Back to Journals » OncoTargets and Therapy » Volume 17
Progerin Inhibits the Proliferation and Migration of Melanoma Cells by Regulating the Expression of Paxillin
Authors Liu W, Huang X, Luo W, Liu X , Chen W
Received 29 September 2023
Accepted for publication 25 February 2024
Published 22 March 2024 Volume 2024:17 Pages 227—242
DOI https://doi.org/10.2147/OTT.S442504
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Weixian Liu,1,2 Xinxian Huang,1– 3 Weizhao Luo,1,2 Xinguang Liu,1,2 Weichun Chen1,2
1Guangdong Provincial Key Laboratory of Medical Molecular Diagnostics, Institute of Aging Research, Guangdong Medical University, Dongguan, People’s Republic of China; 2Institute of Biochemistry & Molecular Biology, Guangdong Medical University, Zhanjiang, People’s Republic of China; 3School of Medical Technology, Guangdong Medical University, Dongguan, People’s Republic of China
Correspondence: Xinguang Liu; Weichun Chen, Tel +86-13922926543 ; +86-13694959795, Email [email protected]; [email protected]
Objective: Progerin, the underlying cause of Hutchinson-Gilford Progeria Syndrome (HGPS), has been extensively studied for its impact on normal cells and premature aging patients. However, there is a lack of research on its specific effects on tumor cells. Melanoma is one of the most common malignant tumors with high morbidity and mortality. This study aimed to elucidate the potential therapeutic role of progerin in melanoma.
Materials and Methods: We constructed the melanoma A375 cell line and M14 cell line with stable expression of progerin. The expression of progerin, paxillin, and epithelial-mesenchymal transition (EMT) marker proteins in each cell group was measured using Western blot. The migration, proliferation, and cell cycle of cancer cells were assessed using the transwell assay, wound healing assay, colony formation assay, CCK 8 assay, and flow cytometry. RT-qPCR technology was used to examine the impact of progerin overexpression on microRNA expression. Finally, we transfected paxillin into the progerin overexpression cell group to verify whether progerin regulates the phenotype of tumor cells through paxillin.
Results: Our study demonstrated that overexpression of progerin leads to decreased expression of paxillin and inhibits cancer cell migration, proliferation, EMT process and cell cycle progression. Additionally, rescue experiments revealed that the migration, proliferation ability, and EMT marker protein expression in progerin overexpressing cancer cells could be partially restored by transfecting a plasmid containing the paxillin gene. Mechanistic investigations further revealed that progerin achieves this inhibition of paxillin expression by upregulating miR-212.
Conclusion: This study reveals that progerin may inhibit the migration and proliferation of melanoma cells through the miR-212/paxillin axis, which provides a new approach for the future treatment of this disease.
Keywords: progerin, paxillin, migration, proliferation, melanoma
Introduction
Melanoma is a prevalent type of tumor that has been on the rise in recent years,1 posing a significant threat to human life. Currently, treatment options for melanoma consist of targeted therapy, immunotherapy, and surgical resection. However, these approaches have certain limitations. It is worth noting that aging, much like cancer, is a separate yet interconnected phenomenon. Senescence is both a tumor suppressor response and a cancer promoter.2,3
Hutchinson-Gilford Progeria Syndrome (HGPS) is a rare progeria disease, which is an ideal model for studying aging, and the average life expectancy of patients is only 14.6 years.4 HGPS is caused by a single base mutation (G608G) in the LMNA gene. This mutation leads to the permanent farnesylation of the lamin A precursor, resulting in the production of progerin.5–7 In comparison to lamin A, progerin is deficient in 50 amino acid residues in its C-terminus, resulting in the absence of the ZMPSTE24 protease cleavage site. This absence enables progerin to retain a farnesyl group at its C-terminus.8 The production of progerin triggers a cascade of events, including nuclear deformity, accumulation of DNA damage, and disruption of chromatin organization. These events lead to accelerated aging of cells, ultimately resulting in premature aging of patients.9–11 As individuals age and accumulate DNA damage, the likelihood of developing tumors gradually increases. However, it is worth noting that individuals with HGPS do not develop childhood tumors.12 The HGPS model provides a unique opportunity to investigate the connection between aging and tumors. Consequently, many scholars have become interested in the potential of using cellular senescence induction as a method to treat cancer. Research has demonstrated that the administration of multiple chemotherapy drugs that induce senescence can effectively trigger senescence in cancer cells. Additionally, activating the senescence response through p53 activation, inhibition of c-MYC in tumors, or treatment with cyclin-dependent kinase (CDK) inhibitors has also shown promise in suppressing tumors.13,14 Considering the function of progerin and the observed correlation between aging and tumor development, it is plausible to hypothesize that introducing progerin into tumor cells could potentially inhibit tumor growth.
Paxillin is a multi-domain adapter protein, mainly composed of five leucine–aspartic acid (LD) domains at the N-terminal, four cysteine–histidine-rich Lin11, Isl-1, and Mec-3 (LIM) domains at the C-terminal, and a proline-rich sequence and some serine and tyrosine residues. It is the main component of focal adhesions, which can recruit a variety of signaling molecules in intracellular signaling and participate in a variety of signaling cascade reactions.15,16 The unique structure and function of paxillin contribute to its crucial role in cancer development. Numerous studies have consistently demonstrated that paxillin is frequently dysregulated in various types of malignant tumors,17 including breast cancer,18 gastric cancer,19 and colorectal cancer. Furthermore, its dysregulation is closely associated with unfavorable prognosis, tumor occurrence, and metastasis. Pan-cancer analysis revealed that paxillin exhibits distinct regulatory mechanisms in various tumor types. The aberrant expression of paxillin is associated with the clinical prognosis and immune infiltration in different tumor types.20 Therefore, paxillin holds promise as a potential biomarker for tumor prognosis.
However, the role of progerin in melanoma is still not understood. This study utilizes progerin-overexpressing melanoma cells as a research model to explore the effect of progerin on paxillin expression and tumor cell migration and proliferation, and further identify the relevant molecular mechanisms to provide certain therapeutic reference value for the treatment of melanoma.
Materials and Methods
Cell Culture
Both A375 and M14 cell lines were obtained from the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in DMEM medium (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA) and maintained in a 5% CO2 incubator at 37°C. The cells were passaged at a ratio of 1:3. All cells showed epithelial cell-like growth characteristics.
Lentiviral Infection
The lentivirus was obtained from Genechem (Shanghai, China). Following the manufacturer’s instructions, 5×104 A375 and M14 cells were seeded in 24-well plates one day prior to the experiment. After culturing overnight, the amount of virus with virus titer MOI=30 was added to infect the cells, and the fluorescence intensity and infection efficiency were observed after 72 h. The cells were then transferred to 6-well plates and screened with puromycin to establish stable expression cell lines.
Wound Healing Assay
To initiate the experiment, harvest cells in the logarithmic growth phase and resuspend them. Inoculate 5×105 cells into each well of a 6-well plate and place the plate in an incubator set at 37°C and 5% CO2. Next, using a 200 µL gun tip, create three scratches perpendicular to the bottom of the plate with consistent force. After gently washing the cells with PBS, add serum-free medium. Finally, capture photographs of the cells at 0 h and 48 h using an inverted microscope (Olympus, Tokyo, Japan) at 40× magnification.
Transwell Migration Assay
A375 cells or M14 cells were resuspended in serum-free medium to achieve a cell concentration of 2×105 cells/mL. Next, 250 µL of A375 cells or M14 cells suspension were inoculated into the upper chamber of a 24-well Transwell (Corning, USA). Subsequently, 750 µL of culture medium containing 20% FBS was added to the lower chamber. The cells were cultured in a 37°C, 5% CO2 incubator for 24 hours. The chamber was removed and rinsed three times with PBS. It was then fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The number of cells that passed through the membrane was counted.
Cell Proliferation Assay
The cell proliferation ability was detected by CCK8 method. Take the cells in the logarithmic growth phase and add them to the medium to resuspend to prepare a cell concentration of 2×104 cells/mL. Then, 100 μL of the suspension was inoculated in a 96-well plate. The detection points were set at 0 h, 24 h, 48 h, and 72 h. At each detection point, 10 µL of CCK8 reagent (Tojin, Japan) was added to each well and incubated for 2 h. The absorbance value at a wavelength of 450 nm was then measured.
Colony Formation Assay
After counting the cells, 500 cells per well were inoculated in a 6-well plate. Then, 2 mL of medium containing 10% FBS was added for culturing purposes over a period of 10 days. The medium was changed every three days. Following this, the cells were washed three times with PBS, fixed with 4% paraformaldehyde and stained with 0.1% crystal violet, and then count the visible colonies (≥50 cells). Finally, the cells were dried and photographed using a camera (Sony, Japan).
Western Blot Assay
Cell lysates were prepared in RIPA buffer with protease inhibitors and then separated on 12% SDS-PAGE gels. The separated proteins were electroblotted onto PVDF membranes (Millipore, USA). The membrane was blocked with 5% milk in TBST and then incubated with specific primary antibodies: LaminA/C (1:1000, ab169532, Abcam, USA), Paxillin (1:1000, #2542, Cell Signaling, USA), Flag (1:1000, #TA180144, OriGene, USA), N-cadherin (1:2000, 66219-1-Ig, Proteintech, USA), E-cadherin (1:5000, 20874-1-AP, Proteintech, China), vimentin (1:10000, 60330-1-1g, Proteintech, USA), and GAPDH (1:10000, 60004-1-Ig, Proteintech, USA). The primary antibody incubation was performed at 4°C. Subsequently, the membrane was incubated with either HRP-conjugated secondary antibody mouse (1:2000, A0216, Beyotime, China) or secondary antibody rabbit (1:2000, A0208, Beyotime, China) at room temperature. Finally, the protein bands were visualized using the ECL Western Blot Analysis System (Pierce, USA).
RT-qPCR Analysis
Total RNA was extracted from cancer cells using TRIzol reagent (Invitrogen, USA). Total RNA was reverse transcribed using the All-in-One miRNA RT-qPCR Detection Kit (GeneCopoeia, USA) and RT-qPCR was performed in a LightCycler 96 System (Roche) following the manufacturer’s protocol. For qPCR analysis, U6 was used as the internal reference for miRNA, and the specific primers are listed in Table 1.The relative expression of genes was determined using the 2−ΔΔCT method.
 |
Table 1 Related Primer Sequences |
Flow Cytometry Analysis
The cell cycle of each group was assessed using a cell cycle detection kit (Beyotime, China). Cells in six-well plates were collected in pre-cooled PBS and fixed overnight at 4°C with 70% ethanol. The staining solution was prepared following the manufacturer’s instructions. Then, 500 µL of the staining solution was added to each tube of cells, and the tubes were incubated at 37°C in the dark for 30 minutes. Flow cytometry analysis was performed using a Becton Dickinson FACSscan and analyzed with FlowJo software.
Dual-Luciferase Reporter Assay
The PXN 3’UTR fragments with and without miR-212 binding sites were synthesized and cloned into the luciferase reporter vector pmirGLO (Promega). These fragments were respectively referred to as PXN 3’UTR-WT and PXN 3’UTR-Mut. The luciferase reporter vector was co-transfected into 293T cells with miR-212 mimic or negative control precursor using Lipofectamine 3000 reagent (Invitrogen). According to the manufacturer’s instructions, the relative luciferase activity was measured with the Dual Luciferase Reporter Assay Kit (Promega).
Cell Transfection
Paxillin plasmids were obtained from Gene Pharma (Shanghai, China). To create A375 and M14 cell lines with stable overexpression of paxillin, pEX-6-paxillin or pEX-6 plasmids were transfected into A375 and M14 cells using Lipofectamine 3000 reagent (Invitrogen) following the manufacturer’s instructions. The transfected cells were then treated with G418 for 4 weeks.
Statistical Analysis
Statistical analysis was performed using SPSS 25.0 (IBM, USA) and GraphPad Prism 8.0 (GraphPad Software, USA), and data are shown as mean ± standard deviation (SD). Statistical results were analyzed using one-way analysis of variance (ANOVA) and Student’s t-test. P<0.05 was considered statistically significant.
Results
Progerin Inhibits the Migration of A375 Cells and M14 Cells
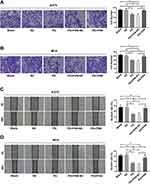
In a previous study conducted by our research group, it was demonstrated that progerin has the ability to inhibit the biological function of lung cancer cells.21 Building upon these findings, we sought to further investigate the impact of progerin on melanoma. To achieve this, we infected A375 cells and M14 cells with a lentivirus carrying the progerin gene. Through transwell experiments, we observed a significant reduction in the migration ability of cancer cells in the progerin (PG) group compared to the blank control group and negative control (NC) group (Figure 1A and B). Additionally, wound healing experiments further demonstrated that progerin had a significant inhibitory effect on the migration ability of A375 cells and M14 cells (Figure 1C and D). These findings indicate that progerin has the potential to inhibit the migration of cancer cells.
Progerin Alters the Expression of Epithelial-Mesenchymal Transition (EMT) Hallmark Proteins
To investigate the aforementioned phenomena, we conducted a study on EMT-related proteins. Given that EMT plays a crucial role in various physiological and pathological processes, such as wound healing and cancer metastasis,22 we formulated a hypothesis that progerin can reduce the migration ability of cancer cells by changing the EMT process in the context of inhibiting paxillin expression. Our findings from Western blot analysis revealed that the overexpression of progerin in A375 cells resulted in an upregulation of the epithelial cell marker E-cadherin and a downregulation of the mesenchymal markers vimentin and N-cadherin (Figure 2A), all of which are associated with EMT. Similarly, in the M14 cell group, the overexpression of progerin led to comparable alterations in EMT-related marker proteins (Figure 2B).
Progerin Inhibited the Proliferation Ability of A375 Cells and M14 Cells
Through clone formation experiments, it was observed that the overexpression of progerin significantly reduced the proliferation ability of A375 cells and M14 cells when compared to the blank group and negative control group (Figure 3A and B). Further analysis using the CCK 8 assay confirmed that overexpression of progerin can decrease the proliferative ability of A375 cells and M14 cells (Figure 3C and D). Additionally, flow cytometry analysis revealed that the overexpression of progerin increased the number of cells in the G1 phase and decreased the number of cells in the S phase of A375 cells and M14 cells, when compared to the blank control group and negative control group (Figure 3E). These findings suggest that progerin has an inhibitory effect on the proliferation of tumor cells.
Overexpression of Progerin Inhibits the Expression of Paxillin in A375 and M14 Cells
In a previous study conducted by our research group,21 we discovered that the expression of paxillin was down-regulated in lung cancer A549 cells that overexpressed progerin, as determined by protein microarray analysis. An increasing number of studies have discovered that paxillin is frequently dysregulated in various human malignancies, and its abnormal expression is related to the clinical prognosis and immune infiltration of different tumor types.15,20 Therefore, to further explore whether paxillin plays a role in the effect of progerin on melanoma cells, we investigated the association of progerin and paxillin in melanoma. Similarly, in A375 cells and M14 cells, paxillin expression was down-regulated in progerin group compared with blank group and negative control group (Figure 4A and B).
Paxillin Partially Rescues the Migration Ability of Progerin-Overexpressing Cancer Cells
To investigate whether progerin inhibits the migration of cancer cells by influencing the expression of paxillin, we conducted a study where we transfected the progerin overexpression cell group with a plasmid containing the paxillin gene. Through transwell experiments, it was found that the paxillin overexpression group (PG-PXN) was compared with the progerin overexpression group and plasmid negative control group (PXN-NC). Transfection of paxillin partially rescued the migration ability of A375 cells and M14 cells (Figure 5A and B). Additionally, the wound healing experiment demonstrated that paxillin transfection also partially rescued the migration ability of cancer cells affected by progerin (Figure 5C and D). In summary, progerin can inhibit the migration of melanoma cells by inhibiting the expression of paxillin.
Paxillin Partially Rescues the Expression Levels of EMT Marker Proteins in Progerin-Overexpressing Cancer Cells
In order to verify whether progerin affects the EMT process through paxillin, we transfected the plasmid containing paxillin gene in the progerin overexpression group. Western blot analysis revealed that overexpressing paxillin effectively restored the expression of EMT marker proteins in progerin-expressing A375 cells (Figure 6A). Similarly, in the M14 cell group, overexpression of paxillin rescued the expression of EMT marker proteins (Figure 6B). These findings indicate that progerin inhibits the EMT process by regulating the expression of paxillin.
Paxillin Partially Rescues the Proliferation Ability of Progerin-Overexpressing Cancer Cells
In order to prove whether progerin inhibits cancer cell proliferation and cell cycle progression by inhibiting paxillin expression, we performed proliferation rescue experiments. Through clone formation experiments, we found that overexpression of paxillin partially rescued the proliferation ability of progerin-affected A375 and M14 cells (Figure 7A and B). Subsequent CCK 8 experiments also indicated that overexpression of paxillin partially rescued the proliferation ability of progerin-expressing cancer cells (Figure 7C and D). Moreover, flow cytometry analysis revealed that transfection of paxillin partially restored the cell cycle progression of progerin-expressing cancer cells (Figure 7E). In conclusion, the inhibition of paxillin expression by progerin hampers the proliferation of melanoma cells.
Progerin Affects Melanoma Progression by Regulating the miR-212/Paxillin Axis
Accumulating evidence shows that miRNA can promote tumor malignant progression by regulating the expression of paxillin.23 Therefore, in order to further explore how progerin regulates paxillin, we focused on the relationship between progerin/miRNA/paxillin. We utilized RT-qPCR to partially detect the relative expression of paxillin-related miRNAs. The results showed that in some detected miRNAs, overexpression of progerin increased the expression levels of miR-212 in A375 cells and M14 cells (Figure 8A and B). Additionally, transfection of miR-212 mimic and miR-212 inhibitor in A375 cells and M14 cells respectively revealed that miR-212 mimic suppressed the expression of paxillin, whereas miR-212 inhibitor had the opposite effect (Figure 8C and D). Furthermore, bioinformatics analysis and dual luciferase experiments provided evidence that miR-212 can decrease the luciferase activity of the WT group (Figure 8E), suggesting that paxillin is a target gene of miR-212. We further transfected miR-212 inhibitor into progerin overexpressing cells and found that miR-212 inhibitor could partially rescue the expression of paxillin (Figure 8F and G), which indicated that progerin may regulate the migration and proliferation of melanoma cells through the miR-212/paxillin axis.
Discussion
Melanoma is a skin cancer formed by malignant melanocytes, the incidence of which is increasing rapidly worldwide.1 Although chemotherapy is widely used for treating melanoma, its effectiveness is limited due to severe side effects. As a result, there is an urgent need for a new treatment approach. In recent years, many studies have shown that aging has a suppressive effect on tumors. Studies have shown that under the action of carcinogenic factors, progerin can play a role in resisting tumors.24 The accumulation of prelamin A has been found to decrease the invasion of lung cancer and breast cancer, thereby exerting an anti-tumor effect.25
In this study, we investigated the impact of progerin on melanoma A375 cells and M14 cells, specifically examining its effects on tumor cell metastasis and proliferation. According to research, lamin plays a role in the phenotypic switching process of melanoma. This process can increase the likelihood of cancer cells metastasizing.26 Metastasis is a leading cause of cancer-related mortality, and the migration of cancer cells to neighboring tissues is a critical initial step in this process. Thus, evaluating the migratory capacity of tumors is crucial for assessing their malignancy. EMT is involved in the dissolution of adhesion between cells, the loss of apical and basal polarity, and the reorganization of the cytoskeleton, so EMT also plays a key role in cancer cell metastasis.27,28 Our study revealed that progerin has the ability to hinder the migration of A375 cells and M14 cells, as well as alter the expression levels of EMT marker proteins including N-cadherin, E-cadherin, and vimentin. Furthermore, the strong adaptability of cancer cells to environmental changes also promotes their growth. An abnormality in cell cycle progression is also a fundamental mechanism of tumorigenesis. At the same time, the abnormality of cell cycle progression is one of the basic mechanisms of tumorigenesis. Cancer cells primarily acquire the ability for continuous cell division by disrupting the control mechanism of the cell cycle.29 Previous studies have demonstrated that progerin can induce G1/S arrest in cancer cells through the CDK4-pRB pathway.21 And our study also showed that progerin can not only significantly reduce the proliferation ability of A375 cells and M14 cells, but also arrest the cell cycle of A375 cells and M14 cells in G1/S phase.
Paxillin, a vital component of focal adhesion and a signaling scaffold molecule, has been found to be abnormally expressed in numerous types of cancers. And this abnormal expression of paxillin is closely associated with prognosis, metastasis, survival, angiogenesis, and other aspects of malignant tumors. According to research, inhibiting the FAK-paxillin signaling pathway has been found to hinder the growth and metastasis capability of melanoma cells.30,31 Knockdown of paxillin has been shown to reduce cancer cell metastasis by impairing N-cadherin-mediated adhesion.32 In another study, it was also shown that the expression of paxillin can inhibit the EMT process in melanoma cells, thereby inhibiting the migration of tumor cells.33 Therefore, paxillin may promote cancer cell metastasis by regulating the EMT process. In a previous study, it was discovered that the interaction of cold-associated tyrosine phosphorylated protein 1 with paxillin can also promote the anchorage-independent growth of cervical cancer HeLa cells.34 And the metastatic potential of tumor cells is associated with their ability to grow independently of anchorage. At the same time, paxillin can also regulate the cell cycle process by participating in the integrin β1-mediated AKT pathway.35 Based on previous research conducted by our research group,21 our hypothesis was whether progerin has an impact on the expression of paxillin, thus affecting tumor progression. To investigate this unknown question, we conducted experiments where we overexpressed progerin in melanoma A375 cells and M14 cells, and examined its effect on the expression of paxillin. The results revealed that progerin can inhibit the expression of paxillin. At the same time, we conducted rescue experiments which demonstrated that transfection of paxillin could partially rescue the migration, proliferation, EMT process, and cell cycle progression of progerin-expressing cancer cells. These findings indicate that paxillin plays a crucial role in the ability of progerin to hinder the migration and proliferation of melanoma cells.
MicroRNA is a type of endogenous small RNA that has the ability to bind to the mRNA of target genes.36 This binding can either inhibit the translation of the mRNA or induce its degradation, thereby playing a role in various physiological and pathological processes. Numerous studies have demonstrated that certain non-coding RNAs can regulate the expression of paxillin, thereby influencing the occurrence and progression of tumors. It has been reported that miR-216b, miR-137, and lncRNA XIST can respectively inhibit the development of gastric cancer, colorectal cancer, and non-small cell lung cancer by regulating the expression of paxillin.37–39 In this study, overexpression of progerin increased the expression levels of miR-212 in A375 cells and M14 cells. Through dual luciferase experiments, we identified paxillin as the target of miR-212. To further investigate the relationship between progerin and paxillin, we conducted experiments where we transfected miR-212 inhibitor into progerin-expressing cancer cells. The results showed that the transfection of miR-212 inhibitor significantly up-regulated paxillin, suggesting that progerin may regulate the expression of paxillin through miR-212. However, the mechanism by which progerin affects the expression of miR-212 remains unclear. Studies have shown that the expression of progerin is associated with RNA interference mechanisms and cell cycle regulation. Progerin affects the levels and subnuclear localization of multiple transcriptional regulators, as well as changes in chromatin organization, potentially leading to epigenetic alterations in progeria cells.40 Furthermore, analysis of miRNA expression profiles in HGPS fibroblasts and control fibroblasts has demonstrated that the accumulation of progerin can result in the overexpression of certain miRNA genes, which may be related to the effect of progerin on epigenetic modification.41 These findings suggest that the introduction of progerin into tumor cells could potentially impact gene expression through alterations in epigenetic modifications, including dysregulation of microRNAs.
Therefore, further exploration is needed to understand the specific mechanism by which progerin regulates miRNA expression. Additionally, animal experiments should be conducted to validate the impact of progerin on the malignant development of melanoma. In summary, our research results are anticipated to offer valuable insights for the future treatment of melanoma and the development of anticancer drugs targeting paxillin.
Conclusions
Our results demonstrate that the overexpression of progerin hinders the migration and proliferation of cancer cells by suppressing paxillin expression in melanoma A375 and M14 cells. Additionally, it alters the expression of EMT signature proteins and the distribution of cancer cell cycles. Further investigations into the mechanism suggest that progerin may impede tumor progression through the miR-212/paxillin axis. These significant findings offer novel insights for the development of future therapeutic strategies targeting melanoma.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (81971329; 81671399).
Disclosure
The authors declare that they have no conflict of interest.
References
1. Ahmadi KF, Ramezanpour A. Clustering trends of melanoma incidence and mortality: a worldwide assessment from 1995 to 2019. Australas J Dermatol. 2022;63:e206–e17. doi:10.1111/ajd.13882
2. Lopes-Paciencia S, Saint-Germain E, Rowell MC, Ruiz AF, Kalegari P, Ferbeyre G. The senescence-associated secretory phenotype and its regulation. Cytokine. 2019;117:15–22. doi:10.1016/j.cyto.2019.01.013
3. Loo TM, Miyata K, Tanaka Y, Takahashi A. Cellular senescence and senescence-associated secretory phenotype via the cGAS-STING signaling pathway in cancer. Cancer Sci. 2020;111(2):304–311. doi:10.1111/cas.14266
4. Ullrich NJ, Kieran MW, Miller DT, et al. Neurologic features of Hutchinson-Gilford progeria syndrome after lonafarnib treatment. Neurology. 2013;81(5):427–430. doi:10.1212/WNL.0b013e31829d85c0
5. Eriksson M, Brown WT, Gordon LB, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423(6937):293–298. doi:10.1038/nature01629
6. De Sandre-Giovannoli A, Bernard R, Cau P, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300(5628):2055. doi:10.1126/science.1084125
7. Gonzalo S, Coll-Bonfill N. Genomic instability and innate immune responses to self-DNA in progeria. Geroscience. 2019;41(3):255–266. doi:10.1007/s11357-019-00082-2
8. Piekarowicz K, Machowska M, Dzianisava V, Rzepecki R. Hutchinson-Gilford Progeria Syndrome-Current Status and Prospects for Gene Therapy Treatment. Cells. 2019;9(1):8. doi:10.3390/cells9010008
9. Gordon LB, Rothman FG, Lopez-Otin C, Misteli T. Progeria: a paradigm for translational medicine. Cell. 2014;156(3):400–407. doi:10.1016/j.cell.2013.12.028
10. Prokocimer M, Barkan R, Gruenbaum Y. Hutchinson-Gilford progeria syndrome through the lens of transcription. Aging Cell. 2013;12(4):533–543. doi:10.1111/acel.12070
11. Gonzalo S, Kreienkamp R. DNA repair defects and genome instability in Hutchinson-Gilford Progeria Syndrome. Curr Opin Cell Biol. 2015;34:75–83. doi:10.1016/j.ceb.2015.05.007
12. Kubben N, Misteli T. Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nat Rev Mol Cell Biol. 2017;18(10):595–609. doi:10.1038/nrm.2017.68
13. Chang BD, Broude EV, Dokmanovic M, et al. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res. 1999;59(15):3761–3767.
14. Acosta JC, Gil J. Senescence: a new weapon for cancer therapy. Trends Cell Biol. 2012;22(4):211–219. doi:10.1016/j.tcb.2011.11.006
15. Alpha KM, Xu W, Turner CE. Paxillin family of focal adhesion adaptor proteins and regulation of cancer cell invasion. Int Rev Cell Mol Biol. 2020;355:1–52. doi:10.1016/bs.ircmb.2020.05.003
16. Lopez-Colome AM, Lee-Rivera I, Benavides-Hidalgo R, Lopez E. Paxillin: a crossroad in pathological cell migration. J Hematol Oncol. 2017;10(1):50. doi:10.1186/s13045-017-0418-y
17. Cai H, Zhang T, Tang WX, Li SL. Expression of paxillin in breast cancer cell with high and low metastatic potentiality. Sichuan Da Xue Xue Bao Yi Xue Ban. 2010;41(1):91–94.
18. Chen DL, Wang ZQ, Ren C, et al. Abnormal expression of paxillin correlates with tumor progression and poor survival in patients with gastric cancer. J Transl Med. 2013;11(1):277. doi:10.1186/1479-5876-11-277
19. Yang HJ, Chen JZ, Zhang WL, Ding YQ. Focal adhesion plaque associated cytoskeletons are involved in the invasion and metastasis of human colorectal carcinoma. Cancer Invest. 2010;28(2):127–134. doi:10.3109/07357900903147184
20. Chen Y, Zhao H, Xiao Y, et al. Pan-cancer analysis reveals an immunological role and prognostic potential of PXN in human cancer. Aging (Albany NY). 2021;13(12):16248–16266. doi:10.18632/aging.203154
21. Hu XT, Song HC, Yu H, Wu ZC, Liu XG, Chen WC. Overexpression of Progerin Results in Impaired Proliferation and Invasion of Non-Small Cell Lung Cancer Cells. Onco Targets Ther. 2020;13:2629–2642. doi:10.2147/OTT.S237016
22. Huang Y, Hong W, Wei X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J Hematol Oncol. 2022;15.
23. Liu W, Huang X, Luo W, Liu X, Chen W. The Role of Paxillin Aberrant Expression in Cancer and Its Potential as a Target for Cancer Therapy. Int J Mol Sci. 2023;24. doi:10.3390/ijms25010024
24. Fernandez P, Scaffidi P, Markert E, Lee JH, Rane S, Misteli T. Transformation resistance in a premature aging disorder identifies a tumor-protective function of BRD4. Cell Rep. 2014;9:248–260. doi:10.1016/j.celrep.2014.08.069
25. de la Rosa J, Freije JM, Cabanillas R, et al. Prelamin A causes progeria through cell-extrinsic mechanisms and prevents cancer invasion. Nat Commun. 2013;4(1):2268. doi:10.1038/ncomms3268
26. Lionetti FM, La Porta CAM. Nuclear biophysical changes during human melanoma plasticity. Cells Tissues Organs. 2022.
27. Cao H, Xu E, Liu H, Wan L, Lai M. Epithelial-mesenchymal transition in colorectal cancer metastasis: a system review. Pathol Res Pract. 2015;211(8):557–569. doi:10.1016/j.prp.2015.05.010
28. Mizukoshi K, Okazawa Y, Haeno H, et al. Metastatic seeding of human colon cancer cell clusters expressing the hybrid epithelial/mesenchymal state. Int, J, Cancer. 2020;146(9):2547–2562. doi:10.1002/ijc.32672
29. Matthews HK, Bertoli C, de Bruin RAM. Cell cycle control in cancer. Nat Rev Mol Cell Biol. 2022;23(1):74–88. doi:10.1038/s41580-021-00404-3
30. Mousson A, Legrand M, Steffan T, et al. Inhibiting FAK-Paxillin Interaction Reduces Migration and Invadopodia-Mediated Matrix Degradation in Metastatic Melanoma Cells. Cancers (Basel);2021. 13. doi:10.3390/cancers14010013
31. Wj CS, Wu Q, Qian J, Yang C, Bo P. Genistein inhibits the growth and regulates the migration and invasion abilities of melanoma cells via the FAK/paxillin and MAPK pathways. Oncotarget. 2017;8:21674–21691. doi:10.18632/oncotarget.15535
32. Athanasopoulou A, Aroukatos P, Nakas D, Repanti M, Papadaki H, Bravou V. Decreased ezrin and paxillin expression in human urothelial bladder tumors correlate with tumor progression. Urol Oncol. 2013;31(6):836–842. doi:10.1016/j.urolonc.2011.07.003
33. Cai FF, Xu HR, Yu SH, et al. ADT-OH inhibits malignant melanoma metastasis in mice via suppressing CSE/CBS and FAK/Paxillin signaling pathway. Acta Pharmacol Sin. 2022;43(7):1829–1842. doi:10.1038/s41401-021-00799-x
34. Yoo SM, Latifkar A, Cerione RA, Antonyak MA. Cool-associated Tyrosine-phosphorylated Protein 1 Is Required for the Anchorage-independent Growth of Cervical Carcinoma Cells by Binding Paxillin and Promoting AKT Activation. J Biol Chem. 2017;292(9):3947–3957. doi:10.1074/jbc.M116.769190
35. Xie J, Guo T, Zhong Z, et al. ITGB1 Drives Hepatocellular Carcinoma Progression by Modulating Cell Cycle Process Through PXN/YWHAZ/AKT Pathways. Front Cell Dev Biol. 2021;9:711149. doi:10.3389/fcell.2021.711149
36. Wang Z, Xie W, Guan H. The diagnostic, prognostic role and molecular mechanism of miR-328 in human cancer. Biomed Pharmacother. 2023;157:114031. doi:10.1016/j.biopha.2022.114031
37. Liu X, Xu D, Xu X, Xue Q, Gao X, Tang C. MiR-216b regulates the tumorigenesis of gastric cancer by targeting PXN. Pathol Res Pract. 2021;218:153325. doi:10.1016/j.prp.2020.153325
38. Chen DL, Wang DS, Wu WJ, et al. Overexpression of paxillin induced by miR-137 suppression promotes tumor progression and metastasis in colorectal cancer. Carcinogenesis. 2013;34:803–811. doi:10.1093/carcin/bgs400
39. Jiang H, Zhang H, Hu X, Li W. Knockdown of long non-coding RNA XIST inhibits cell viability and invasion by regulating miR-137/PXN axis in non-small cell lung cancer. Int J Biol Macromol. 2018;111:623–631. doi:10.1016/j.ijbiomac.2018.01.022
40. Ashapkin VV, Kutueva LI, Kireev II. Are There Common Mechanisms Between the Hutchinson-Gilford Progeria Syndrome and Natural Aging? Front Genet. 2019;10:455. doi:10.3389/fgene.2019.00455
41. Frankel D, Delecourt V, Novoa-Del-Toro EM, et al. miR-376a-3p and miR-376b-3p overexpression in Hutchinson-Gilford progeria fibroblasts inhibits cell proliferation and induces premature senescence. iScience. 2022;25:103757. doi:10.1016/j.isci.2022.103757
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.