves can better tolerate extensive liver resection, which in turn reduces the impact of MVI on postoperative recurrence and survival. Moreover, good liver function reserve after recurrence permits reoperation, microwave ablation, or TACE. Thus, the effect of extensive hepatectomy on postoperative 5-year OS cannot be explained solely by the liver function reserve.
We found that postoperative PRS was influenced significantly by the post-recurrence treatment modality. The implementation of effective therapeutic strategies is important to prolong survival after curative hepatectomy. However, previous prognostic nomograms did not take these factors into consideration. In this study, PRS was predicted with differentiation of ER and LR at different sites after different post-recurrence treatments, rather than simple comparison of post-recurrence treatments. Re-resection and microwave ablation were found to be associated with improved 3 and 5-year PRS. The significant correlation between post-recurrence treatment and PRS indicates that patient survival can be improved by repeated treatment after recurrence. Curative treatments, such as re-resection and microwave ablation, lead to better survival outcomes, as confirmed in this study and a previous study.55 However, clinical decisions about the post-recurrence treatment modality can be difficult to make due to the lack of universally recognized consensus.56 The significant correlation between post-recurrence treatment and PRS indicates that patient survival can be improved by repeated treatment after recurrence.
This study revealed no significant difference in OS between curative resection and conservative treatment, although curative resection was superior to immunotargeted drug therapy for patients with extrahepatic ER. Moreover, no significant difference was found in the benefits provided by TACE, immunotargeted drug therapy, and conservative treatment in these patients. Thus, various therapeutic approaches for extrahepatic ER and LR after curative hepatectomy need to be evaluated in further large-sample multicenter studies. However, few studies have shown beneficial effects of isolated extrahepatic recurrence resection.56 In our study, TACE was found to be an inferior post-recurrence modality for patients who could not tolerate secondary excision or microwave ablation. Radiotherapy is an effective and safe therapeutic approach for patients with HCC after recurrence, even for those with inferior vena cava/right atrium tumor thrombi.57 Immunotargeted drug therapy has been applied in a small proportion of cases due to its limited cost effectiveness. Thus, further studies are needed to evaluate the efficacy of different and combined treatment modalities for patients with recurrence after curative hepatectomy.
The γ-GGT level was found to affect 3- and 5-year OS after recurrence in this study. It is a cell membrane-bound enzyme that plays crucial roles in glutathione metabolic processes. The pretreatment serum γ-GGT level reflects the chronic inflammatory status of HCC, especially that caused by HBV.58 HBV infection is the main etiology of HCC in China.2 In this study, 82.6% of patients were HBV infected and some patients showed HBsAg or hepatitis B antigen serological conversion. Further clinical studies are needed to verify the effects of γ-GGT, determine cut-off values for this indicator, and assess its prognostic performance for patients with HCC.
Study Limitations
This study has several limitations. a) The proposed nomograms were based on single-center retrospective and prospective data. Given the low prevalence of 5-year OS, we could not create an external validation group; instead, we internally validated the nomogram. External validation in large-sample multicenter prospective studies is required. b) The proposed nomograms may be suitable primarily for patients with HBV-related HCC; such patients comprised the majority of our sample. Thus, the nomograms should be applied cautiously to HCC populations with HCV infection, non-alcoholic steatohepatitis, and other etiological backgrounds. c) In this study, decisions about the postoperative recurrence management strategies adopted were based primarily on existing guidelines, with consideration of patients’ clinical status and recurrent tumor characteristics. However, management decisions may have been affected by patients’ preference for less-invasive treatment and a lesser financial burden, which may have introduced bias. In addition, we considered only primary postoperative recurrence treatment modalities, although some patients received sequential treatments. The use of multiple and/or sequential treatment modalities may provide additional benefits that were not accounted for, which would reduce the quality of the evidence obtained in this study.
Conclusion
In this study, novel nomograms for the prediction of 3- and 5-year OS of patients with HCC after curative hepatectomy performed well in the context of real clinical practice in China. These nomograms were constructed with the distinction of postoperative ER and LR and the inclusion of post-recurrence treatment modality, which improves predictive efficacy for OS. The current study illustrates the importance not only of distinguishing ER and LR but also of comparing PRS according to recurrence site and treatment type. Our novel nomograms showed better calibration ability and prognostic performance than previously proposed systems and nomograms.
Data Sharing Statement
All data are available from the corresponding author, Wei XU, upon reasonable request. The data are not publicly available due to privacy and ethical restrictions.
Ethics Approval and Consent to Participate
This study was reviewed and approved by the Institutional Review Board of Hunan Provincial People’s Hospital (The First Hospital Affiliated with Hunan Normal University; no. 2015-01). For patients recruited retrospectively between January 2012 and December 2014, ethical approval was waived in view of the retrospective nature of the study; the patients’ privacy was ensured and the data were anonymized or maintained with confidentiality. Patients recruited prospectively between January 2015 and June 2019 provided written informed consent prior to study inclusion.
Funding
This study was supported by funds from the Education Department (project no. 17C0969) and Health Commission (project no. 20200074) of Hunan Province.
Disclosure
The authors declare that they have no competing interest.
References
1. Wong MCS, Huang JLW, George J, et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019;16:57–73.
2. Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi:10.3322/caac.21387
4. Cho CS, Gonen M, Shia J, et al. A novel prognostic nomogram is more accurate than conventional staging systems for predicting survival after resection of hepatocellular carcinoma. J Am Coll Surg. 2008;206:281–291. doi:10.1016/j.jamcollsurg.2007.07.031
5. Huang S, Huang GQ, Zhu GQ, et al. Establishment and validation of SSCLIP scoring system to estimate survival in hepatocellular carcinoma patients who received curative liver resection. PLoS One. 2015;10:e0129000. doi:10.1371/journal.pone.0129000
6. Shim JH, Jun MJ, Han S, et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg. 2015;261:939–946. doi:10.1097/SLA.0000000000000747
7. Li Y, Xia Y, Li J, et al. Prognostic nomograms for pre- and postoperative predictions of long-term survival for patients who underwent liver resection for huge hepatocellular carcinoma. J Am Coll Surg. 2015;221:962–74.e4. doi:10.1016/j.jamcollsurg.2015.08.003
8. Shen J, He L, Li C, et al. Prognostic nomograms for patients with resectable hepatocellular carcinoma incorporating systemic inflammation and tumor characteristics. Oncotarget. 2016;7:80783–80793. doi:10.18632/oncotarget.13038
9. Li J, Zhou J, Yang PH, et al. Nomograms for survival prediction in patients undergoing liver resection for hepatitis B virus related early stage hepatocellular carcinoma. Eur J Cancer. 2016;62:86–95. doi:10.1016/j.ejca.2016.04.011
10. Yang P, Qiu J, Li J, et al. Nomograms for pre- and postoperative prediction of long-term survival for patients who underwent hepatectomy for multiple hepatocellular carcinomas. Ann Surg. 2016;263:778–786. doi:10.1097/SLA.0000000000001339
11. Hu H, Han XK, Long XR, et al. Prognostic nomogram for post-surgical treatment with adjuvant TACE in hepatitis B virus-related hepatocellular carcinoma. Oncotarget. 2016;7:58302–58314. doi:10.18632/oncotarget.11078
12. Hsu CY, Liu PH, Hsia CY, et al. Surgical resection is better than transarterial chemoembolization for patients with hepatocellular carcinoma beyond the Milan Criteria: a prognostic nomogram study. Ann Surg Oncol. 2016;23(3):994–1002. doi:10.1245/s10434-015-4929-x
13. Torzilli G, Donadon M, Belghiti J, et al. Predicting individual survival after hepatectomy for hepatocellular carcinoma: a novel nomogram from the “HCC East & West Study Group”. J Gastrointest Surg. 2016;20:1154–1162. doi:10.1007/s11605-016-3132-0
14. Fu YP, Ni XC, Yi Y, et al. A novel and validated Inflammation-Based Score (IBS) predicts survival in patients with hepatocellular carcinoma following curative surgical resection: a STROBE-Compliant article. Medicine (Baltimore). 2016;95:e2784. doi:10.1097/MD.0000000000002784
15. Feng LH, Dong H, Lau WY, et al. Novel microvascular invasion-based prognostic nomograms to predict survival outcomes in patients after R0 resection for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2017;143:293–303. doi:10.1007/s00432-016-2286-1
16. Shen J, He L, Li C, et al. Nomograms to predict the individual survival of patients with solitary hepatocellular carcinoma after hepatectomy. Gut Liver. 2017;11:684–692. doi:10.5009/gnl16465
17. Fu YP, Yi Y, Huang JL, et al. Prognostic nomograms stratify survival of patients with hepatocellular carcinoma without portal vein tumor thrombosis after curative resection. Oncologist. 2017;22:561–569. doi:10.1634/theoncologist.2016-0231
18. Zhang W, Tan Y, Shen S, et al. Prognostic nomogram for hepatocellular carcinoma in adolescent and young adult patients after hepatectomy. Oncotarget. 2017;8:106393–106404. doi:10.18632/oncotarget.18192
19. Jing CY, Fu YP, Zheng SS, et al. Prognostic nomogram for patients with hepatocellular carcinoma underwent adjuvant transarterial chemoembolization following curative resection. Medicine (Baltimore). 2017;96(11):e6140. doi:10.1097/MD.0000000000006140
20. Sun HC, Xie L, Yang XR, et al. Shanghai score: a prognostic and adjuvant treatment-evaluating system constructed for Chinese patients with hepatocellular carcinoma after curative resection. Chin Med J (Engl). 2017;130(22):2650–2660. doi:10.4103/0366-6999.218019
21. Zheng BH, Liu LZ, Zhang ZZ, et al. Radiomics score: a potential prognostic imaging feature for postoperative survival of solitary HCC patients. BMC Cancer. 2018;18(1):1148. doi:10.1186/s12885-018-5024-z
22. Huang JL, Fu YP, Jing CY, et al. A novel and validated prognostic nomogram based on liver fibrosis and tumor burden for patients with hepatocellular carcinoma after curative resection. J Surg Oncol. 2018;117:625–633. doi:10.1002/jso.24895
23. Gan W, Yi Y, Fu Y, et al. Fibrinogen and C-reactive protein score is a prognostic index for patients with hepatocellular carcinoma undergoing curative resection: a prognostic nomogram study. J Cancer. 2018;9:148–156. doi:10.7150/jca.22246
24. Cai BB, Shi KQ, Li P, et al. A nomogram integrating hepatic reserve and tumor characteristics for hepatocellular carcinoma following curative liver resection. Clin Chim Acta. 2018;485:187–194. doi:10.1016/j.cca.2018.06.020
25. Cao Y, Jiang Z, Wang S, Zhang H, Jiang Y, Lv L. Prediction of long-term survival rates in patients undergoing curative resection for solitary hepatocellular carcinoma. Oncol Lett. 2018;15:2574–2582.
26. Hu Y, You S, Yang Z, Cheng S. Nomogram predicting survival of hepatocellular carcinoma with portal vein tumour thrombus after curative resection. ANZ J Surg. 2019;89:E20–E25. doi:10.1111/ans.14708
27. Wang Y, Sun K, Shen J, et al. Novel prognostic nomograms based on inflammation-related markers for patients with hepatocellular carcinoma underwent hepatectomy. Cancer Res Treat. 2019;51:1464–1478. doi:10.4143/crt.2018.657
28. Kim JM, Kwon CHD, Joh JW, et al. Nomograms in hepatectomy patients with hepatitis B virus-related hepatocellular carcinoma. J Gastrointest Surg. 2019;23:1559–1567.
29. Chen L, Cai BB, Zhou CJ, et al. A sample model established by S-index predicting overall survival after curative resection of primary hepatocellular carcinoma. Cancer Manag Res. 2019;11:693–703. doi:10.2147/CMAR.S193593
30. Lin ZX, Ruan DY, Jia CC, et al. Controlling nutritional status (CONUT) score-based nomogram to predict overall survival of patients with HBV-associated hepatocellular carcinoma after curative hepatectomy. Clin Transl Oncol. 2020;22:370–380. doi:10.1007/s12094-019-02137-4
31. Huang ZY, Liang BY, Xiong M, et al. Long-term outcomes of repeat hepatic resection in patients with recurrent hepatocellular carcinoma and analysis of recurrent types and their prognosis: a single-center experience in China. Ann Surg Oncol. 2012;19:2515–2525. doi:10.1245/s10434-012-2269-7
32. Ochiai T, Ikoma H, Okamoto K, et al. Clinicopathologic features and risk factors for extrahepatic recurrences of hepatocellular carcinoma after curative resection. World J Surg. 2012;36:136–143. doi:10.1007/s00268-011-1317-y
33. Meniconi RL, Komatsu S, Perdigao F, Boëlle PY, Soubrane O, Scatton O. Recurrent hepatocellular carcinoma: a Western strategy that emphasizes the impact of pathologic profile of the first resection. Surgery. 2015;157:454–462. doi:10.1016/j.surg.2014.10.011
34. Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947–955. doi:10.1097/SLA.0000000000000710
35. Notake T, Kobayashi A, Shinkawa H, et al. Nomogram predicting long-term survival after the diagnosis of intrahepatic recurrence of hepatocellular carcinoma following an initial liver resection. Int J Clin Oncol. 2017;22:715–725. doi:10.1007/s10147-017-1114-1
36. He W, Peng B, Tang Y, et al. Nomogram to predict survival of patients with recurrence of hepatocellular carcinoma after surgery. Clin Gastroenterol Hepatol. 2018;16:756–64.e10. doi:10.1016/j.cgh.2017.12.002
37. Saito R, Amemiya H, Hosomura N, et al. Prognostic factors for post-recurrent survival in hepatocellular carcinoma after curative resection. Anticancer Res. 2019;39(6):3033–3038. doi:10.21873/anticanres.13436
38. Xiao H, Chen ZB, Jin HL, et al. Treatment selection of recurrent hepatocellular carcinoma with microvascular invasion at the initial hepatectomy. Am J Transl Res. 2019;11:1864–1875.
39. Bruix J, Sherman M. American Association for the study of liver diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi:10.1002/hep.24199
40. Kao WY, Chiou YY, Hung HH, et al. Risk factors for long-term prognosis in hepatocellular carcinoma after radiofrequency ablation therapy: the clinical implication of aspartate aminotransferase-platelet ratio index. Eur J Gastroenterol Hepatol. 2011;23:528–536.
41. Toyoda H, Kumada T, Tada T, Kaneoka Y, Maeda A. A laboratory marker, FIB-4 index, as a predictor for long-term outcomes of hepatocellular carcinoma patients after curative hepatic resection. Surgery. 2015;157:699–707. doi:10.1016/j.surg.2014.10.022
42. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi:10.1200/JCO.2014.57.9151
43. Lemoine M, Shimakawa Y, Nayagam S, et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65:1369–1376. doi:10.1136/gutjnl-2015-309260
44. Kamarajah SK, Frankel TL, Sonnenday C, Cho CS, Nathan H. Critical evaluation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with Hepatocellular Carcinoma (HCC): a Surveillance, Epidemiology, End Results (SEER) analysis. J Surg Oncol. 2018;117:644–650. doi:10.1002/jso.24908
45. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi:10.1016/S0140-6736(18)30010-2
46. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146(1691–700):e3.
47. Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi:10.1002/1097-0142(195405)7:3<462::AID-CNCR2820070308>3.0.CO;2-E
48. Cong WM, Bu H, Chen J, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22:9279–9287. doi:10.3748/wjg.v22.i42.9279
49. Bedossa P. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15–20. doi:10.1002/hep.1840200104
50. Couinaud C. Anatomic principles of left and right regulated hepatectomy: technics (in French). J Chir (Paris). 1954;70:933–966.
51. Shindoh J, Hasegawa K, Inoue Y, et al. Risk factors of post-operative recurrence and adequate surgical approach to improve long-term outcomes of hepatocellular carcinoma. HPB (Oxford). 2013;15:31–39. doi:10.1111/j.1477-2574.2012.00552.x
52. DeOliveira ML, Winter JM, Schafer M, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:
53. Poon RT. Differentiating early and late recurrences after resection of HCC in cirrhotic patients: implications on surveillance, prevention, and treatment strategies. Ann Surg Oncol. 2009;16:792–794. doi:10.1245/s10434-009-0330-y
54. Wang Q, Lin L, Yoo S, et al. Impact of non-neoplastic vs intratumoural hepatitis B viral DNA and replication on hepatocellular carcinoma recurrence. Br J Cancer. 2016;115:841–847. doi:10.1038/bjc.2016.239
55. Erridge S, Pucher PH, Markar SR, et al. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br J Surg. 2017;104:1433–1442. doi:10.1002/bjs.10597
56. Wen T, Jin C, Facciorusso A, et al. Multidisciplinary management of recurrent and metastatic hepatocellular carcinoma after resection: an international expert consensus. Hepatobiliary Surg Nutr. 2018;7:353–371. doi:10.21037/hbsn.2018.08.01
57. Lou J, Li Y, Liang K, et al. Hypofractionated radiotherapy as a salvage treatment for recurrent hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: a multi-center analysis. BMC Cancer. 2019;19:668. doi:10.1186/s12885-019-5870-3
58. Sun P, Li Y, Chang L, Tian X. Prognostic and clinicopathological significance of Gamma-Glutamyltransferase in patients with hepatocellular carcinoma: A PRISMA-compliant meta-analysis. Medicine (Baltimore). 2019;98:e15603. doi:10.1097/MD.0000000000015603
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



