Back to Journals » Journal of Hepatocellular Carcinoma » Volume 11
Prognostic Effect of Sarcopenia in Hepatocellular Carcinoma Patients Targeted with Interventional Therapy Combined with Immunotherapy and Targeted Therapy
Authors Yang H , Cong T, Luo Y , Yang C, Ren J, Li X
Received 13 October 2023
Accepted for publication 11 January 2024
Published 24 January 2024 Volume 2024:11 Pages 175—189
DOI https://doi.org/10.2147/JHC.S444530
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Hongcai Yang,* Tianhao Cong,* Yingen Luo, Chao Yang, Jinrui Ren, Xiao Li
Department of Interventional Therapy, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiao Li, Department of Interventional Therapy, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, People’s Republic of China, Tel +86-13910309111, Fax +86-028-88092354, Email [email protected]
Objective: To investigated the association between sarcopenia and the prognosis and adverse events of hepatocellular carcinoma (HCC) patients undergoing interventional therapy combined with immunotherapy and targeted therapy.
Methods: Between January 2019 and December 2022, patients with unresectable HCC who received interventional therapy combined with immunotherapy and targeted therapy were included in this study. Total skeletal muscle area at the L3 level was normalized for height in m2 as the skeletal muscle index (SMI). All patients were divided into low and high SMI group according to the median SMI.
Results: Ninety-six consecutive patients were included eventually, with 49 patients in the high-SMI group and 47 patients in the low-SMI group. In the low-SMI group, the median overall survival (OS) was 459.00 days (95% CI, 334.76– 583.24 days), and the 3-, 6-, and 12-month OS rates were 100%, 89.4% and 68.1%, respectively. In the high-SMI group, the median OS was not reached, and the 3-, 6-, and 12-month OS rates were 100%, 98% and 79.5%, respectively (p< 0.05). SMI and Barcelona Clinic Liver Cancer (BCLC) C stage were independent prognostic factors for OS (p< 0.05). In the low-SMI group, 26 patients had treatment-related adverse events (TRAEs), resulting in dose adjustment or treatment suspension for 10 patients. In the high-SMI group, 33 patients had TRAEs, and 18 patients received dose adjustment or treatment suspension; the between-group difference was nonsignificant (p> 0.05).
Conclusion: SMI is associated with the prognosis of HCC patients receiving interventional therapy combined with immunotherapy and targeted therapy, and sarcopenia is an independent risk factor for OS. However, sarcopenia does not seem to predict the occurrence of adverse events.
Keywords: hepatocellular carcinoma, sarcopenia, immune checkpoint inhibitors, tyrosine kinase inhibitors, transarterial chemoembolization
Corrigendum for this paper has been published.
Introduction
Primary liver cancer is the sixth most common cancer and the third leading cause of cancer death in the world,1 and hepatocellular carcinoma (HCC) accounts for 75%-85%. Since approximately half of HCC patients are diagnosed at an advanced stage, the 5-year survival rate is only 18%.2,3 In recent years, studies on the treatment of advanced HCC have emerged one after another, with studies on interventional therapy combined with immune checkpoint inhibitors (ICIs) and tyrosine kinase inhibitors (TKIs), which was referred to triple therapy in the following text, being particularly prominent. The latest research shows that this combination therapy can effectively improve the survival rate of patients with advanced HCC, yielding encouraging results.4–6 Many studies have also revealed the mechanism of this combination therapy from various aspects.7 Although these results are promising, we are still unable to confirm the indicators that can precisely predict the response of patients to triple therapy. The purpose of this study is to accurately stratify patients and improve treatment efficiency. On the other hand, for those patients who may have a poor prognosis despite the use of triple therapy, we can correct negative factors in time and expand the benefits of triple therapy.
Several prognostic biomarkers or staging systems have been developed to predict the prognosis of patients undergoing triple therapy, including hematological markers, infiltrating lymphocytes, vascular endothelial growth factor, Barcelona Clinic Liver Cancer (BCLC) stage, and Child‒Pugh score.4,8,9 However, most of the above parameters evaluated only one aspect related to the disease, such as lesion condition, organ function, and lack of consideration for the overall nutrition and functional performance of the patient.10 However, in addition to the cancer stage and organ function, the nutritional status and functional performance of patients will also affect the course of the disease and prognosis.11 Therefore, it is necessary to consider the nutrition and functional performance of patients when evaluating the prognosis or the course of the disease.
Sarcopenia is a progressive and generalized skeletal muscle disorder involving the accelerated loss of muscle mass and function.11 Although no consensus has been reached on the variables to be included or the cutoff points in sarcopenia, various studies have shown that sarcopenia is associated with poorer prognosis in patients with liver cirrhosis, liver cancer, colorectal cancer and lung cancer.12–15 In the field of liver cancer, Yang et al16 showed the effect of sarcopenia on the prognosis of patients with hepatocellular carcinoma after surgical resection. Loosen et al17 found that sarcopenia is a poor prognostic factor in patients with HCC who received transarterial chemoembolization (TACE). Studies by Uojima et al18 have also shown that sarcopenia is related to the tolerability of lenvatinib use and the resulting prognosis in HCC patients. A meta analysis has indicated that a low skeletal muscle mass is common among HCC patients undergoing systemic therapy, including immunotherapy and targeted therapy, and is associated with poorer survival outcomes.19 Nonetheless, there are few studies on the effects of sarcopenia on HCC patients undergoing triple therapy. Consequently, in this study, we aimed to explore the relationship between sarcopenia and the prognosis of HCC patients receiving triple therapy.
Methods
Patients
Between January 2019 and December 2022, patients diagnosed with HCC who received triple treatment (TACE or HAIC combined with TKIs and ICIs) from Cancer Hospital Chinese Academy of Medical Sciences were included in this retrospective study. The diagnosis of HCC was based on imaging and serum AFP findings or on histological assessment. The ethics committee approved the ethics of the study, and all participants signed informed consent for treatment. The inclusion criteria were as follows: (a) ≥ 18 years old, (b) estimated survival time > 30 days, (c) Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 0–1, (d) liver function rated as Child‒Pugh B or C class, and (e) at least one measurable target lesion evaluated by Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1) and modified RECIST (mRECIST). The exclusion criteria were as follows: (a) history of other malignant tumors and (b) incomplete clinical data or loss to follow-up (Figure 1).
 |
Figure 1 Flow chart of research design. |
Clinical Parameters
Clinical characteristics, including baseline characteristics, demographic information, hepatitis, cirrhosis, tumor burden, vascular invasion and extrahepatic metastasis, BCLC stage, and Child‒Pugh class, were collected through medical records and follow-up. Laboratory test results, including those for white blood cells (WBCs), red blood cells (RBCs), hemoglobin (Hb), platelets (PLTs), prothrombin time (PT), albumin (ALB), alkaline phosphatase (ALP), international normalized ratio (INR), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), direct bilirubin (DBIL), glucose (GLU), creatinine (Cre), and gamma-glutamyltransferase (GGT), were also analyzed.
Treatment Procedures
The TACE procedure was as follows: with the patients under local anesthesia, right femoral access was established using a 5-Fr vessel access kit (Radiofocus; Terumo) by the Seldinger method. Angiographies of the superior mesenteric and common hepatic arteries using a 0.035-inch hydrophilic wire and a 5-Fr Rösch hepatic catheter. Then, we infused 50 mg/m2 lobaplatin over 15 min through a 2.4-Fr microcatheter into the proper, lobar or segmental hepatic artery. Subsequently, lobaplatin was admixed at a 1:1 ratio in an iodized oil emulsion and infused through the microcatheter into the feeding artery at the segmental, subsegmental, or more peripheral level. Finally, if necessary, additional embolization with gelation sponge or polyvinyl alcohol particles (350–560 μm) was performed until stasis or near stasis of arterial flow was achieved. In terms of hepatic arterial infusion chemotherapy with fluorouracil, leucovorin, and oxaliplatin (FOLFOX-HAIC), a 5-Fr catheter was inserted into the celiac trunk or superior mesenteric artery for arteriography. Then, a 2.7 Fr microcatheter was superselected into the tumor- and thrombus-feeding arteries. The interventional radiologists confirmed blood flow into the gastroduodenal artery by microcatheter angiography and embolized the route with a coil or microcoil to prevent chemotherapeutic drug reflux to the stomach and duodenum. The peripheral part of the catheter exposed outside was covered with sterile gauze and fastened on the thigh skin with rubberized fabric and bandage. Chemotherapeutic agents were infused within 2 days after catheter insertion. The following chemotherapeutic agents were sequentially infused into the hepatic artery by connecting an arterial pump: oxaliplatin (85 mg/m2 for 2–4 hours), leucovorin (400 mg/m2 for 2 hours), and fluorouracil (400 mg/m2 for 1 hour and another 2400 mg/m2 for more than 46 hours). Repeated TACE was conducted according to need. The treatment interval of HAIC was 4–6 weeks.
Additionally, before or after interventional treatment within 1 month, patients received TKIs and ICIs. TKIs included lenvatinib (8 mg/day or 12 mg/day), sorafenib (800 mg/day) or regorafenib (80–160 mg/day). The included ICIs were toripalimab (3 mg/kg every 2 weeks intravenously) and camrelizumab (200 mg every 3 weeks intravenously). The medications allowed for adjustment according to toxicity or disease state.
The treatment plan for each patient is individually tailored by a multidisciplinary team consisting of two interventional radiologists, two oncologists, and one radiologist. This team takes into account the patient’s imaging results, serological manifestations, and tolerance to treatment. Adjustments to the treatment plan or drug dosages may be made in the event of disease progression or if the patient experiences intolerable side effects.
Definition
Based on the latest CT images within one month before triple therapy, the total cross-sectional areas of skeletal muscle, visceral adipose tissue and subcutaneous adipose tissue at the L3 level for each patient were measured independently by two researchers in a blinded fashion. Core Slicer (www.coreslicer.com) was used to identify and quantify skeletal muscle, subcutaneous adipose tissue, visceral adipose tissue and their total cross-sectional area in Hounsfield units (HU). The threshold range was defined as follows: skeletal muscle was defined as −29 HU to 150 HU, subcutaneous adipose tissue was defined as −190 HU to −30 HU, and visceral adipose tissue was defined as −150 HU to −50 HU (Figure 2A and B).
As an internationally recognized standard for the evaluation of sarcopenia, SMI is considered to represent the level of muscle mass.16,20 Therefore, in our research, we used skeletal muscle index (SMI) as the criterion for assessing sarcopenia. Notably, although several academic organizations have published diagnostic criteria for sarcopenia, an agreement has not been reached.20–23 In terms of previous studies, the cut-off point of SMI may be the median, the optimal cut-off value, or according to the recommendations of academic society groups, which may differ depending on the number of patients or by patient ethnicity.23 In this study, to reduce the influence of factors other than SMI, we defined the median value of SMI as the cutoff value for sarcopenia. Body mass index (BMI) was defined as weight (kg)/height (m2), and SMI was defined as the total cross-sectional skeletal muscle area in the L3 plane (cm2)/height (m2). Finally, based on the level of SMI, patients were divided into two groups: a high-SMI group and a low-SMI group.
Research Endpoint
The primary endpoint of our study was to investigate the influence of sarcopenia on the prognosis of HCC patients treated with triple therapy. OS was defined as the number of days from therapy initiation to either death or December 30, 2022, whichever came first. The secondary endpoint is to observe the relationship between sarcopenia and the incidence of key adverse events (AEs) in triple therapy. AEs were evaluated according to Common terminology criteria for adverse of US National Cancer Institute (NCI).24
Statistical Analysis
For continuous parametric data, an independent-samples t test was performed for comparison. For categorical parametric variables, we used Pearson’s chi-square test, Yates’s correction for continuity and Fisher’s exact test. Nonparametric data were compared with the Mann‒Whitney U-test. Correlation analyses were performed using the Pearson correlation coefficient. Using the R packages “survival” and “survfit” to analyze the prognostic difference. The “maxstat” R package was used to identify the optimal cutoff value of SMI. Log rank test was used to evaluate the statistical difference. Furthermore, univariate and multivariate Cox regression analyses were used to explore independent prognostic factors associated with triple therapy, and parameters with p values < 0.05 in the univariate analysis were included in the multivariate analysis. All statistical analyses were performed using SPSS (version 25.0, Chicago, IL, USA) and R (version 4.2.0).
Result
Patient Characteristics
After strict exclusion, a total of 96 patients were included in this study. The baseline characteristics of all included patients are shown in Table 1. A total of 87 (90.63%) patients were male, and 9 (9.38%) patients were female. The mean age of all patients was 57.04±9.96 years (range: 31–79 years) and the mean BMI was 22.75±3.54 kg/m2 (range: 12.54–30.41 kg/m2). Of all the patients, 40 (41.67%) patients had BCLC stage B disease, 56 (58.33%) had BCLC stage C disease. A total of 85 (88.54%) patients suffered from cirrhosis. Additionally, a total of 20 (20.83%) patients had extrahepatic metastasis, and 37 (38.54%) patients had macrovascular invasion. The mean SMI was 45.55±6.79 cm2/m2 (range: 33.0 9–63.33 cm2/m2) and the median values of SMI as the cutoff value for sarcopenia were 45.37 cm2/m2 in male patients and 36.33 cm2/m2 in female patients, respectively.
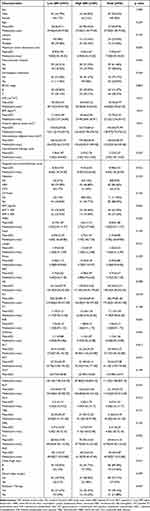 |
Table 1 The baseline characteristics of all included patients |
Efficacy and Safety
The median follow-up was 680 days (range, 567–793 days). During the study period, the median number of interventional procedures per patient was 2 cycles (range, 1–13 cycles), and the median number of immunotherapy cycles was 8 cycles (range, 1–26 cycles). During the follow-up, 50 (47.9%) patients died. The median OS of all patients was 553 days (95% CI, 422–684 days), and the 3-, 6-, and 12-month OS rates were 100%, 92.7%, and 73.9%, respectively.
According to the median of SMI (45.37 cm2/m2 in male, 36.33 cm2/m2 in female), the patients were divided into two groups: high SMI (n = 49) and low SMI (n = 47). The mean SMI of the low- and high-SMI groups was 40.35±3.07 (range: 33.09–44.96) and 50.54±5.52 (range: 36.33–63.33), respectively.
During the study period, 59 (61.5%) patients had treatment-related AEs (TRAEs). Specifically, the most common TRAE was fatigue, with 25 (26.0%) patients, followed by decreased appetite, hypertension and abnormal liver function, with 21 (21.9%), 19 (19.8%) and 17 (17.7%) patients developing related symptoms, respectively (Table 2). In addition, 28 (29.2%) patients underwent adjustment or discontinuation of the drug due to TRAEs.
 |
Table 2 Treatment-related adverse events for all grades |
Sarcopenia and Disease Characteristics
As shown in Table 1, in the baseline comparison between the two groups, the SMI, BMI, areas of visceral and subcutaneous adipose tissue of the low-SMI group patients were significantly lower than those of the high-SMI group (p < 0.05), and the maximum tumor dimension in the low-SMI group was significantly higher than that in the high-SMI group (p < 0.05). The levels of PLT, AST and ALP in the low-SMI group were higher than those in the high-SMI group (p < 0.05). Furthermore, Pearson’s chi-square test was performed to test the correlation between SMI and the indicators that showed significant differences between the two groups. As shown in Table 3, there was a significant positive correlation between SMI and BMI, areas of visceral adipose tissue, and subcutaneous adipose tissue (correlation coefficients: 0.502, 0.371, and 0.312, respectively, p < 0.05) and a negative correlation between SMI and maximum tumor dimension, levels of PLT, and ALP (correlation coefficients: −0.354, −0.312, and −0.205, respectively, p < 0.05).
 |
Table 3 The correlation between SMI and BMI, maximum tumor dimension, the total cross-sectional areas of visceral adipose tissue and subcutaneous adipose tissue, PLT, AST, ALP |
Sarcopenia and Prognosis
Kaplan‒Meier survival analysis showed that the OS of high-SMI group was significantly higher than the low-SMI group (Figure 3A, p < 0.05). For the low-SMI group, the median OS was 459.00 days (95% CI, 334.76–583.24 days), and the 3-, 6-, and 12-month OS rates were 100%, 89.4% and 68.1%, respectively. In terms of the high-SMI group, the median OS was not reached, and the 3-, 6-, and 12-month OS rates were 100%, 98.0% and 79.5%, respectively. Figure 3B exhibited the relationship in detail between SMI and the prognosis of patients. To further elaborate the effect of SMI on the prognosis of HCC patients with triple therapy, univariate and multivariate Cox regression analyses were conducted. The results showed that SMI [HR: 0.945 (95% CI, 0.898–0.995)] and BCLC C stage [HR: 2.508 (95% CI, 1.307–4.633)] were independent prognostic factors for HCC patients with triple therapy (Table 4, p < 0.05).
 |
Table 4 Univariate and multivariate analyses of prognostic factors affecting overall survival |
Taking into account that SMI was the independent prognostic factor and that choosing the median SMI as the cutoff value for sarcopenia may not effectively distinguish the patients, the optimal cutoff value (43.55 cm2/m2 in male, 34.93 cm2/m2 in female) was used as the critical point of sarcopenia. Under such conditions, the OS of the low-SMI group was significantly reduced (Figure 4, p < 0.05), the median OS in low-SMI group was 404.00 days (95% CI, 352.73–455.27 days), and multivariate Cox regression showed that sarcopenia (SMI < 43.55 cm2/m2 in male, < 34.93 cm2/m2 in female) was an independent negative prognostic factor [HR: 1.942 (95% CI, 1.030–3.661)].
 |
Figure 4 When the optimal cutoff value was selected as the cutoff value for sarcopenia, the difference in overall survival between the high and low SMI groups. |
Sarcopenia and Treatment-Related Adverse Events
In the low-SMI group, 26 patients had TRAEs, and 33 patients had TRAEs in the high-SMI group. There was no significant difference in the number of TRAEs between the two groups (p = 0.226). As shown in Table 2, the most common TRAEs were fatigue, decreased appetite, hypertension and abnormal liver function in both groups. Notably, there were 10 patients in the low-SMI group and 18 patients in the high-SMI group with dose adjustment or treatment suspension due to TRAEs, with no significant difference between the two groups (p = 0.96). The specific symptoms of those who received dose adjustment or treatment suspension were mostly comprehensive, such as abnormal liver function, hyperbilirubinemia, eczema and diarrhea.
Discussion
This study demonstrated that sarcopenia was an independent predictor for HCC patients treated with triple therapy by using a cohort of 96 patients undergoing interventional therapy combined with TKIs and ICIs. In addition, by analyzing the incidence of TRAEs in the two groups and comparing the dose adjustment or treatment suspension caused by TRAEs between the two groups, we found that sarcopenia may not be effective in predicting the occurrence of adverse reactions. To the best of our knowledge, this is the first study to investigate the correlation between sarcopenia and the prognosis of HCC patients treated with triple therapy.
In terms of systemic therapy, several studies have indicated a relationship between the survival rates of HCC patients undergoing systemic therapy treatment and sarcopenia.25–27 A meta-analysis incorporating 11 studies revealed that low skeletal muscle mass is associated with poorer OS and shorter treatment failure time in HCC patients receiving sorafenib or lenvatinib therapy.28 However, the majority of these studies were primarily focused on targeted monotherapies, such as sorafenib or lenvatinib. Regarding the correlation between immunotherapy or immune-targeted combination therapy and sarcopenia, multiple studies have presented varying perspectives.29–32 Kuo et al19 conducted a meta-analysis on the aforementioned studies, ultimately indicating the widespread presence of low skeletal muscle mass in HCC patients undergoing different systemic treatment types, significantly correlating with adverse prognosis. Compared to our study, despite differences in the cut-off values used in various studies, the majority utilized L3-SMI to assess muscle mass, consistent with our research methodology.
As the treatment of HCC has entered the era of molecular and immunotherapy, in recent years, interventional therapy combined with TKIs and ICIs has achieved encouraging results.4,33,34 However, the application of triple therapy is limited to some extent due to related cost factors.35 In addition, based on the great heterogeneity of HCC, such as the heterogeneity of immune checkpoints and molecular target expression or the heterogeneity of TACE effectiveness,36 triple therapy can only benefit some patients, while others not only do not attain the desired prognosis but also face adverse events and costs of treatment. To achieve personalized medical care, people are committed to investigating biomarkers that can predict the prognosis of triple therapy, such as tumor mutational burden (TMB), hematological markers, infiltrating lymphocytes, and BCLC stage.4,8,9 Several studies have suggested that extrahepatic metastasis is an independent risk factor influencing the prognosis of patients undergoing triple therapy.34,37 Yu et al38 found that assessing the response of portal vein tumor thrombosis (PVTT) can predict the prognosis of HCC patients with PVTT undergoing transarterial interventional therapy combined with tyrosine kinase inhibitors with anti-PD-1 antibodies. Unfortunately, there are currently few biomarkers that are effective enough, and most of the predictive parameters seem to reflect only one single aspect of disease and cannot be associated with more information.39 Sarcopenia is a comprehensive process involving inflammation and protein and energy metabolism. It has been reported that sarcopenia is associated with poor prognosis in HCC patients, including surgical treatment, molecular targeted therapy, and interventional therapy.16–18,23,40,41 In this study, we found that there was a significant negative correlation between SMI and maximum tumor dimension, PLT, and ALP, and we speculated that SMI may be related to liver synthesis and catabolism and tumor characteristics. Although the underlying mechanism is not fully elucidated, protein catabolism in diseases such as cancer can lead to significant loss of muscle mass and can be seen in up to 40% of patients with liver cirrhosis.42 Additionally, molecular changes in sarcopenic muscle involve comprehensive and complex signaling pathways, such as the synthesis of insulin-like growth factor 1 by the liver and target of rapamycin.11 It has also been reported that muscle-derived cytokines released from skeletal muscle contain interleukin-15 (IL-15), which can improve the therapeutic effect of ICIs by elevating the proportion of circulating natural killer cells and CD8+ T cells.43–45 Consequently, sarcopenia is a comprehensive parameter that may reflect the patient’s systemic status, tumor characteristics and liver function at the same time, which can effectively predict the prognosis of HCC patients with triple therapy.
In terms of TRAEs, although some studies have suggested a link between SMI and the occurrence of severe adverse events in HCC patients undergoing targeted therapy,18 major studies on immune therapy or immune-targeted combination therapy have not reported a significant association between SMI and TRAEs.29,30,32,46,47 Among these, the study by Toshida et al29 suggested that in HCC patients undergoing atezolizumab plus bevacizumab treatment, there was no significant difference in the occurrence rates of adverse events (any grade) and adverse events exceeding Grade 3 between the Non-Sarcopenia group and the Sarcopenia group. Similarly, Hiroaki et al30 reported in their study that there was no significant correlation between SMI and the occurrence of TRAEs in HCC patients undergoing atezolizumab plus bevacizumab treatment. Additionally, Nalee et al47 indicated that a comparable incidence of adverse events was observed between patients with and without sarcopenia in HCC patients undergoing nivolumab treatment. These findings align with the results of our study. The differences in results compared to solely targeted therapy may stem from variations in the occurrence rates of TRAEs between combination therapy and single-targeted therapy itself or the inherent selection bias from retrospective studies, which necessitates further refinement through additional meta-analyses or prospective studies.
The present study has some limitations. First, it is a retrospective study with a relatively small sample size. On the one hand, triple therapy has not been widely used in the clinic; on the other hand, the treatment of patients will be changed due to adverse events or disease progression, and we are unable to conduct a subgroup analysis of patients with different combination regimens. However, we defined triple therapy as a combination of targeted therapy and immunotherapy within one month before or after interventional therapy. The impact of treatment intervals and treatment changes on the results was minimized, and there was no significant difference in the treatment cycle or other treatment modalities between the two groups. Furthermore, because the sample size of female participants was small, the cohort of female patients did not obtain effective results in the subgroup analysis. Together, larger confirmatory prospective clinical studies involving different triple therapy regimens to avoid further potential confounders are necessary, and we hope that this study will result in novel ideas and directions.
Conclusion
Our research has found that SMI is associated with the prognosis of HCC patients with triple therapy, and sarcopenia is an independent risk factor for overall survival, which is of great assistance to personalized medical treatment for HCC patients and the development of novel targets. However, sarcopenia does not seem to predict the occurrence of adverse events.
Abbreviations
AEs, adverse events; BMI, body mass index; BCLC, barcelona clinic liver cancer; HU, hounsfield units; HCC, hepatocellular carcinoma; ICIs, immune checkpoint inhibitors; SMI, skeletal muscle index; TKIs, tyrosine kinase inhibitors; TACE, transarterial chemoembolization; TRAEs, treatment-related adverse events.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by Hongcai Yang and Tianhao Cong, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Cancer Hospital of the Chinese Academy of Medical Sciences. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Statement of Ethics
This is a retrospective study and involved no intervention, and all data was deidentified, the ethics committee waived the requirement for informed consent. Patient data and confidentiality were respected by the Declaration of Helsinki.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Hongcai Yang and Tianhao Cong contributed equally to this work and are co-first authors. Each author made substantial contributions to the work presented, whether it was through conception, study design, execution, data collection, analysis, and interpretation, or in all of these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
3. Kim BH, Lim YS, Kim EY, et al. Temporal improvement in survival of patients with hepatocellular carcinoma in a hepatitis B virus-endemic population. J Gastroenterol Hepatol. 2018;33(2):475–483. doi:10.1111/jgh.13848
4. Lai Z, He M, Bu X, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: a biomolecular exploratory, Phase II trial. Eur J Cancer. 2022;174:68–77. doi:10.1016/j.ejca.2022.07.005
5. Zhang T, Zhang J, Zhang X, Mu H, Yu G, Xing W. Triple combination therapy comprising angiogenesis inhibitors, anti-PD-1 antibodies, and hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2021;39(15_suppl):e16124–e16124. doi:10.1200/JCO.2021.39.15_suppl.e16124
6. Gu S, Tan Y, Zhou H, et al. Combination of donafenib and anti-PD-1 antibody plus trans-arterial chemoembolization (TACE) in unresectable hepatocellular carcinoma (uHCC). J Clin Oncol. 2022;40(16_suppl):e16197–e16197. doi:10.1200/JCO.2022.40.16_suppl.e16197
7. Salem R, Greten TF. Interventional radiology meets immuno-oncology for hepatocellular carcinoma. J Hepatol. 2022. doi:10.1016/j.jhep.2022.08.003
8. Goodman AM, Kato S, Bazhenova L, et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther. 2017;16(11):2598–2608. doi:10.1158/1535-7163.mct-17-0386
9. Zou Y, Zou X, Zheng S, et al. Efficacy and predictive factors of immune checkpoint inhibitors in metastatic breast cancer: a systematic review and meta-analysis. Ther Adv Med Oncol. 2020;12:1758835920940928. doi:10.1177/1758835920940928
10. Vitale A, Farinati F, Noaro G, et al. Restaging Patients With Hepatocellular Carcinoma Before Additional Treatment Decisions: a Multicenter Cohort Study. Hepatology. 2018;68(4):1232–1244. doi:10.1002/hep.30185
11. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636–2646. doi:10.1016/S0140-6736(19)31138-9
12. Ebadi M, Montano-Loza AJ. Clinical relevance of skeletal muscle abnormalities in patients with cirrhosis. Dig Liver Dis. 2019;51(11):1493–1499. doi:10.1016/j.dld.2019.05.034
13. Nakamura R, Inage Y, Tobita R, et al. Sarcopenia in Resected NSCLC: effect on Postoperative Outcomes. J Thorac Oncol. 2018;13(7):895–903. doi:10.1016/j.jtho.2018.04.035
14. Berardi G, Antonelli G, Colasanti M, et al. Association of Sarcopenia and Body Composition With Short-term Outcomes After Liver Resection for Malignant Tumors. JAMA Surg. 2020;155(11):56.
15. Miyamoto Y, Baba Y, Sakamoto Y, et al. Sarcopenia is a Negative Prognostic Factor After Curative Resection of Colorectal Cancer. Ann Surg Oncol. 2015;22(8):2663–2668. doi:10.1245/s10434-014-4281-6
16. Yang J, Chen K, Zheng C, et al. Impact of sarcopenia on outcomes of patients undergoing liver resection for hepatocellular carcinoma. J Cachexia Sarcopenia Muscle. 2022;13(5):2383–2392. doi:10.1002/jcsm.13040
17. Loosen SH, Schulze-Hagen M, Bruners P, et al. Sarcopenia Is a Negative Prognostic Factor in Patients Undergoing Transarterial Chemoembolization (TACE) for Hepatic Malignancies. Cancers. 2019;11(10):1503.
18. Uojima H, Chuma M, Tanaka Y, et al. Skeletal Muscle Mass Influences Tolerability and Prognosis in Hepatocellular Carcinoma Patients Treated with Lenvatinib. Liver Cancer. 2020;9(2):193–206. doi:10.1159/000504604
19. Kuo MH, Tseng CW, Hsu CS, et al. Prevalence and Effect of Low Skeletal Muscle Mass among Hepatocellular Carcinoma Patients Undergoing Systemic Therapy: a Systematic Review and Meta-Analysis. Cancers. 2023;15(9):67.
20. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601. doi:10.1093/ageing/afz046
21. Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21(3):300–307 e302. doi:10.1016/j.jamda.2019.12.012
22. Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016;46(10):951–963. doi:10.1111/hepr.12774
23. Yamasaki T, Saeki I, Yamauchi Y, et al. Management of Systemic Therapies and Hepatic Arterial Infusion Chemotherapy in Patients with Advanced Hepatocellular Carcinoma Based on Sarcopenia Assessment. Liver Cancer. 2022;11(4):329–340. doi:10.1159/000522389
24. Kkf C, Mitchell SA, Chan N, Ang E, Tam W, Kanesvaran R. Linguistic validation of the simplified Chinese version of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). BMC Cancer. 2020;20(1):1153. doi:10.1186/s12885-020-07631-5
25. Hiraoka A, Kumada T, Kariyama K, et al. Clinical importance of muscle volume in lenvatinib treatment for hepatocellular carcinoma: analysis adjusted with inverse probability weighting. J Gastroenterol Hepatol. 2021;36(7):1812–1819. doi:10.1111/jgh.15336
26. Saeki I, Yamasaki T, Maeda M, et al. No Muscle Depletion with High Visceral Fat as a Novel Beneficial Biomarker of Sorafenib for Hepatocellular Carcinoma. Liver Cancer. 2018;7(4):359–371. doi:10.1159/000487858
27. Antonelli G, Gigante E, Iavarone M, et al. Sarcopenia is associated with reduced survival in patients with advanced hepatocellular carcinoma undergoing sorafenib treatment. United Eur Gastroenterol J. 2018;6(7):1039–1048. doi:10.1177/2050640618781188
28. Guan J, Yang Q, Chen C, Wang G, Zhu H. Prognostic value of low skeletal muscle mass in hepatocellular carcinoma patients treated with sorafenib or lenvatinib: a meta-analysis. EXCLI J. 2021;20:1–16. doi:10.17179/excli2020-3111
29. Toshida K, Itoh S, Tomiyama T, et al. Comparison of the prognostic effect of sarcopenia on atezolizumab plus bevacizumab and lenvatinib therapy in hepatocellular carcinoma patients. JGH Open. 2022;6(7):477–486. doi:10.1002/jgh3.12777
30. Matsumoto H, Tsuchiya K, Nakanishi H, et al. Clinical Usefulness of Monitoring Muscle Volume during Atezolizumab Plus Bevacizumab Therapy in Patients with Unresectable Hepatocellular Carcinoma. Cancers. 2022;14(14):454.
31. Guo Y, Ren Y, Wu F, Dong X, Zheng C. Prognostic impact of sarcopenia in patients with hepatocellular carcinoma treated with PD-1 inhibitor. Therap Adv Gastroenterol. 2022;15:17562848221142417. doi:10.1177/17562848221142417
32. Akce M, Liu Y, Zakka K, et al. Impact of Sarcopenia, BMI, and Inflammatory Biomarkers on Survival in Advanced Hepatocellular Carcinoma Treated With Anti-PD-1 Antibody. Am J Clin Oncol. 2021;44(2):74–81. doi:10.1097/COC.0000000000000787
33. Ke Q, Xin F, Fang H, Zeng Y, Wang L, Liu J. The Significance of Transarterial Chemo(Embolization) Combined With Tyrosine Kinase Inhibitors and Immune Checkpoint Inhibitors for Unresectable Hepatocellular Carcinoma in the Era of Systemic Therapy: a Systematic Review. Front Immunol. 2022;13:913464. doi:10.3389/fimmu.2022.913464
34. Xin Y, Cao F, Yang H, et al. Efficacy and safety of atezolizumab plus bevacizumab combined with hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma. Front Immunol. 2022;13:929141. doi:10.3389/fimmu.2022.929141
35. Jorgensen JT. Companion and Complementary Diagnostics: clinical and Regulatory Perspectives. Trends Cancer. 2016;2(12):706–712. doi:10.1016/j.trecan.2016.10.013
36. Takenaka Y, Oya R, Takemoto N, Inohara H. Predictive impact of sarcopenia in solid cancers treated with immune checkpoint inhibitors: a meta-analysis. J Cachexia Sarcopenia Muscle. 2021;12(5):1122–1135. doi:10.1002/jcsm.12755
37. Fu Y, Peng W, Zhang W, et al. Induction therapy with hepatic arterial infusion chemotherapy enhances the efficacy of lenvatinib and pd1 inhibitors in treating hepatocellular carcinoma patients with portal vein tumor thrombosis. J Gastroenterol. 2023;58(4):413–424. doi:10.1007/s00535-023-01976-x
38. Yu W, Liu W, Zhang K, Chen S, Wang X. Transarterial interventional therapy combined with tyrosine kinase inhibitors with or without anti-PD-1 antibodies as initial treatment for hepatocellular carcinoma with major portal vein tumor thrombosis: a single-center retrospective study. Cancer Immunol Immunother. 2023;72(11):3609–3619. doi:10.1007/s00262-023-03511-6
39. Aoyagi T, Terracina KP, Raza A, Matsubara H, Takabe K. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol. 2015;7(4):17–29. doi:10.4251/wjgo.v7.i4.17
40. C-H W, Liang P-C, Hsu C-H, Chang F-T, Shao -Y-Y, Ting-Fang Shih T. Total skeletal, psoas and rectus abdominis muscle mass as prognostic factors for patients with advanced hepatocellular carcinoma. JFormos Med Assoc. 2021;120(1, Part 2):559–566. doi:10.1016/j.jfma.2020.07.005
41. Saeki I, Yamasaki T, Yamauchi Y, et al. Skeletal Muscle Volume Is an Independent Predictor of Survival after Sorafenib Treatment Failure for Hepatocellular Carcinoma. Cancers. 2021;13(9):2247.
42. Dasarathy S. Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle. 2012;3(4):225–237. doi:10.1007/s13539-012-0069-3
43. Dalamaga M. Interplay of adipokines and myokines in cancer pathophysiology: emerging therapeutic implications. World J Exp Med. 2013;3(3):26–33. doi:10.5493/wjem.v3.i3.26
44. Waldmann TA, Dubois S, Miljkovic MD, Conlon KC. IL-15 in the Combination Immunotherapy of Cancer. Front Immunol. 2020;11:868. doi:10.3389/fimmu.2020.00868
45. Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res. 2010;16(24):6019–6028. doi:10.1158/1078-0432.CCR-10-1966
46. Chen BB, Liang PC, Shih TT, et al. Sarcopenia and myosteatosis are associated with survival in patients receiving immunotherapy for advanced hepatocellular carcinoma. Eur Radiol. 2023;33(1):512–522. doi:10.1007/s00330-022-08980-4
47. Kim N, Yu JI, Park HC, et al. Incorporating sarcopenia and inflammation with radiation therapy in patients with hepatocellular carcinoma treated with nivolumab. Cancer Immunol Immunother. 2021;70(6):1593–1603. doi:10.1007/s00262-020-02794-3
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


