Back to Journals » International Journal of General Medicine » Volume 16
Prognostic Factors and Outcomes in Advanced Stage Lung Cancer Patients with COVID-19 Omicron Variant Infection
Authors Zhao Z, Han X, You Y, Zhang J, Nie K, Ji Y
Received 24 August 2023
Accepted for publication 13 December 2023
Published 15 December 2023 Volume 2023:16 Pages 5947—5953
DOI https://doi.org/10.2147/IJGM.S436917
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Zhimei Zhao,1,* Xiang Han,1,* Yunhong You,1 Jiankang Zhang,2 Keke Nie,1 Youxin Ji1
1Department of Oncology, Affiliated Qingdao Central Hospital of Qingdao University, Qingdao Cancer Hospital, Qingdao City, People’s Republic of China; 2Department of Medicine, Qingdao Medical College, Qingdao University, Qingdao City, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Keke Nie; Youxin Ji, Department of Oncology, Affiliated Qingdao Central Hospital of Qingdao University, Qingdao Cancer Hospital, 127 Si Liu South Road, Shibei District, Qingdao City, 266042, People’s Republic of China, Tel + (86)532-6866-5078, Fax + (86)532-8486-3506, Email [email protected]; [email protected]
Background: We study the characteristics and outcomes in lung cancer patients with COVID-19 Omicron variant infection.
Methods: Hospitalized lung cancer patients with advanced-stage disease and laboratory-confirmed COVID-19 Omicron infection were included. Pneumonitis involving at least 25% of lung parenchyma on CT scans, accompanied by symptoms and oxygen saturation below 93%, were criteria for enrollment. Pneumonitis severity was graded using CTCAE v5.0. Treatment included Paxlovid, prednisolone, anticoagulation, and ventilation. Initial data, radiographic findings, and outcomes were compared. Logistic regression was employed to determine risk factors for in-hospital mortality.
Results: Fifteen patients (median age: 65 years; 80.0% males) were included. 73.3% improved and were discharged, 20.0% died, and 6.7% remained intubated. Initial symptoms included cough (100.0%), fever (73.3%), and shortness of breath (53.3%). Symptoms resolved in discharged patients. Median fever duration was 3.5 days, and respiratory symptom recovery took 26 days. Three patients died due to respiratory failure from Omicron pneumonia. Lower oxygen saturation, reduced lymphocyte/neutrophil ratio on day 7, and diffuse bilateral lung lesions were poor prognostic factors.
Conclusion: This study underscores the importance of prompt intervention and early diagnosis for lung cancer patients infected with the COVID-19 Omicron variant. Lower oxygen saturation, decreased lymphocyte/neutrophil ratio on day 7, and diffuse lung lesions on CT scans were associated with worse outcomes. Clinicians should prioritize timely and comprehensive management to improve survival rates in this population.
Keywords: COVID-19 omicronvariant, lung cancer, oxygen saturation, lymphocyte and neutrophils ratio, chest CT scan
Introduction
The pandemic of coronavirus disease 2019 (COVID‐19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has been ongoing since December 2019 and has impacted more than 397 million individuals globally, resulting in a staggering death toll exceeding 5 million as of February 2022.1 In November 2021, a new variant of concern, Omicron, emerged within the SARS-CoV-2 lineage, presenting heightened transmissibility and the concerning potential for re-infecting individuals previously exposed to COVID-19.2 The Omicron variant is notably highly contagious and has exhibited the capability to re-infect individuals who had recently recovered from COVID-19.2
Recent investigations have unveiled distinct patterns in the characteristics and outcomes of patients hospitalized with COVID-19 under the influence of the Omicron variant compared to prior viral variants, showcasing a notable decrease in disease severity and mortality rates.2,3 Data indicate an exacerbation rate of up to 13.08% among Omicron-infected patients. Moreover, individuals who have cancer, coupled with multiple comorbidities including type 2 diabetes, obesity, and cardiovascular diseases that weakened immune systems, are predisposed to experiencing worse COVID-19 infections and outcomes.4,5 Notably, individuals with lung cancer harbor an accumulation of risk factors contributing to COVID-19 morbidity and mortality, including advanced age, a multitude of comorbidities, a history of smoking, lung damage associated with the tumor, and the immunosuppressive effects of cancer treatments.2,6,7
Nevertheless, there remains a gap in understanding the precise clinical trajectory of COVID-19 Omicron variant infection, particularly concerning the relationships between patient outcomes, clinical laboratory parameters, and features indicative of interstitial lung disease on CT scans.8 Consequently, it is of utmost importance to uncover predictive markers and risk factors that can empower clinicians to accurately identify life-threatening conditions in lung cancer patients afflicted with COVID-19 Omicron infection.
Patients andMethods
Between December 15, 2022, and January 30, 2023, we enrolled patients with stage IV lung cancer who had laboratory-confirmed COVID-19 Omicron infection and exhibited viral pneumonitis affecting a minimum of 25% of lung parenchyma as seen on chest CT scans. Eligible patients presented with evident symptoms and oxygen saturation levels below 93%, as assessed by a finger-tip oximeter at rest and without supplementary oxygen, leading to their hospitalization. The severity of pneumonitis was assessed according to CTCAE v5.0 criteria9 as follows: G1 denoting asymptomatic cases with lesions localized to one lung lobe or involving <25% of lung parenchyma on chest CT scan; G2 indicating symptomatic cases with lesions localized to multiple lung lobes or involving 25%–50% of lung parenchyma; G3 representing severe cases with lesions encompassing >50% of lung parenchyma and hampering daily activities, necessitating oxygen supplementation; and G4 defining life-threatening cases with lesions extending across all lobes of both lungs, causing substantial respiratory compromise.
Confirmation of COVID-19 Omicron variant infection was achieved through a test kit (Beijing Kinghawk Pharmaceutical Co. Ltd, Beijing, China) using reverse transcriptase-polymerase chain reaction (RT-PCR) in all patients.10 Viral pneumonitis involvement on chest CT scan was diagnosed independently by two radiologists. Oxygen saturation levels were continuously monitored using a finger-tip oximeter (Mindray uMEC12 Patient Monitor, Shenzhen, China) during rest, ambulation, and with supplemental oxygen. All cases were categorized as Grade 2 or higher COVID-19 Omicron-induced pneumonitis based on diagnostic and treatment guidelines.9,11,12
Discharge criteria included maintaining a normal body temperature for at least three consecutive days, resolution of clinical respiratory symptoms, substantial improvement in inflammation evidenced by chest CT, and achieving two consecutive negative results for SARS-CoV-2 RNA, with intervals of at least 24 hr apart.
Data are presented as medians or averages. Risk factors associated with mortality are explored using both univariable and multivariable logistic regression analyses. Continuous variables were compared using t-tests or Fisher’s exact test. Statistical analyses were performed using SigmaPlot 14.0 (Systat Software Inc., USA), with a significance level set at P < 0.05.
This study obtained ethical approval from the Ethics Committee of the Affiliated Qingdao Central Hospital of Qingdao University and was conducted in accordance with the provisions of Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws. Informed consents for disclosing patient information were obtained from all study participants.
Results
A total of 15 cases of lung cancer infected with the Omicron variant were included in our study. The median age was 65 years (range: 43–86), with 80.0% of the cases being male. The most common histology observed was non-small-cell lung cancer (n=12, 80%), and all cases were in stage IV (n=15, 100%). Among the patients, 11 (73.3%) were able to recover and were discharged, 3 patients (20.0%) unfortunately succumbed to the illness, and 1 patient (6.7%) required persistent intubation and assisted mechanical ventilation (Table 1). The prevailing initial respiratory symptoms included cough in all 15 cases (100.0%), fever in 11 cases (73.3%), and shortness of breath in 8 cases (53.3%). Fatigue, diarrhea, and loss of taste or smell were frequently observed during the first 2 weeks of infection. Notably, atypical respiratory symptoms are often exacerbated by the presence of underlying lung cancer. The median duration of fever was 3.5 days, and the median time for recovery from respiratory symptoms was 26 days.
 |
Table 1 Clinical Characteristics |
The median oxygen saturation (mOS) at rest without oxygen inhalation on day 1 and day 7 are detailed in Table 1. A comparison of the average oxygen saturation on day 1 and day 7 between the group of patients who died or remained unrecovered (P2, P3, P4, and P8) and the group of recovered patients revealed values of 76% vs 71% and 89% vs 95%, respectively, indicating a significant difference (P<0.001). Blood work was conducted on each patient twice a week, with white cell count, neutrophil count, lymphocyte count, and C-reactive protein levels assessed on day 1 and day 7 (Table 2). The average lymphocyte/neutrophil ratio on day 7, compared to day 1, exhibited a decrease of 20.93% in the group of patients who died or remained unrecovered (P2, P3, P4, and P8), while it increased by 0.15% in the fully recovered patients. This difference is statistically significant (P<0.001). However, no evident correlation was found between changes in blood hemoglobin, eosinophilic granulocyte count, basophilic granulocyte count, and tumor marker levels with patients’ prognosis (Data not shown).
 |
Table 2 Blood Cells and C-Reactive Protein Changes |
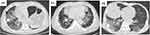
Upon admission, all patients presented with lung infiltrations as observed through chest CT scans. Based on the appearance of these scans, three distinct patterns emerged. Pattern 1 shows diffuse lung infiltrations affecting both lungs, characterized by multiple bilateral ground glass opacities mixed with large areas of consolidation (Figure 1). All three patients in this pattern were admitted in a critically ill state; despite aggressive treatment involving antiviral agents, high-dose steroids, and antibiotics, two of them ultimately succumbed. The remaining patient required intubation and assisted mechanical ventilation.
Pattern 2 demonstrated multiple ground glass opacities with a predominant sub-pleural distribution in both lungs on non-contrast chest CT scans (Figure 2). In this subset of patients, only P2 died due to respiratory failure, while the other five patients fully recovered after receiving treatment. Notably, advanced age proved to be a poor prognostic factor in this pattern. The median duration of in-hospital treatment for this group was 25 days.
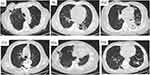 |
Figure 2 Sub-pleural pattern. Non-contrast chest CT scan showing multiple ground glass opacities with a predominant sub-pleural distribution in both lungs (Black arrow). |
Pattern 3 showed multiple ground glass opacities distributed in the central areas of both lungs on non-contrast chest CT scans (Figure 3). In this subset of patients, no patients died and all the six patients recovered after receiving treatment. The median duration of in-hospital treatment for this group was 18 days.
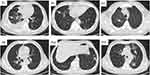 |
Figure 3 Pulmonary parenchyma pattern. Non-contrast chest CT scan showing multiple ground glass opacities distributed in the central areas of both lungs (White arrow). |
Discussion
Studies have shown that SARS-CoV-2 causes the release of pro-inflammatory cytokines such as interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α, which are mediators of lung inflammation, lung damage, fever, and fibrosis; various chemokines have been found to play an important role in disease progression.13
Patients with cancer are notably more vulnerable to COVID-19 than the general population, experiencing more rapid deterioration compared to those without cancer.14 Among cancer types, individuals with lung cancer appear to face the highest risk of mortality due to COVID-19 infection.15 Lung cancer patients who developed irAEs while receiving ICIs had a significantly higher incidence of COVID-19 pneumonia compared to those without irAEs, and lung cancer patients receiving ICIs who become infected with COVID-19 may face a higher risk of immune hyperactivation and cytokine storm.16 Clinical manifestations of COVID-19 exhibit remarkable heterogeneity, intricately tied to the dynamic interplay between the virus and the host’s physiological response.17 The central objectives for managing lung cancer patients infected with COVID-19 revolve around minimizing complications and reducing mortality rates.18 Notably, lung cancer patients are predominantly elderly, often afflicted with concurrent obstructive or restrictive lung diseases, compounding the risk of unfavorable outcomes in the context of COVID-19 infection. Moreover, an elevated expression of angiotensin-converting enzyme 2 (ACE2) has been observed in lung carcinoma compared to normal tissues, amplifying the vulnerability of this patient cohort to COVID-19 infection.19 Luo J. et al20 underscored the impact of smoking status and chronic obstructive pulmonary disease on the severity of COVID-19 infection in lung cancer patients; however, systemic therapies exhibited limited influence. HLA super-types displayed similarity in mild and severe cases of lung cancer patients with COVID-19 infection, and hydroxychloroquine failed to yield improvements. The incidence of severe illness and mortality rates in lung cancer patients with COVID-19 was reported to surge in the context of recent cancer treatment, likely attributed to chemotherapy-induced lymphocytopenia, which impairs cell-mediated immunity and further exacerbates virus-induced damage.21
In our study, the average oxygen saturation on day 1 and day 7 remained at 76% and 71%, respectively, for the 3 patients who succumbed to the infection and the one unrecovered patient. Notably, these levels did not improve significantly despite aggressive treatment. In contrast, a critical determinant for survival was the improvement in oxygen saturation by day 7 for the 11 recovered patients. Furthermore, we identified a significant decline of 20.93% in the lymphocyte/neutrophils ratio on day 7 for the non-surviving patients, alongside a marginal increase of 0.15% for the recovered patients. Thus, the increase in the L/N ratio emerged as a predictive marker for recovery, potentially outweighing the significance of lymphocytopenia as a predictor. Notably, among the three patients who displayed diffuse bilateral ground glass opacities alongside extensive consolidation on non-contrast chest CT scans, two succumbed to the disease despite vigorous treatment, while one remained reliant on intubation and assisted mechanical ventilation. Among the 12 patients with predominantly sub-pleural or central distribution of multiple ground glass opacities on non-contrast chest CT scans, only patient P2 experienced respiratory failure and passed away. Consequently, the location and pattern of lung infiltration emerge as pivotal prognostic determinants. Senior age and high ECOG performance score also bore heightened risk factors.21
This study, to our knowledge, is the first to delineate the prognostic significance of oxygen saturation and L/N ratio changes by day 7, as well as the patterns and distribution of lung infiltration on chest CT scans among lung cancer patients afflicted with COVID-19 Omicron infection. However, certain limitations warrant acknowledgment. This study represents a single-center retrospective analysis with a limited number of participants, potentially giving rise to selection bias. The predictive value of oxygen saturation, L/N ratio changes by day 7, and the patterns evident on chest CT scans necessitate further exploration in larger cohorts.
Conclusions
Lung cancer patients affected by COVID-19 Omicron infection confront elevated in-hospital mortality and protracted recovery periods. Diminished oxygen saturation, coupled with a decreased lymphocyte/neutrophils ratio by day 7, alongside widespread lung lesions on chest CT scans, collectively emerge as potent indicators of an unfavorable prognosis. Timely diagnosis and prompt intervention stand as crucial measures in safeguarding the lives of lung cancer patients contending with COVID-19 Omicron infection.
Ethics Approval
This study received approval from the Ethics Committee of the Affiliated Qingdao Central Hospital of Qingdao University and was conducted in accordance with the provisions of the Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws.
Data Available
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Consent for Publication
Prior to submitting this manuscript, written informed consent was obtained from the patients for the publication of their medical case details and any accompanying images.
Acknowledgment
We extend our sincere gratitude to Qing Yan and Ye Wang for their exceptional assistance with the laboratory work pertaining to the diagnosis of COVID-19 Omicron infection at the Department of Central Laboratory, Affiliated Central Hospital of Qingdao University. We also express our appreciation to Professor Wendy Wang of the Feinstein Institute for Medical Research and Professor John Califra in New York for their valuable contributions in editing the manuscript.
Funding
This study was not funded by any external sources.
Disclosure
The authors affirm that they have no conflicts of interest to disclose.
References
1. Hadj Hassine I. Covid-19 vaccines and variants of concern: a review. Rev Med Virol. 2022;32(4). doi:10.1002/rmv.2313
2. Zhu -F-F, Gu -B-B, Jin Y-J, et al. Risk Factors for Radiological Progression Within Admissive One Week in the Hospitalized COVID-19 Omicron Variant-Infected Patients. Infect Drug Resist. 2022;15:7127–7137. doi:10.2147/idr.s388696
3. Guarnera A, Santini E, Podda P. COVID-19 Pneumonia and Lung Cancer: a Challenge for the Radiologist Review of the Main Radiological Features, Differential Diagnosis and Overlapping Pathologies. Tomography. 2022;8:513–528. doi:10.3390/tomography8010041
4. Wang QQ, Berger NA, Xu R. Analyses of Risk, Racial Disparity, and Outcomes among US Patients with Cancer and COVID-19 Infection. JAMA Oncol. 2021;7(2):220–227. doi:10.1001/jamaoncol.2020.6178
5. Rogado J, Pangua C, Serrano-Montero G, et al. Covid-19 and lung cancer: a greater fatality rate? Lung Cancer. 2020;146:19–22. doi:10.1016/j.lungcan.2020.05.034
6. Kasymjanova G, Anwar A, Cohen V, et al. The impact of COVID-19 on the diagnosis and treatment of lung cancer at a Canadian academic center: a retrospective chart review. Curr Oncol. 2021;28(6):4247–4255. doi:10.3390/curroncol28060360
7. Greenberg A, Anand G, Sinclair G. Lung Cancer Treatment in COVID-19–Positive Patients: guidelines and Gestalt. JCO Oncol Pract. 2020;16(9):615–617. doi:10.1200/op.20.00511
8. Passaro A, Bestvina C, Velez Velez M, Garassino MC, Garon E, Peters S. Severity of COVID-19 in patients with lung cancer: evidence and challenges. J Immunother Cancer. 2021;9. doi:10.1136/jitc-2020-002266
9. N. Cancer Institute, Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) 5.0; 2017. Available from: https://www.meddra.org/.
10. Bazargan M, Elahi R, Esmaeilzadeh A. OMICRON: virology, immunopathogenesis, and laboratory diagnosis. J Gene Med. 2022;24(7):1. doi:10.1002/jgm.3435
11. Ying-Hui J, Qing-Yuan Z, Zhi-Yong P, et al. Chemoprophylaxis, diagnosis, treatments, and discharge management of COVID-19: an evidence-based clinical practice guideline (updated version). Med J Chinese People’s Lib Army. 2020;45:1003–1029. doi:10.11855/j.issn.0577-7402.2020.10.01
12. Thompson JA, Schneider BJ, Brahmer J, Management of Toxicities, NCCN Guidel. 2.2022; 2022. Available from: https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf.
13. Verma G. Immunomodulatory approaches in managing lung inflammation in COVID ‐ 19: a double ‐ edge sword. Immunity Inflammation Dis. 2023:1–15. doi:10.1002/iid3.1020
14. Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi:10.1016/S1470-2045(20)30096-6
15. Horn L, Garassino M. COVID-19 in patients with cancer: managing a pandemic within a pandemic. Nat Rev Clin Oncol. 2021;18(1):18–19. doi:10.1038/s41571-020-00441-5
16. Che K, Hong C, He Y, Peng D, Zeng Z. Association of immune-related adverse events with COVID-19 pneumonia in lung cancer patients receiving immune checkpoint inhibitors: a cross-sectional study in China. BMC cancer. 2023;1–8.
17. Catania C, Stati V, Spitaleri G. Interstitial pneumonitis in the COVID-19 era: a difficult differential diagnosis in patients with lung cancer. Tumori. 2021;107(3):267–269. doi:10.1177/0300891620951863
18. Dingemans AMC, Soo RA, Jazieh AR, et al. Treatment Guidance for Patients With Lung Cancer During the Coronavirus 2019 Pandemic. J Thorac Oncol. 2020;15(7):1119–1136. doi:10.1016/j.jtho.2020.05.001
19. Zhang H, Quek K, Chen R, et al. Expression of the SAR2-Cov-2 receptor ACE2 reveals the susceptibility of COVID-19 in non-small cell lung cancer. J Cancer. 2020;11(18):5289–5292. doi:10.7150/jca.49462
20. Luo J, Rizvi H, Preeshagul IR, et al. COVID-19 in patients with lung cancer. Ann Oncol. 2020;31(10):1386–1396. doi:10.1016/j.annonc.2020.06.007
21. Peixoto D, Callia JPB, Bittencourt MS, et al. Clinical presentation and in-hospital prognosis of lung cancer patients presenting with suspected and confirmed COVID-19. Brazilian J Med Biol Res. 2022;55:1–7. doi:10.1590/1414-431X2022e12140
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.