Back to Journals » Cancer Management and Research » Volume 15
Prognostic Nomogram for Lymph-Node Metastasis in Oral Squamous Cell Carcinoma (OSCC) Using Immunohistochemical Marker D2-40
Authors Sharma A, Natarajan S, Manaktala N , Boaz K, KP N, Lewis A , Yellapurkar S
Received 8 April 2023
Accepted for publication 24 July 2023
Published 1 September 2023 Volume 2023:15 Pages 929—936
DOI https://doi.org/10.2147/CMAR.S408772
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Sanjeev K. Srivastava
Ankita Sharma, Srikant Natarajan, Nidhi Manaktala, Karen Boaz, Nandita KP, Amitha Lewis, Shweta Yellapurkar
Department of Oral Pathology, Manipal College of Dental Sciences Mangalore, Manipal Academy of Higher Education, Manipal, Karnataka, India
Correspondence: Karen Boaz, Department of Oral Pathology, Manipal College of Dental Sciences Mangalore, Manipal Academy of Higher Education, Manipal, Karnataka, India, Email [email protected]
Introduction: Nomograms are proven in “individualized risk prediction” in sarcomas and breast and prostate cancers. Incorporating immunohistochemical markers and histopathological parameters can enhance accuracy of these graphical representations of statistical predictive models concerning metastasis. D2-40, a monoclonal antibody to podoplanin (regulator of motility expressed in malignant epithelial cells), dually predicts metastatic potential of tumour by estimating the motile tumour phenotype and by detecting lymphatic vessels/density, both essential to metastasis in OSCC. Thus, we propose a model that incorporates D2-40 immunostaining of individual tumour cells (ITC) too with other variables (seen in H+E staining) as a predictive nomogram.
Methods: Sixty cases of OSCC were selected with equal number of cases (n=30) of pN0 and pN+ status. Bryne’s grading of invasive front of tumour (ITF) was done on H+E-stained slides followed by D2-40 immunostaining for ITCs at ITF and lymphatic vessels. Multivariate regression analysis was used to generate the nomogram of LNM where the predictive contribution of each covariate, namely depth of invasion, D2-40-stained ITCs, gender, histological grade, and worst pattern of invasion (WPOI), was plotted on a scale of 1– 100 points.
Results: The nomogram showed that the strongest variable in OSCC was the WPOI in H+E-stained section followed by D2-40-positive ITCs and gender.
Discussion: Our predictive nomogram for LNM in OSCC surprisingly showed that a tumour with lower score of WPOI (islands vs ITC) showed numerous D2-40-positive ITCs, drastically increasing the probability of metastasis. The concept of “individualized risk prediction” can be used to predict lymph node metastasis using a variety of histopathological criteria that can be visualized in routine and immunohistochemical staining in OSCC with the aid of a nomogram.
Keywords: nomogram, individualized risk prediction, D2-40, lymph node metastasis in OSCC, podoplanin
Introduction
Metastasis/micrometastasis to the cervical lymph nodes is known to be the single most adverse prognosticator in oral squamous cell carcinoma.1 Various efforts have been directed towards establishing reproducibility and uniformity in assessment of parameters governing lymph node metastasis in different tumours at a clinical, radiologic, and microscopic level.2
Numerous statistical and mathematical models have been put forth in assessment of factors influencing prognosis in a host of tumours. One such tool is the nomogram, which is a user-friendly graphical representation of a statistical predictive model that generates a numerical probability of a clinical event. A nomogram can take into account several variables to predict an outcome of interest for an individual patient.3
This statistical method gives objectivity to evaluation of risk factors. Additionally, a combination of histopathological parameters and immunohistochemical staining might enhance accuracy of predictive models concerning lymph node metastasis in OSCC.
Dysregulation of cellular motility forms the core of the process of metastasis. Podoplanin is a molecule that regulates epithelial cell motility, while D2-40, a monoclonal antibody to podoplanin, is a 40 kDa surface sialo-glycoprotein expressed in malignant epithelial cells which modulates the actin filaments in the cytoplasm leading to formation of filopodia.4 Podoplanin is found on the leading edge of tumour cells and is thought to influence their migratory and invasive properties by regulating the cell shape and control of the intercellular adhesion as well as motility by influencing the polarity complexes and supporting platelet aggregation.5,6 Thus, D2-40 has found application in independently estimating the motility index of a tumour, with higher motility resulting in increased chances of metastases to lymph nodes and other organs.
The cytoskeleton of an epithelial cell, made up of the actin cytoskeleton, the microtubule network, and the intermediate filaments, provides structural framework and mechanical strength to maintain cell shape. A continuous reorganization of the actin is a pre-requisite for the morphology, migration, and invasion of cancer cells.7 The axis or polarization of an epithelial cell is determined by the spatial arrangement of molecules, receptors, and skeletal elements such as microtubules and actin filaments within the cell. The most influential polarity proteins, Par, are associated with Rho-GTPases (Rho → Ras associated homologous proteins), that regulate the functioning of microtubules and actin, and are important regulators in cell processes such as cell adhesion, migration, polarity, and membrane trafficking. Podoplanin aids RhoA (a Rho-GTPase which is an important regulator of stress fibre formation), which stimulates the up-regulation of proteins involved in epithelial–mesenchymal transition (EMT) as well as transcriptional repressors such as snail, involved in the down-regulation of epithelial proteins such as E-cadherin.
In human epithelial cells, podoplanin expression correlates with the redistribution of ezrin to membrane projections and the formation of actin-rich filopodia.5 The ERM (ezrin, radixin, moesin) proteins provide an essential, regulated link between integral membrane proteins and the actin cytoskeleton and are involved in co-ordinating cell shape, motility, and adhesion. Additionally, enhanced RhoA activity regulates ROCK (Rho-associated protein kinase) to sustain ERM protein phosphorylation and stabilization in an active conformation, strengthening their relationship with podoplanin. Ezrin, once recruited to the actin cytoskeleton, induces the formation of surface protrusions/filopodia.
D2-40 plays a dual role in predicting the metastatic potential of a tumour. It also detects lymphatic vessels by recognizing podoplanin, which is a small transmembrane mucin-like protein present on all lymphatic vessels.8 Consequently, lymphatic vessel density and cellular motility have been established to be vital to the process of metastasis in OSCC.
Thus, based on the established physiological role of the podoplanin molecule in cellular motility, we suggest a model that incorporates D2-40 immunohistochemical staining, along with other variables that are routinely analysed in H+E staining, as a predictive nomogram for lymph node metastasis and thus prognosis in OSCC.
Materials and Methods
The study was conducted after approval from the Institutional Ethics Committee, Manipal College of Dental Sciences, Mangalore, Manipal Academy of Higher Education (Ref no. 14134), and each patient in the study has signed a consent for investigations sequential to planned biopsy/surgery. Institutional and Department permissions were taken before retrieving slides/sections for the study.
Study Samples
Formalin-fixed, paraffin-embedded tissue sections of 60 cases of oral squamous cell carcinoma (lymph node histopathology data available) were retrieved from the departmental archives, and lymph node status was confirmed histologically using H+E-stained sections. Equal numbers of cases (n=30) each of pN0 and pN+ status were selected for immunohistochemical staining with D2-40. The histological grading was done at the invasive tumour front using Bryne’s grading criteria (from 1992) on the H+E-stained slides (Figure 1), and the corresponding section was subjected to immunohistochemical staining. The individual tumour cells (ITC) were considered as a separate criterion, and scores were designated as 0=Absent; 1=Present (ITC being dissociated cells either in groups of cells (n<15) or as single cells according to the pattern of invasion defined according to Bryne’s grading system, from 1992).
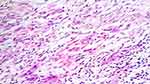 |
Figure 1 ITCs at advancing front of tumour in poorly differentiated OSCC of tongue; pN1 status (H+E stain; ×100). |
IHC Staining Methodology and Analysis
The evaluation of immunohistochemical staining of D2-40-stained lymphatic vessels (Figure 2) and cellular (cytoplasmic) staining of cells at the invasive front of tumour (Figure 3) was done. The expression was evaluated, and the predominant pattern of staining of the tumour was assigned a score using a slight modification of the Funayama et al method, which is described below.9
 |
Figure 2 Strong IHC expression of D2-40 in lymphatic vessels (arrows). |
 |
Figure 3 D2-40 staining (limited to basal layer) in moderately differentiated OSCC with pN1mi+ status (×400). |
- Score of 0 assigned for no staining
- Score of 1 assigned for positive reaction in the cell border restricted to only the 1st basal layer or cell layer immediately adjacent to the basement membrane
- Score of 2 assigned for positive staining in the basal and few supra-basal layers, but not complete staining of the tumour
- Score of 3 assigned for complete tumour staining
The lymphatic vessels were counted in 5 HPF (×10), and the average number of lymphatic vessels was calculated. The depth of the tumour was assessed using a calibrated reticule in low power magnification (4×) as the distance from the surface of the tumour to the farthest tumour satellite seen.
The nomogram was formulated with all parameters correlating with the pathological status of lymph nodes.
Results
A lymph node metastasis nomogram (Figure 4) was created using multivariate regression analysis using R software. It puts the predictive contribution of each covariate on a scale of points from 1 to 100. We utilized all the parameters that were analysed for correlating tumour behaviour and lymph node metastasis (Table 1).
 |
Table 1 Univariate and Multivariate Binary Logistic Regression Analysis to Predict Lymph Node Metastasis |
The generated nomogram showed the worst pattern of invasion in H+E being the strongest variable (WPOI → worst pattern of invasion is a parameter in Bryne’s grading system which categorizes the dissociating cells as islands (well delineated infiltrating borders)) followed by infiltrating solid cords, small groups/cords of infiltrating cells (n>15) and individual tumour cells. Cellular dissociation in small groups of cells (n<15) was assigned 100 points, followed by ITC stained by D2-40 (60 points) and gender (42 points). The depth of invasion (DOI) and average number of lymphatic vessels received almost equal scores. The remaining variables (continuous and categorical) were assigned a smaller range of score proportional to their effect size. The total point axis represents the maximum potential score and is seen to be associated with a prediction of probability of lymph node metastasis. The total points accumulated by the co-variants relate to the predicted probability of an outcome of interest for the individual patient (Figure 4).
Discussion
A nomogram is a clinical prediction model that is becoming a popular decision aid in predicting cancer risk, prevention, and treatment outcomes.10 Though the utility of nomograms in individualized risk calculation is well recognized in a variety of cancers, such as sarcomas and breast and prostate cancer, not much data for equivalent studies for head and neck tumours (particularly oral cancer) is available in the literature.11 Wang et al studied OSCC cases and Balasubramanian et al studied oral tongue cancer cases and reported that nomograms generated based on the data from the patients can be implemented as successful models in prediction of overall (OS), disease-specific (DSS), loco-regional recurrence-free (LRRFS), and distant recurrence-free survivals (DRFS).12,13 In a study conducted on buccal mucosa cancer patients, Wang et al effectively created a nomogram to stratify patients by risk patients, thus laying emphasis on better individual treatment planning and prognosis assessment.14 Mattavelli et al studied the prognostic implication of pre-operative neutrophil to lymphocyte ratio (NLR) and the effect of different clinico-pathologic factors in a series of primary oral squamous cell carcinomas (OSCC). After developing prognostic nomograms, they found that very low preoperative NLR values had a negative prognostic impact on survival and recurrence. Extra-nodal spread and perineural extension were the most significant clinico-pathologic prognosticators.15
It is well accepted that tumour biology and microenvironment contribute to the disease outcome. Comprehending cancer cell locomotion in OSCC is facilitated by assessment of the pattern of invasion in routine microscopy for grading of these tumours. This prognostic factor, however, lacks objectivity and has moderate reproducibility in oral cancer due to subjective variations.16 The lymph node metastasis status has a direct role in the selection of a therapeutic approach which determines the outcome of the disease.2 We propose that a model like our nomogram corroborated with D2-40 expression will objectively aid in predicting lymph node metastasis. In a study conducted by Parvez et al they laid emphasis on identifying and monitoring lymphovascular density in the peritumoral region to predict the likelihood of metastasis in advanced oral squamous cell carcinoma.17 However, there is another aspect of D2-40 (podoplanin) expression that warrants attention, namely the correlation between tumour expression and increased metastatic potential. By delving deeper into this correlation, this article provides a better understanding of the mechanisms behind tumour metastasis and potentially identifying new targets for therapeutic intervention, ultimately to achieve the goal of improving patient outcomes and reducing the morbidity associated with this aggressive disease.
With this data, we introduce the concept of utilizing “individualized risk prediction” for predicting lymph node metastasis using a host of histopathological parameters that can be visualized in routine and immunohistochemical staining in OSCC. Previously authors have reported the utility of nomograms as an initial method of risk assessment. A combination of various patient factors, such as age, sex, and race, and tumour characteristics, such as anatomic subsite, clinical tumour size, and the presence of clinical and/or radiological nodal metastases, has been used to formulate nomograms to study/predict prognostic behaviour of tumours.11,18 This has been rendered equivalent to the gold standard TNM staging for survival. However, not many of these nomogram models are aimed towards prediction of lymph node metastasis from among other equally significant histopathological parameters.
Current opinion amongst oncologists seems to suggest that histopathological diagnosis may not be the most reliable or accurate predictor of prognosis. The lack of objectivity coupled with lack of sensitive markers hitherto preclude a direct correlation of the patient’s clinical scenario to the histopathological parameters visible on a microscopic glass slide. Sawant et al had employed immunohistochemical markers of cell adhesion in cases of oral cancer to propose that a nomogram constructed on post-surgical OSCC tumour samples would be of value in addition to histopathology for the detection of neck node metastasis in pathologically node-negative patients.2 Podoplanin assessment can be utilized in understanding behaviour of patterns of invasion. Yuan et al found high podoplanin expression in 20 (57%) of the 35 oral and hypopharyngeal SCC, and it was more prevalent in tumours with lymph node metastases, specifically oral cavity tumours.19 Patients with high levels of podoplanin in their tumours had a statistically significant increased rate of lymph node metastases (P=0.0001) and the shortest disease-specific survival (P=0.0004) compared to the other patients.
We propose that a nomogram will predict the probability of metastasis based on the data obtained primarily using histological microscopic parameters. According to our findings, the nomogram gives the highest weightage to the worst pattern of invasion (WPOI) by H+E. Recently, Lakhera et al have provided evidence that WPOI exhibits the strongest correlation with nodal positivity, stage, and lympho-vascular/perineural invasion. This finding highlights the importance of WPOI as a potential prognostic marker in the assessment of cancer progression.20
However, a tumour with a WPOI score of 2 (islands) in H+E may also have ITCs detected by D2-40 which drastically upgrades the probability of metastasis. This can therefore claim advantage over H+E as it helps in detecting the motile tumour phenotype, and in proving the importance of the invasive potential regardless of the histological pattern of invasion used for Bryne’s grading system. A statistical model such as this may enhance the importance of histopathological parameters that are routinely analysed. External validity of our model is a crucial prerequisite to applicability and can only be assessed by confirming results in an adequate number of tissue samples. Based on our data, we hypothesize that this model will impress upon a concept that will quantify the role of various histopathological parameters that have been established for facilitating the complex process of metastasis. This would ultimately assist the clinician in predicting OSCC post-operative risk and survival and may facilitate the streamlining of the treatment aimed towards specific tumour phenotypes governing cellular motility and lymphatic vessel density, furthermore propelling the advent and use of precision medicine in oncology.21
Conclusion
Our predictive nomogram for LNM in OSCC surprisingly showed that a tumour with lower score of WPOI (islands vs ITC) showed numerous D2-40-positive ITCs, drastically increasing the probability of metastasis. The proposed nomogram is a prediction model that can assist the clinician in predicting OSCC post-operative risk and survival.
Data Sharing Statement
Data are available upon request to Dr Karen Boaz (at [email protected]). The findings of this study were presented at the ESMO (European Society of Medical Oncology) Asia Congress 2022, Singapore. The poster’s abstract was published in “Poster Abstracts” in Annals of Oncology: https://doi.org/10.1016/j.annonc.2022.10.273.
Ethics Approval and Consent
The given study was commenced after approval from the Institutional Ethics Committee, Manipal College of Dental Sciences, Mangalore, Manipal Academy of Higher Education (Ref no. 14134). Each patient in the study signed a consent for investigations sequential to planned biopsy/surgery.
Consent for Publication
The consent obtained for performing the tissue biopsy also included consent for further investigations and subsequent publication. Nomogram, being a statistical evaluation of a previously performed tissue study, did not require any human participants' consent. However, requisite permissions were obtained from the Institution and Department before retrieving slides/sections for this study with the patient’s identity being concealed at all times of the study.
Disclosure
The authors declare that they have no competing interest in this work.
References
1. Dhawan I, Sandhu SV, Bhandari R, Sood N, Bhullar RK, Sethi N. Detection of cervical lymph node micrometastasis and isolated tumor cells in oral squamous cell carcinoma using immunohistochemistry and serial sectioning. J Oral Maxillofac Pathol. 2016;20(3):436. doi:10.4103/0973-029X.190946
2. Sawant SS, Dongre H, Ahire C, et al. A nomogram for predicting the risk of neck node metastasis in pathologically node-negative oral cavity carcinoma. Oral Dis. 2017;23(8):1087–1098. doi:10.1111/odi.12696
3. Hamada T, Nakai Y, Yasunaga H, et al. Prognostic nomogram for nonresectable pancreatic cancer treated with gemcitabine-based chemotherapy. Br J Cancer. 2014;110(8):1943–1949. doi:10.1038/bjc.2014.131
4. Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9(4):261–272. doi:10.1016/j.ccr.2006.03.010
5. Lowe KL, Navarro-Nunez L, Watson SP. Platelet CLEC-2 and podoplanin in cancer metastasis. Thromb Res. 2012;129:S30–S37. doi:10.1016/S0049-3848(12)70013-0
6. Gandalovičová A, Vomastek T, Rosel D, Brábek J. Cell polarity signaling in the plasticity of cancer cell invasiveness. Oncotarget. 2016;7(18):25022–25049. doi:10.18632/oncotarget.7214
7. Sun B, Fang Y, Li Z, Chen Z, Xiang J. Role of cellular cytoskeleton in epithelial-mesenchymal transition process during cancer progression. Biomed Rep. 2015;3(5):603–610. doi:10.3892/br.2015.494
8. Mäkinen T, Norrmen C, Petrova TV. Molecular mechanisms of lymphatic vascular development. Cell Mol Life Sci. 2007;64(15):1915–1929. doi:10.1007/s00018-007-7040-z
9. Funayama A, Cheng J, Maruyama S, et al. Enhanced expression of podoplanin in oral carcinomas in situ and squamous cell carcinomas. Pathobiology. 2011;78(3):171–180. doi:10.1159/000324926
10. Wang SJ, Patel SG, Shah JP, et al. An oral cavity carcinoma nomogram to predict benefit of adjuvant radiotherapy. JAMA Otolaryngol Head Neck Surg. 2013;139(6):554–559. doi:10.1001/jamaoto.2013.3001
11. Montero PH, Yu C, Palmer FL, et al. Nomograms for preoperative prediction of prognosis in patients with oral cavity squamous cell carcinoma. Cancer. 2014;120(2):214–221. doi:10.1002/cncr.28407
12. Wang F, Zhang H, Wen J, et al. Nomograms forecasting long-term overall and cancer-specific survival of patients with oral squamous cell carcinoma. Cancer Med. 2018;7(4):943–952. doi:10.1002/cam4.1216
13. Wang F, Wen J, Cao S, et al. Nomogram predicting long-term overall and cancer-specific survival of patients with buccal mucosa cancer. BMC Oral Health. 2022;22(1):138. doi:10.1186/s12903-022-02147-9
14. Balasubramanian D, Subramaniam N, Missale F, et al. Predictive nomograms for oral tongue squamous cell carcinoma applying the American Joint Committee on Cancer/Union Internationale Contre le Cancer 8th edition staging system. Head Neck. 2021;43(4):1043–1055. doi:10.1002/hed.26554
15. Mattavelli D, Lombardi D, Missale F, et al. Prognostic nomograms in oral squamous cell carcinoma: the negative impact of low neutrophil to lymphocyte ratio. Front Oncol. 2019;9:339. doi:10.3389/fonc.2019.00339
16. Beggan C, Fives C, O’Leary G, Sheahan P, Heffron CC, Feeley L. Pattern of invasion and lymphovascular invasion in squamous cell carcinoma of the floor of the mouth: an interobserver variability study. Histopathology. 2016;69(6):914–920. doi:10.1111/his.13014
17. Parvez K, Arora V, Wadhwa N. Peri-tumoral lymphovascular density by antipodoplanin antibody D2-40, as a predictor of nodal metastasis in oral squamous cell carcinoma. Physician. 2020;6(3):1–11. doi:10.38192/1.6.3.6
18. Bobdey S, Balasubramaniam G, Mishra P. Nomogram prediction for survival of patients with oral cavity squamous cell carcinoma. Head Neck. 2016;38(12):1826–1831. doi:10.1002/hed.24507
19. Yuan P, Temam S, El-Naggar A, et al. Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer. 2006;107(3):563–569. doi:10.1002/cncr.22061
20. Lakhera KK, Nama Y, Maan P, et al. Worst pattern of invasion as a predictor of nodal metastasis in early-stage oral squamous cell carcinoma. Indian J Surg Oncol. 2023;14(1):160–168. doi:10.1007/s13193-022-01639-y
21. Zhang XY, Xie S, Wang DC, Shan XF, Cai ZG. Prognosis and nomogram prediction for patients with oral squamous cell carcinoma: a Cohort Study. Diagnostics. 2023;13(10):1768. doi:10.3390/diagnostics13101768
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

