Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Prognosis Prediction of CRAFITY Score in HCC Undergoing TACE Combined with PD-(L)1 Inhibitors and Molecular Targeted Therapy
Authors Hu ZX, Xu XY, Wang Z, Huang JT, Li WC, Zhang S, Shen J, Zhong BY , Zhu XL
Received 3 October 2023
Accepted for publication 10 November 2023
Published 21 November 2023 Volume 2023:10 Pages 2073—2082
DOI https://doi.org/10.2147/JHC.S439660
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Imam Waked
Ze-Xin Hu,* Xiao-Yang Xu,* Ze Wang,* Jin-Tao Huang, Wan-Ci Li, Shuai Zhang, Jian Shen, Bin-Yan Zhong, Xiao-Li Zhu
Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiao-Li Zhu; Bin-Yan Zhong, Department of Interventional Radiology, The First Affiliated Hospital of Soochow University, 188 Shizi St, Suzhou, 215006, People’s Republic of China, Tel/Fax +86 512 67972173, Email [email protected]; [email protected]
Background: The CRAFITY (C-reactive protein and alpha-fetoprotein in immunotherapy) score has demonstrated prognostic significance in hepatocellular carcinoma (HCC) patients undergoing immunotherapy. The study aimed to validate accuracy of CRAFITY score on predicting prognosis for patients with HCC treated with transarterial chemoembolization (TACE) combined with PD-(L)1 inhibitors and molecular targeted therapy.
Methods: Eighty-five HCC patients who underwent TACE in combination with molecular targeted therapy (MTT) and PD-(L)1 Inhibitors were consecutively enrolled from November 2019 to November 2022. Patients were divided into CRAFITY 0 score (n=32), CRAFITY 1 score (n=31), and CRAFITY 2 score (n=22), respectively. The primary outcomes were overall survival (OS) and progression-free survival (PFS), and the secondary outcomes included tumor response rate and treatment-related adverse events (TRAEs). Factors affecting survival were identified via Cox regression analysis.
Results: The median overall survival (OS) for HCC patients with CRAFITY scores of 0, 1, and 2 was 33.4 months (95% confidence interval [CI]: 27.1– 39.7), 34.5 months (95% CI: 23.1– 45.9), and 24.2 months (95% CI: 13.9– 39.3), respectively, there were statistical differences among the three groups (p< 0.05). The progression-free survival (PFS) was 14.1 months (95% CI: 10.0– 18.2), 14.1 months (95% CI: 9.0– 19.2), and 9.3 months (95% CI: 7.2– 11.4) for patients with CRAFITY scores of 1, 2, and 3, respectively, with a significant difference between the three groups (p< 0.05). In patients with CRAFITY scores of 1, 2, and 3, the disease control rates (DCR) were 94%, 84%, and 73%, respectively (p < 0.05), while the overall response rates (ORR) were 78.1%, 67.7%, and 59.1%, respectively (p = 0.318). A higher CRAFITY score showed a correlation with an increased frequency of fatigue and grade 3 fever (p< 0.05). Moreover, CRAFITY 2 score was an independent risk factor for both OS (HR = 2.610(1.281– 4.564), p = 0.014) and PFS (HR = 2.419(1.281– 4.564), p = 0.006).
Conclusion: The CRAFITY score may provide an efficient predictive capacity for prognosis in HCC patients undergoing TACE combined with PD-(L)1 inhibitors and molecular targeted therapy.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, C‑reactive protein and alpha‑fetoprotein in immunotherapy score, PD-(L)1 inhibitors, tyrosine kinase inhibitors, combination therapy
Introduction
Liver cancer is the sixth most common cancer and the third leading cause of cancer-related deaths in the world. The most common type is HCC, accounting for approximately 75–85% of cases, with a five-year survival rate of only about 18%.1 Due to its insidious onset, over 80% of patients are diagnosed at an advanced stage,2 making surgical resection or ablation for curative intent no longer feasible.
TACE is the standard care for intermediate HCC while it is the most widely used treatment approach Across all stages.3–5 Following the publication of IMbrave150,6 notable congruent outcomes have been observed in the Phase 2/3 study ORIENT-327 conducted in China. This study integrated the PD-1 antibody sintilimab with the bevacizumab biosimilar (IBI305) for the treatment of advanced HCC. Recently, the superiority of the combination of the anti-PD-1 antibody camrelizumab and the VEGFR2-targeted TKI rivoceranib over sorafenib was demonstrated in the CARES-310.8 This international Phase 3 trial was conducted globally in 13 countries and regions. It received approval from local or central institutional review boards and ethics committees. The combination treatment with an anti-PD-(L)1 agent and anti-VEGF and/or oral tyrosine kinase inhibitors (TKIs) has been recommended in the first-line setting through the aforementioned milestone studies.
By embolizing tumor-feeding arteries, TACE results in necrosis of the tumor tissue, leading to acute hypoxia. This results in an increase in VEGF and the expression of PD-L1 on immune cells and tumor cells, while reducing the release of immunosuppressive factors.9,10 Consequently, the regime of TACE with anti-PD-(L)1 and MTT was widely adopted in real-world, and the effectiveness was validated by several studies.11–13 However, the benefit population of patients undergoing this combination therapy remains uncertain due to tumor heterogeneity. Recently, the C-reactive protein (CRP) and AFP in immunotherapy (CRAFITY) score has been shown to effectively predict the survival of HCC patients receiving monotherapy and Combination therapy based on immunotherapy.14–17 The CRAFITY score was defined as follows: CRAFITY 0 represented an AFP level below 100 ng/mL and a CRP level of less than 1 mg/dl, CRAFITY 1 represented an AFP level of at least 100 ng/mL or a CRP level of at least 1 mg/dl, and CRAFITY 2 represented both an AFP level over 100 ng/mL and a CRP level of more than 1 mg/dl. Yet, the predictive role of the CRAFITY score in HCC patients undergoing TACE combine with PD-(L)1 inhibitors and MTT has not been reported.
This study aimed to evaluate the correlation between the CRAFITY score and the prognosis of patients undergoing TACE in combination with PD-(L)1 inhibitors and MTT.
Methods
Patients
This study screened a total of 110 patients who received TACE combined with MTT and immunotherapies at the First Affiliated Hospital of Soochow University from November 2019 to November 2022. The inclusion criteria were as follows: 1) ages between 18 and 75 years old; 2) histologically or clinically confirmed diagnosis of HCC with BCLC B or C 3) Child-Pugh score ≤7 points, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; 4) initiation of MTT and Immune checkpoint inhibitors (ICIs) within one month before or after TACE; 5) at least one measurable target lesion in accordance with mRECIST. The exclusion criteria were as follows: 1) history of other systemic treatments; 2) presence of severe complications such as cardiorespiratory, renal, or coagulation disorders, decompensated cirrhosis, etc.; 3) concurrent other malignancies; 4) history of immunodeficiency disorders or organ transplantation; 5) incomplete medical history data.
Eighty-five patients were included in this study and divided into three groups finally, which could achieve either 0 (AFP < 100 ng/mL and CRP < 1 mg/dl), 1 (either AFP >100 ng/mL or CRP >1 mg/dl), or 2 (AFP >100 ng/mL and CRP >1 mg/dl) points according to the definition,14 both indicators were obtained from the baseline data. Based on the Declaration of Helsinki, the study protocol was approved by the ethical committee of the First Affiliated Hospital of Soochow University. Requirement to obtain written informed consent was waived due to its retrospective nature, and we stated that patient data was strictly confidential. The process of this study is shown in Figure 1.
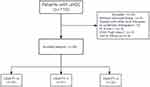 |
Figure 1 Flowchart of the patient selection process. Abbreviations: uHCC, unresectable hepatocellular carcinoma; PS, performance status. |
Transarterial Chemoembolization Procedure
Patients were offered either conventional TACE (cTACE) or drug-eluting beads TACE (DEB-TACE) based on preferences and tumor characteristics. The procedures were performed by physicians with at least 10 years of experience in interventional radiology. Each patient underwent superselective embolization using microcatheters. For patients with large tumor burdens or multifocal tumors in bilobes, the TACE treatment was performed in multiple sessions. “On-demand” TACE treatments were based on the postoperative results of contrast-enhanced CT or MRI and tumor marker reassessment to determine the need for additional treatment.
MTTs and ICIs Administration
Molecular targeted (TKI or anti-VEGF) agents including sorafenib, lenvatinib and bevacizumab were all administrated with their standard dose.18–20 Oral agents interrupted for two days before and after each TACE session if no obvious symptoms occurred after TACE. Dose modifications were permitted according to the label. ICIs including sintilimab, atezolizumab were administrated based on their standard dose and frequency.7 Dose reduction was not allowed, but interruption of ICIs due to adverse events was allowed. Patients discontinued the combination treatment in the case of unacceptable toxicity or disease progression.
Follow Up
All enrolled patients were regularly followed up. Enhanced MRI/CT evaluations were conducted 30–40 days after the initial TACE treatment and subsequently repeated every 3 months. The laboratory tests were performed every 3 weeks before Intravenous administration of ICIs, encompassing complete blood count, comprehensive biochemical analysis, tumor markers, thyroid function, chest pain panel, and full immunological profiling, HBV-DNA load. Tumor response assessment was performed by two experienced independent radiologists with over five years of expertise, following the guidelines of mRECIST. Patient follow-up was consistently maintained until either the occurrence of mortality or the conclusion of the study on June 1, 2023.
Outcomes
Primary outcomes included overall survival (OS) and progression-free survival (PFS). OS defined as the time from the initiation of combination therapies to death from any cause or last follow-up; PFS defined as the time from the initiation of TACE procedure to first tumor progression or death. Secondary outcomes included objective response rate (ORR), disease control rate (DCR), and treatment related adverse events (TRAEs). TRAEs were evaluated according to Common Terminology Criteria for Adverse Events version 5.0 (CTCAE v5.0).21
Statistical Analysis
Categorical variables were compared using the χ2 test or Fisher’s exact test. Continuous variables were compared using either the t-test for normally distributed variables or the Mann–Whitney U-test for variables with a non-normal distribution. Characteristics were summarized using median with interquartile range for continuous variables with abnormal distribution, and using frequencies with proportions for categorical variables. The survival analysis was carried out using the Kaplan–Meier method and compared using the Log rank test. Multivariable Cox regression analysis to identify factors independently associated with PFS and OS. p< 0.05 was considered statistically significant. All the above statistical analyses were performed using SPSS version 26.0 (IBM, NY) and R software (http://www.r-project.org).
Result
Baseline Characteristics
From November 2019 to November 2022, 110 patients with uHCC were recruited and 25 patients were excluded. Finally, 85 patients were enrolled in this study and were observed either until death or the last follow-up date (June 1, 2023) for living patients. Based on preoperative baseline data, 32 patients were assigned to the CRAFITY 0 score group, 31 patients were assigned to the CRAFITY 1 score group, and the remaining 22 were assigned to the CRAFITY 2 score group (Figure 1). Table 1 shows the baseline characteristics of 85 HCC patients, with a mean age of 60.6 ± 9.9 years and 74 (87.1%) male patients. The majority of cases had a history of hepatitis B(n=73,85.9%) infection and cirrhosis (n=66,77.6%). The Child–Pugh stage of A or B was found in 63 (74.1%), 22 (25.9%) patients, respectively. More than half of the patients were at the Barcelona Clinic Liver Cancer (BCLC) stage C(n=43,50.6%). A small proportion presented with extrahepatic metastases (n=16,18.8%). The median tumor diameter was 7.2 (4.4~10.0), with unilobar and bilobar distributions in 50 cases (58.8%) and 35 cases (41.2%), respectively. TACE procedure was performed three times in each of the three groups. Significant differences among the three groups were observed in terms of tumor diameter, age, and BCLC stage (p<0.05).
 |
Table 1 Baseline Demographic and Clinical Characteristics of Enrolled Patients |
Survival Assessment
The median follow-up duration was 28.3month. At the time of last follow-up date (June 1, 2023), 45 patients had died and 62 patients were found disease progression in the entire cohort. The median OS and PFS of the entire cohort are 30.1 months and 11.3 months, respectively.
The survival times of different CRAFITY score groups were compared. The median OS for HCC patients with CRAFITY scores of 0, 1, and 2 was 33.4 months (95% confidence interval [CI]: 27.1–39.7), 34.5 months (95% CI: 23.1–45.9), and 24.2 months (95% CI: 13.9–39.3), respectively. Notably, statistically significant differences were detected among these three groups (p<0.05). Furthermore, there was a significant difference between the CRAFITY 2 score group and the other groups (p=0.005, Figure 2A), while no statistically significant variance was observed between the CRAFITY 1 group and the CRAFITY 2 group (p=0.510). The median progression-free survival (PFS) was 14.1 months (95% CI: 10.0–18.2), 14.1 months (95% CI: 9.0–19.2), and 9.3 months (95% CI: 7.2–11.4) for patients with CRAFITY scores of 1, 2, and 3, respectively, with a significant difference between the three groups (p=0.031, Figure 2B).
 |
Figure 2 (A) Kaplan–Meier curve for overall survival among the three groups. (B) Kaplan–Meier curve for progression-free survival among the three groups. |
Considering the non-significant difference between CRAFITY 0 and 1 group, the two groups were combined (CRAFITY<2 group, n = 63) and compared with the CRAFITY 2 group further. The mOS and mPFS of CRAFITY <2 group was 33.4 months(95% CI: 26.8–40.0) and 14.1 month (95% CI: 11.6–16.6), respectively, both showed a significant difference between the CRAFITY 2 score group (p<0.01, p<0.001, Figure 3A and B).
 |
Figure 3 (A) Kaplan–Meier curve for overall survival between CRAFITY<2 group and CRAFITY 2 group. (B) Kaplan–Meier curve for progression-free survival between CRAFITY<2 group and CRAFITY 2 group. |
Tumor Response
The best tumor response rates are shown in Table 2. The ORR and DCR of the entire cohort was 69.4% and 89.7%. CR, PR, SD and PD were observed in 10 (11.8%), 49 (57.6%), 13 (15.3%) and 13 (15.3%) patients, respectively. Notably, Lower CRAFITY scores are associated with better tumor responses. Specifically, the ORR of the CRAFITY 0, 1, and 2 scores was 78.1%, 67.7%, 59.1%, respectively (p=0.318). In terms of the DCR, patients with CRAFITY scores of 0, 1, and 2 achieved rates of 96.6%, 88.9%, and 84.6%, respectively, which demonstrated a statistically significant difference (p<0.05).
 |
Table 2 Tumor Response for Each Group |
Safety Assessment
The details of TRAEs are summarized in Table 3. All reported AEs were mild and well-tolerated, with no treatment-related deaths occurring during the study. Grade 3 liver injuries were more likely to occur in CRAFITY 2 score (p<0.05), moreover, grades 1–2 fatigue is more likely to occur in this group (p<0.05). No significant differences in additional AEs were observed among the three groups. In terms of treatment discontinuation, two patients ceased targeted therapy due to gastrointestinal bleeding, one due to gastrointestinal perforation, and another due to severe hand-foot syndrome. Among those who discontinued immunotherapy, one patient experienced immune-related ocular inflammation, another had immune-related pituitary inflammation, and a third developed immune-related myocarditis. Of note, the symptoms in these patients were effectively managed with appropriate symptomatic treatment following medication discontinuation.
 |
Table 3 Treatment-Related Adverse Events |
Prognostic Factors for OS and PFS
We conducted a multivariate Cox proportional hazards regression analysis to ascertain the independent prognostic indicators for overall survival (OS) and progression-free survival (PFS). As presented in Table 4, the results indicated that CRAFITY 2 score was an independent risk factor for both OS (HR = 2.610(1.281–4.564), p=0.014) and PFS (HR = 2.419(1.281–4.564), p=0.006). Meanwhile, BCLC stage was a significant independent risk factor for PFS (HR = 2.013(1.159–3.496), p=0.013).
 |
Table 4 Multivariate Analyses Factors Associated with OS and PFS |
Evaluating the Predictive Performance of the CRAFITY Score in Comparison to Other Inflammation Markers
Comparisons of C-index were performed. The CRAFITY score exhibited the best predictive performance with a C-index of 0.705 (95% CI: 0.647–0.782) for OS and 0.598 (95% CI: 0.508–0.648) for PFS, significantly surpassing other inflammation markers. The C-index of all models and the comparisons between these models are displayed in the Supplementary Table 1.
Discussion
The TACE-ICIs-MTT combination regime shows promise for enhancing uHCC prognosis, with growing real-world application. However, this treatment approach may not be suitable for all individuals; thus, it is imperative to find biomarkers that can identify the beneficiary population undergoing this combination regime. In our study, we found that the CRFITY score efficiently stratifies the prognostic outcomes of the treatment population under this combination regimen, both mOS and mPFS showed great significances between CRAFITY <2 group and CRAFITY 2 group (p<0.01, p<0.001). Although, the stratification of CRFITY scores at 0 and 1 points did not show significant differences (p=0.510), which has been reported in subgroup analysis of a previous study.16 Potential reasons for the above findings could be the relatively small number of cases, additionally, the application of TACE could contribute to the increased complexity of the tumor microenvironment. Lower CRAFITY scores are associated with better tumor responses, which was consistent with the previous studies.14,15 We further investigated that CARFITY 2 score was the independent predictor for both OS and PFS of patients after TACE-ICIs-MTT combination treatment.
A tolerable safety profile was observed in this study. Due to the increased complexity of AEs with the addition of immunotherapy, early identification of immune-related adverse reactions is of significant importance in determining whether patients can achieve favorable treatment outcomes.22,23 In this study, fatigue and Grade 3 liver injury were more likely to occur in CRAFITY 2 score. However, there were no statistically significant differences in immune-related AEs among the three groups, suggesting the need for further research to explore this problem.
The benefits of TACE in patients with HCC have been confirmed by several studies. A systematic analysis involving 10,108 individuals24 demonstrated that the median overall survival (mOS) for HCC patients treated with TACE was 19.4 months, with a 5-year survival rate of 32.4%. In order to stratify the prognosis of patients undergoing TACE treatment, the “six and twelve” score was successfully developed by Wang et al25 and wildly applied.26 Meanwhile, some inflammatory markers and scores have been proven to have a certain effect of prediction in the above patients.27,28 With the success of the IMbrave-150 trial,6 systematic therapy based on ICIs was becoming the standard treatment for advanced HCC patients, despite some studies29,30 identifying certain indicators and markers that can predict the prognosis of combination therapy, there is still a lack of high-level evidence. Recently, the TACE-ICIs-MTT combination regime has been widely used, covering a majority of patients in BCLC stage B and C, and achieved impressive therapeutic results.11–13 While some studies have indicated that the Neutrophil-to-Lymphocyte Ratio (NLR) can predict the prognosis of patients undergoing this combination therapy,31,32 the level of evidence supporting this remains relatively low. In our study, comparisons were made between CRAFITY and other inflammatory markers, and the results indicated that CRAFITY exhibited superior predictive performance in the treatment regime.
AFP and CRP are significant prognostic indicators in HCC,33–36 AFP, in particular, is widely employed as a serum biomarker in HCC management and stands as the sole biomarker guiding treatment decisions.37 A higher AFP level was related to a more invasive HCC. Emerging evidence suggests that AFP is associated with up-regulation of VEGF signaling,38 moreover, it might impair macrophage function, leading to decreased functions of phagocytosis and antigen presentation,39 enabling the escape of hepatoma cells from the host’s lymphocytes immune surveillance. Current studies found that the reduction in the serum AFP level after anti-PD-1 therapy was associated with a favorable prognosis in advanced HCC.36,40 Chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) and cirrhosis can lead to AFP elevation in HCC, previous studies have confirmed the prognostic significance of stratifying AFP levels in HCC patients with HBV infection.41 The HIMALAYA study42 suggested that HBV‑related HCC patients responded better to immune checkpoint inhibitors. The majority of patients in our study had HBV infection and received antiviral treatment.
CRP functions as an acute-phase protein and serves as a widely acknowledged indicator of cancer-induced systemic inflammation, a condition often evidenced in clinical symptoms.43 Moreover, inflammation gives rise to numerous tumor-facilitating effects, encompassing the stimulation of cancer cell proliferation, initiation of metastatic processes, promotion of angiogenesis, and suppression of adaptive immunity in tumor microenvironment.44 In non-small-cell lung cancer patients, high CRP was associated with PD-L1 positivity, suggesting that CRP may impair the efficacy of immunotherapy. Recently, it has been shown in small studies that high CRP level was associated with a poor outcome when treated PD-1 immune checkpoint blockade in patients with advanced non-small-cell lung cancer.45 More recently, tissue samples from the Checkmate 040 Trial46 demonstrated that inflammatory factors associated with CRP were associated with objective responses and OS among patients treated with Nivolumab.
In light of above findings, combining CRP and AFP to predict outcomes in HCC patients during the era of immunotherapy appears to be reasonable. The CRAFITY score was successfully developed by Bernhard Scheiner et al to predict prognosis of patients with HCC treated with PD-(L)1 inhibitors, and it validated in populations receiving atezolizumab plus bevacizumab or tyrosine kinase inhibitor plus immunotherapy combinations.15,47 Guan et al16 showed the CRAFITY score was a superior predictor for patients treated with locoregional‑immunotherapy; however, there was a relatively small proportion of patients who underwent TACE-ICIs-MTT in their study. To our knowledge, this is the first study using the CRAFITY score to stratify the prognosis of the patients receiving TACE- ICIs-MTT combination regime.
Limitation
There are several limitations in the study. Firstly, this study is a single-center retrospective cohort study and the selection bias cannot be avoided. Secondly, the total sample size of our study was relatively small compared to previous studies. Thirdly, there was a lack of an external cohort available to validate the predictive value of the CRAFITY score in HCC patients undergoing TACE-ICIs-MTT regime.
Conclusion
The CRAFITY score may provide an efficient predictive capacity for prognosis in HCC patients undergoing TACE combined with PD-(L)1 Inhibitors and Molecular Targeted Therapy. More large-scale, randomized, controlled clinical trials are needed to confirm the above conclusion.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
2. Vogel A, Meyer T, Sapisochin G, et al. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362. doi:10.1016/S0140-6736(22)01200-4
3. Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35(9):2155–2166. doi:10.1111/liv.12818
4. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
5. Llovet JM, Villanueva A, Marrero JA, et al. Trial design and endpoints in hepatocellular carcinoma: AASLD consensus conference. Hepatology. 2021;73(Suppl 1):158–191. doi:10.1002/hep.31327
6. Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi:10.1016/j.jhep.2021.11.030
7. Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. 2021;22(7):977–990. doi:10.1016/S1470-2045(21)00252-7
8. Qin S, Chan SL, Gu S, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. 2023;402:1133–1146. doi:10.1016/S0140-6736(23)00961-3
9. Zhong BY, Jin ZC, Chen JJ, et al. Role of transarterial chemoembolization in the treatment of hepatocellular carcinoma. J Clin Transl Hepatol. 2023;11(2):480–489. doi:10.14218/JCTH.2022.00293
10. Palmer DH, Malagari K, Kulik LM. Role of locoregional therapies in the wake of systemic therapy. J Hepatol. 2020;72(2):277–287. doi:10.1016/j.jhep.2019.09.023
11. Zhu HD, Li HL, Huang MS, et al. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. 2023;8(1):58. doi:10.1038/s41392-022-01235-0
12. Jin ZC, Zhong BY, Chen JJ, et al. Real-world efficacy and safety of TACE plus camrelizumab and apatinib in patients with HCC (CHANCE2211): a propensity score matching study. Eur Radiol. 2023. doi:10.1007/s00330-023-09754-2
13. Duan X, Li H, Kuang D, et al. Transcatheter arterial chemoembolization plus apatinib with or without camrelizumab for unresectable hepatocellular carcinoma: a multicenter retrospective cohort study. Hepatol Int. 2023;17(4):915–926. doi:10.1007/s12072-023-10519-8
14. Scheiner B, Pomej K, Kirstein MM, et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy - development and validation of the CRAFITY score. J Hepatol. 2022;76(2):353–363. doi:10.1016/j.jhep.2021.09.035
15. Yang Y, Ouyang J, Zhou Y, et al. The CRAFITY score: a promising prognostic predictor for patients with hepatocellular carcinoma treated with tyrosine kinase inhibitor and immunotherapy combinations. J Hepatol. 2022;77(2):574–576. doi:10.1016/j.jhep.2022.03.018
16. Guan R, Mei J, Lin W, et al. Is the CRAFITY score a superior predictor of prognosis and adverse events in hepatocellular carcinoma patients treated with locoregional-immunotherapy. Hepatol Int. 2023;17:1279–1288. doi:10.1007/s12072-023-10535-8
17. Hatanaka T, Kakizaki S, Hiraoka A, et al. Prognostic impact of C-reactive protein and alpha-fetoprotein in immunotherapy score in hepatocellular carcinoma patients treated with atezolizumab plus bevacizumab: a multicenter retrospective study. Hepatol Int. 2022;16(5):1150–1160. doi:10.1007/s12072-022-10358-z
18. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
19. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
20. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
21. Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014;106(9). doi:10.1093/jnci/dju244
22. Sangro B, Chan SL, Meyer T, et al. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J Hepatol. 2020;72(2):320–341. doi:10.1016/j.jhep.2019.10.021
23. Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–172. doi:10.1038/s41571-021-00573-2
24. Lencioni R, De Baere T, Soulen MC, et al. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–116. doi:10.1002/hep.28453
25. Wang Q, Xia D, Bai W, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: a multicentre observational study. J Hepatol. 2019;70(5):893–903. doi:10.1016/j.jhep.2019.01.013
26. Adhoute X, Penaranda G, Raoul JL, et al. ”Six-and-twelve” score for outcome prediction of hepatocellular carcinoma following transarterial chemoembolization. In-depth analysis from a multicenter French cohort. World J Hepatol. 2020;12(8):525–532. doi:10.4254/wjh.v12.i8.525
27. Mcnally ME, Martinez A, Khabiri H, et al. Inflammatory markers are associated with outcome in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. Ann Surg Oncol. 2013;20(3):923–928. doi:10.1245/s10434-012-2639-1
28. Liu PH, Hsu CY, Hsia CY, et al. ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD era. J Gastroenterol Hepatol. 2017;32(4):879–886. doi:10.1111/jgh.13608
29. Campani C, Bamba-Funck J, Campion B, et al. Baseline ALBI score and early variation of serum AFP predicts outcomes in patients with HCC treated by atezolizumab-bevacizumab. Liver Int. 2023;43(3):708–717. doi:10.1111/liv.15487
30. Wu YL, Fulgenzi C, D’alessio A, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as prognostic biomarkers in unresectable hepatocellular carcinoma treated with atezolizumab plus bevacizumab. Cancers (Basel). 2022;14(23). doi:10.3390/cancers14235834
31. Zheng X, Qian K. Neutrophil-to-lymphocyte ratio predicts therapy outcomes of transarterial chemoembolization combined with tyrosine kinase inhibitors plus programmed cell death ligand 1 antibody for unresectable hepatocellular carcinoma. Anticancer Drugs. 2023;34(6):775–782. doi:10.1097/CAD.0000000000001458
32. Li S, Wu J, Wu J, et al. Prediction of early treatment response to the combination therapy of TACE plus lenvatinib and anti-PD-1 antibody immunotherapy for unresectable hepatocellular carcinoma: multicenter retrospective study. Front Immunol. 2023;14:1109771. doi:10.3389/fimmu.2023.1109771
33. Sieghart W, Pinter M, Hucke F, et al. Single determination of C-reactive protein at the time of diagnosis predicts long-term outcome of patients with hepatocellular carcinoma. Hepatology. 2013;57(6):2224–2234. doi:10.1002/hep.26057
34. Meischl T, Rasoul-Rockenschaub S, Gyori G, et al. C-reactive protein is an independent predictor for hepatocellular carcinoma recurrence after liver transplantation. PLoS One. 2019;14(5):e216677. doi:10.1371/journal.pone.0216677
35. Zheng Y, Zhu M, Li M. Effects of alpha-fetoprotein on the occurrence and progression of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146(10):2439–2446. doi:10.1007/s00432-020-03331-6
36. Shao YY, Liu TH, Hsu C, et al. Early alpha-foetoprotein response associated with treatment efficacy of immune checkpoint inhibitors for advanced hepatocellular carcinoma. Liver Int. 2019;39(11):2184–2189. doi:10.1111/liv.14210
37. Galle PR, Foerster F, Kudo M, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39(12):2214–2229. doi:10.1111/liv.14223
38. Shan YF, Huang YL, Xie YK, et al. Angiogenesis and clinicopathologic characteristics in different hepatocellular carcinoma subtypes defined by EpCAM and alpha-fetoprotein expression status. Med Oncol. 2011;28(4):1012–1016. doi:10.1007/s12032-010-9600-6
39. Atemezem A, Mbemba E, Marfaing R, et al. Human alpha-fetoprotein binds to primary macrophages. Biochem Biophys Res Commun. 2002;296(3):507–514. doi:10.1016/S0006-291X(02)00909-9
40. Sun X, Mei J, Lin W, et al. Reductions in AFP and PIVKA-II can predict the efficiency of anti-PD-1 immunotherapy in HCC patients. BMC Cancer. 2021;21(1):775. doi:10.1186/s12885-021-08428-w
41. Blank S, Wang Q, Fiel MI, et al. Assessing prognostic significance of preoperative alpha-fetoprotein in hepatitis B-associated hepatocellular carcinoma: normal is not the new normal. Ann Surg Oncol. 2014;21(3):986–994. doi:10.1245/s10434-013-3357-z
42. Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1(8). doi:10.1056/EVIDoa2100070
43. Diakos CI, Charles KA, Mcmillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. doi:10.1016/S1470-2045(14)70263-3
44. Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi:10.1038/nature07205
45. Oya Y, Yoshida T, Kuroda H, et al. Predictive clinical parameters for the response of nivolumab in pretreated advanced non-small-cell lung cancer. Oncotarget. 2017;8(61):103117–103128. doi:10.18632/oncotarget.21602
46. Sangro B, Melero I, Wadhawan S, et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J Hepatol. 2020;73(6):1460–1469. doi:10.1016/j.jhep.2020.07.026
47. Teng W, Lin CC, Su CW, et al. Combination of CRAFITY score with Alpha-fetoprotein response predicts a favorable outcome of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. Am J Cancer Res. 2022;12(4):1899–1911.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
