Back to Journals » Journal of Asthma and Allergy » Volume 16
Profiles of Birch Allergen Component Sensitization and Its Association with Pollen Food Allergy Syndrome in Northern China
Authors Wang X , Chen L, Ding J, Wang H, Wang X
Received 24 August 2023
Accepted for publication 7 November 2023
Published 14 November 2023 Volume 2023:16 Pages 1241—1250
DOI https://doi.org/10.2147/JAA.S427764
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Xiaoyan Wang,1,2 Lijia Chen,1 Jiaqi Ding,3 Hongtian Wang,1 Xueyan Wang1,2
1Department of Allergy, Beijing Shijitan Hospital, Capital Medical University, Beijing, 100038, People’s Republic of China; 2Beijing Laboratory of Allergic Diseases, Beijing Municipal Education Commission, Beijing, 100038, People’s Republic of China; 3Department of Otolaryngology, Qingdao Women and Children’s Hospital Affiliated to Qingdao University, Qingdao, Shandong, 0355729, People’s Republic of China
Correspondence: Xueyan Wang, Hongtian Wang, Tieyi Road, No. 10th, Haidian District, Beijing, 100038, People’s Republic of China, Tel +86 13810570961 ; +86 13391836668, Email [email protected]; [email protected]
Purpose: To investigate the major allergen components associated with birch pollen allergy in northern China and elucidate clinical relevance to pollen food allergy syndrome (PFAS).
Methods: Fifty-eight patients were recruited for a cross-sectional study and categorized into two groups: PFAS group and non-PFAS group, as well as apple allergy group and non-apple allergy group. The sIgE levels of birch pollen and its components, namely Bet v 1, Bet v 2, Bet v 4, and Bet v 6, were analyzed.
Results: Among 58 participants, 44 individuals (75.9%) reported PFAS. 32 out of 44 (72.7%) participants reported apple allergy. Bet v 1 exhibited the highest sensitization rate at 82.8%, followed by Bet v 2 (29.3%) and Bet v 6 (1.7%). The combined sensitization rate for Bet v 1 and/or Bet v 2 was 93.1%. A total of 77.6% of the subjects demonstrated sensitization to single component, while 19.0% exhibited sensitization to two components. The sIgE levels of birch pollen and Bet v 1 were significantly elevated in PFAS group compared to non-PFAS group (p=0.001, p< 0.001, respectively), as well as in apple-allergic and non-apple-allergic group (p< 0.001, p< 0.001, respectively). The optimal cut-off values for birch pollen and Bet v 1 sIgE were determined to be 7.09 kUA/L (with a sensitivity of 84.1% and specificity of 78.6%) and 5.11 kUA/L (with a sensitivity of 75.0% and specificity of 85.7%) when diagnosing PFAS. In terms of apple allergy, the optimal cut-off value were 9.40 kUA/L (with a sensitivity of 81.3% and specificity of 76.9%) and 6.53 kUA/L (with a sensitivity of 84.4% and specificity of 84.6%), respectively.
Conclusion: The predominant sensitization pattern is mono-sensitization to Bet v 1, but when considering immunotherapy, Bet v 2 should also be taken into account. Bet v 1 serves as a valuable biomarker for diagnosing PFAS and apple allergy.
Keywords: birch pollen, allergen component, Bet v 1, pollen food allergy syndrome, apple allergy
Introduction
Pollen-related allergic diseases afflict 30% to 40% of the global population.1 Birch belongs to the family Betulaceae and Fagaceae. Birch pollen allergy is a prevalent form of pollinosis worldwide, resulting in significant impairment of health-related quality of life for affected individuals.2–4
Birch is predominantly distributed across Europe, North America and Asia.2,5 The dispersal period of birch pollen ranges from March to May, with a sensitization rate in the general population ranging from 8% to 16%.2,4,6 Birch pollen predominantly elicits allergic rhinitis (AR), allergic conjunctivitis, and asthma. In particular, Class 2 food allergy, also known as pollen food allergy syndrome (PFAS), is becoming increasingly prevalent due to the extensive cross-reactivity between birch pollen and various foods.7–10 PFAS is triggered by cross-reactive allergens present in pollen, uncooked fruits and vegetables, grains, as well as tree nuts.7,11,12 For birch pollen, apple is the food most likely to trigger PFAS.13
Component resolved diagnosis (CRD) can elucidate the molecular mechanisms of allergens and differentiate the phenotypes of birch pollen allergy.14 To date, a total of seven allergen components derived from birch pollen have been identified, with Bet v 1 being recognized as the major allergen. Studies on birch allergen components have demonstrated their usefulness in predicting disease phenotype and guiding birch allergen immunotherapy.15,16
In northern China, the prevalent pollen allergens were weed/grass pollens with a sensitization rate of 24.0% for mugwort and 21.2% for goosefoot, 17.7% for maize, 17.0% for salix.6 In addition to those pollens, birch pollen allergy was comparable to that in Europe, with a prevalence of 11.3%.3,6 Meanwhile, there is a relative lack of research on birch pollen allergy in our country, with only one study conducted in southern China17 and two in northern China.18,19 The previous studies had a restricted sample size and solely concentrated on Bet v 1, disregarding other birch pollen allergen components such as Bet v 2 and Bet v 4. Additionally, the clinical relevance between birch allergen component and food allergy has not been fully investigated. Thus, further investigation is necessary to fully explore the clinical implications of birch pollen allergen components.
The aim of this investigation was to examine the major allergen components and sensitization patterns of birch pollen in northern China, as well as their differences and clinical significance between individuals with and without PFAS.
Materials and Methods
Study Population
A cross-sectional study was conducted, enrolling 58 patients with birch pollen allergy who were admitted to the Department of Allergy at Beijing Shijitan Hospital from March 2022 to February 2023. Inclusion criteria: (1) Patients with a confirmed diagnosis of AR with or without asthma according to Allergic Rhinitis and Its Effects on Asthma (ARIA) or GINA; (2) Serum sIgE levels for birch pollen at least 0.7 kUA/L, with or without sensitization to other pollen allergens; (3) Typical symptoms of spring pollen allergy for a minimum of two years, with or without autumn pollen allergy symptoms. The spring allergic symptoms are induced by tree pollens, including birch, cypress, plane, popolus, willow, ash and others. Similarly, the autumn symptoms are triggered by pollens such as mugwort, scandent hop, goosefoot and ragweed. Exclusion criteria: (1) Previous history of allergen immunotherapy specific to pollen; (2) Present upper respiratory tract infection or chronic sinusitis; (3) Absence of typical spring seasonal allergy symptoms; (4) The serum sIgE tests for birch pollen indicate a level below 0.7 kUA/L.
PFAS and Non-PFAS
Based on a compelling medical history of immediate reactions following the consumption of the suspected food, skin prick tests or food-specific IgE tests, and necessary oral food challenges, patients were categorized into PFAS and non-PFAS groups. According to clinical symptoms following ingestion of allergenic foods, patients were classified into two groups: localized symptom group with oral mucosal symptoms; systemic symptom group with systemic symptoms including urticaria, angioedema, laryngeal edema, respiratory distress, gastrointestinal disorders or circulatory collapse indicative of anaphylaxis. Furthermore, patients were classified into two groups based on their apple allergy status: the group with apple allergy and the group without apple allergy.
Questionnaire
Under the guidance of an allergy specialist, patients or their legal guardians were asked to complete a questionnaire regarding their social demographics, clinical history of AR and asthma, comorbidities, and family medical history. Furthermore, PFAS questionnaire including the related foods (fruits, vegetables, legumes, grains, nuts and others), type and timing of symptoms onset was finished.20
Ethics Statement
Each participant or their legal guardian provided written informed consent, and the Ethics Committee of Beijing Shijitan Hospital, Capital Medical University granted approval for this study (No. 2022–081).
Birch Pollen and Allergen Component sIgE Assay
All enrolled participants underwent ImmunoCAP (ThermoFisher Scientific, Uppsala, Sweden) screening for birch pollen allergen and its components Bet v 1, Bet v 2, Bet v 4, and Bet v 6 in peripheral blood samples. Specific IgE levels greater than 0.35 kUA/L were considered positive.
Statistics Analyses
Statistical analysis of the data was conducted using SPSS 25.0 software package and Prism 8.0 software. Categorical data were presented as numbers (n) and percentages (%), while quantitative data were analyzed using either mean and standard deviation (SD) or median and interquartile range (IQR). The significance of frequencies was calculated using the chi-square test, medians were compared using the Mann–Whitney U-test, and means were compared using the t-test when comparing two different groups. Pearson correlation analysis was conducted. Receiver operating characteristics (ROC) curves were used to assess the diagnostic value of the sIgE levels of birch pollen and its components. The statistical significance was determined using a significance level of p<0.05.
Results
Clinical Characteristics of Study Population
A total of 58 participants (31 males, mean age 30.4 ± 14.4 years) were enrolled in the study. The demographic characteristics are presented in Table 1. Of these, 16 individuals (27.6%) reported pollen allergy symptoms exclusively during spring, while 42 participants (72.4%) experienced symptoms both in spring and autumn. Additionally, 14 subjects (24.1%) had comorbid asthma with AR, whereas the remaining 44 participants (75.9%) only had AR.
 |
Table 1 Demographic Characteristics of Birch Pollen Allergy Subjects Between PFAS and Non-PFAS Group |
In addition, according to the questionnaire results, 44 out of 58 participants (75.9%) reported experiencing PFAS reactions, with fruits being the most common trigger (n = 38, 86.4%), followed by vegetables (n = 26, 59.1%), nuts (n = 11, 25.0%), legumes (n = 8, 18.2%), and grains (n = 5, 11.4%). Among them, 32 out of 44 (72.7%) participants who experienced PFAS reactions reported apple allergy. Based on the severity of symptoms induced by culprit foods, PFAS was categorized into three groups: localized symptom group (n = 23, 52.3%), systemic symptom group (n = 21, 47.7%) (Table 1). All subjects in the PFAS group who presented with systemic symptoms exhibited both spring and autumn pollen allergy symptoms.
Analysis of sIgE for Birch and Its Allergen Components
The overall sensitization rate for the four birch allergen components was 94.8%. Bet v 1 had the highest sensitization rate of 82.8%, followed by Bet v 2 (29.3%) and Bet v 6 (1.7%). All subjects tested negative for Bet v 4. The positive rate of Bet v 1 and/or Bet v 2 was 93.1% (Figure 1A). A significant positive correlation was observed between the serum levels of sIgE for birch pollen and Bet v 1 (r= 0.898, p< 0.001), while no significant correlation was found between the levels of sIgE to birch pollen and sIgE to Bet v 2, Bet v 4, or Bet v 6 (Figure 1B).
Birch Pollen Allergen Component Sensitization Pattern
Among the 58 subjects, 77.6% exhibited sensitization to single allergen component, while 19.0% were sensitized to two components. Four patterns of sensitization were observed, as depicted in Figure 1C. Among those affected, 63.8% exhibited Bet v 1+/Bet v 2- while 10.3% displayed Bet v 2+/Bet v 1-. Additionally, a further 19.0% showed Bet v 1+/Bet v 2+. One patient (1.7%) tested positive for Bet v 6, as illustrated in Figure 1C and Table 1. Furthermore, 5.2% of patients exhibited negative results for all four allergen components. Notably, the prevailing pattern observed was Bet v 1-positive while being negative for both Bet v 2 and Bet v 4 (Figure 1D).
Differences of sIgE Levels Between PFAS and Non-PFAS Group
Birch pollen sIgE levels in patients with PFAS (median 14.45 kUA/L; range 1.13–100 kUA/L) were significantly higher than those of non-PFAS patients (median 3.14 kUA/L; range 0.86–45.70 kUA/L, p=0.001). Similarly, Bet v 1 sIgE levels were significantly elevated in patients with PFAS (median 10.79 kUA/L; range 0–100 kUA/L) compared to non-PFAS patients (median 1.11 kUA/L; range 0.01–40.9 kUA/L, p<0.001) (Figure 2A).
Birch pollen sIgE levels were significantly higher in patients with apple allergy (median 19.65 kUA/L; range 1.13–100 kUA/L) than those without apple allergy (median 4.75 kUA/L; range 0.86–45.70 kUA/L, p<0.001). Similarly, Bet v 1 sIgE levels were significantly elevated in patients with apple allergy (median 13.80 kUA/L; range 0.02–100 kUA/L) compared to non-allergic patients (median 1.80 kUA/L; range 0.00–40.9 kUA/L, p<0.001) (Figure 2B). No statistically significant differences were observed between the two groups in terms of sIgE levels for Bet v 2, Bet v 4, and Bet v 6.
Meanwhile, no statistically significant differences were observed in sIgE levels of birch pollen and its four components between the localized and systemic groups of PFAS subjects.
The Diagnostic Value of Birch Pollen and Bet v 1 for PFAS
The diagnostic efficacy of sIgE levels for various birch pollen allergen components was evaluated using ROC curves, as depicted in Figure 3. ROC analysis revealed that both birch pollen and Bet v 1 sIgE levels had consistent areas under the curve (AUC, 0.791; 95% CI: 0.629–0.952, p=0.001 and AUC, 0.789; 95% CI: 0.648–0.929, p=0.001 respectively) in diagnosing PFAS. The optimal cut-off values for birch pollen sIgE and Bet v 1sIgE were determined to be 7.09 kUA/L (84.1% sensitivity, 78.6% specificity) and 5.11 kUA/L (75.0% sensitivity, 85.7% specificity), respectively (Figure 3A). The AUC of Bet v 2 was found to be insignificant.
Regarding the diagnosis of apple allergy, the AUC values for birch and Bet v 1 sIgE levels were found to be 0.828 (95% CI 0.717–0.939, p<0.001) and 0.847 (95% CI 0.738–0.957, p<0.001), respectively. The optimal cut-off value for birch pollen sIgE was 9.40 kUA/L, with 81.3% sensitivity and 76.9% specificity, and for Bet v 1sIgE, it was 6.53 kUA/L, with 84.4% sensitivity and 84.6% specificity (Figure 3B).
Discussion
Till now, seven allergen components in birch pollen has been identified (Table S1).21 In this study, the major allergen components of birch pollen in northern China were investigated. Bet v 1 was identified as the most predominant allergen, while sensitization to Bet v 2 and Bet v 6 was relatively infrequent.
Bet v 1 and its homologs belong to the family of pathogenesis-related proteins 10 (PR-10).16,22 Our study has demonstrated that Bet v 1 is the predominant allergen component in northern China, which is consistent with the findings of previous studies conducted in Europe, Japan and South Korea.17,18,23–29 The regional differences in the birch pollen allergen sensitization rate are shown in Figure 4 including Japan,25 Korea,30 Italy,24 Switzerland3, Czech,26 Austria,3 France,3 Russia,31 southern China.17 A gradient of Bet v 1 sensitization rate was observed, with a higher prevalence in the northern region and a lower prevalence in the southern region. In Czech Republic,26 89.9% of patients tested positive for Bet v 1, while in southern Italy, the proportion decreased to 58%.3,24 In regions situated further south, such as Uganda, the rate of sensitization to Bet v 1 was observed to be 26%.27 Similar results have been observed in China, with a sensitization rate of 82.4% for Bet v 1 in northern China18 and 82.8% in our study, while the rate was less than 50% in southern China.17
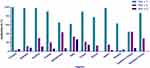 |
Figure 4 Regional variations in sensitization rates to birch allergen components. |
A gradient of sensitization rate is also observed among other birch pollen allergen components. While Bet v 1 predominates in the northern region, Bet v 2 and Bet v 4 are more prevalent in the southern area.24,27 Bet v 2 belongs to the profilin family, thus its sensitization rate is elevated in regions with high prevalence of grass pollen allergy and PFAS. In fact, a study conducted in southern Italy reported a significantly higher Bet v 2 positive rate compared to northern Italy (52.3% vs 6.1%).24 Similarly, another investigation carried out in Guangzhou, southern China17 revealed an even higher Bet v 2 positive rate of 42.3%, surpassing our own findings. Taken into account, when considering the CRD-based precise diagnosis and therapy of birch pollen, it is crucial to take regional variations into consideration.
The sensitization pattern of birch allergen components differs from that of other allergens, such as dust mite, grass or weed pollen, which exhibit multi-sensitization.14,32,33 In 40–60% of individuals allergic to birch pollen, mono-sensitization to Bet v 1 has been reported. This study found that 63.8% of participants were mono-sensitized to Bet v 1. Thus, a birch allergen immunotherapy based on Bet v 1 would suffice for most individuals with birch allergy. However, 10.3% of our participants exhibited mono-sensitivity to Bet v 2, which may be attributed to the high prevalence of weed/grass pollen allergy in northern China. Therefore, a screening of sIgE for Bet v 1 and Bet v 2 is imperative when considering birch allergen immunotherapy to prevent ineffective treatment in individuals with mono-sensitivity to Bet v 2.
Food allergy due to birch pollen-related cross-reactivity was common in Central-Northern Europe and Asia.8,9,34,35 In our study, the prevalence of PFAS in birch pollen allergy subjects was 75.9%, which is comparable to that observed in Europe.36 Bet v 1 frequently cross-reacts with several proteins in Rosaceae fruits, such as apple (Mal d 1), pear (Pyr c 1), apricot (Pru ar 1), cherry (Pru av 1).13,23 Therefore, Bet v 1 sIgE levels has been shown by multiple studies to effectively distinguish between individuals with PFAS or apple allergies and those without food allergies.16,30 Bet v 1 sIgE levels were significantly elevated in patients with PFAS compared to non-PFAS patients in our study which was consistent with previous studies.19,36 The greater the risk of developing food allergy. The present study demonstrated that Bet v 1 sIgE levels exhibited a consistent AUC of 0.789 in diagnosing PFAS, as revealed by ROC analysis. Notably, previous studies reported AUC values of 0.863 for Bet v 1 by Junda Li et al19 and 0.925 in Korean patients,30 which aligns with our findings. The optimal cut-off value of Bet v 1 sIgE level was 15.1kUA/L in Korea,30 17.4 kUA/L in Italy36 and 5.11 kUA/L in our study. The optimal cut-off value may vary across different studies and regions.
The predominant component in apple has been identified as Mal d 1, and there is a strong correlation between the sIgE level of Mal d 1 and Bet v 1.13 In this study, Bet v 1 sIgE levels was higher in apple allergy group with a cut-off value of 6.53 kUA/L (84.4% sensitivity and 84.6% specificity). The symptoms elicited by food proteins similar to Bet v 1 are typically not severe in nature. A multi-center study in southern Europe20 found 70% of PR-10 related PFAS was oral symptoms only. However, more than 40% of subjects in our cohort exhibited generalized symptoms that may not be attributed to Bet v 1. Mal d 3, a lipid transfer protein (LTP), although not considered a major allergic component, has been associated with an elevated risk of severe allergic reactions to apple, particularly in southern European regions such as Spain.37,38 Conversely, in regions abundant with birch trees, sensitization to Mal d 3 may not necessarily indicate severe reactions to apple but rather to peach or nuts.19,39,40 According to a study conducted by Deng et al,41 LTPs (Pru p 3, Mal d 3, etc) have been identified as the primary food allergens associated with mugwort pollen-related food allergy in China due to their homology with Art v 3.42 In our study, five subjects with PFAS were found to be negative for Bet v 1 sIgE with a high sIgE level of mugwort in these patients, suggesting the potential involvement of LTPs in their condition.
There was a debate of clinical significance of Bet v 2. Study of no differences was found in PFAS and non-PFAS group in our study which was consistent with another study in northern China.19 A study on the clinical relevance of Bet v 2 has shown that sensitization to Bet v 2 without prior sensitization to Bet v 1 is not associated with allergic symptoms during birch pollen season, whereas symptoms are present during grass pollen season.43 Thus, sensitization to Bet v 2 primarily reflects cross-reactivity with profilin and may serve as an indicator of poly-sensitization, but lacks clinical relevance in the context of AR. Routine diagnosis and therapy of birch pollen allergy is generally based on the assessment of Bet v 1. However, Bet v 2, a panallergen should be taken into consideration when confirming a diagnosis of birch pollen allergy, and especially when prescribing allergen immunotherapy.
The occurrence of Bet v 4 sensitivity is relatively uncommon, with a prevalence ranging from 5% to 11% among individuals allergic to birch pollen.26 In our study, only one patient had a Bet v 4 sIgE level greater than 0.1 kUA/L, indicating that this component is a minor allergen in the northern region of China. Bet v 4 is homologous to Amb a 9, Amb a 10, Art v 5, and Che a 3 which are minor allergens found in ragweed, mugwort, and goosefoot pollen in China.42 Therefore, poly-sensitization to tree/grass/weed pollen may be attributed to Bet v 4. Bet v 6 is a member of the isoflavone reductase family and may cause cross-reactivity with food allergens in a subset of patients. Our data clearly indicate that the sensitization rate of Bet v 6 is less than 2%, and Bet v 6 does not offer significant diagnostic value. Only one participant in this study exhibited a positive sIgE response to Bet v 6, while testing negative for Bet v 1 and Bet v 2. The patient is currently asymptomatic for food allergies, but presents with multiple seasonal allergies to pollen during the spring and autumn. A larger sample size may be necessary to elucidate the clinical significance of minor allergens, such as Bet v 4 and Bet v 6.
This study has several limitations. First, a relatively small sample size was enrolled in this study with an increasing risk of bias. Second, the diagnosis of PFAS was based on clinical history, questionnaires, and tests for allergen sIgE, open food challenge, instead of a double-blind placebo controlled food challenge. There may be a risk of recollection bias, potentially leading to an exaggeration of food allergic reactions and symptoms. Further investigations involving a large sample size are imperative to comprehensively explore and address these issues in a meticulously designed study.
Conclusion
In conclusion, the present study has demonstrated that Bet v 1 is the predominant allergen component in the northern region of China. The predominant sensitization pattern is mono-sensitization to Bet v 1, but when considering immunotherapy, Bet v 2 should also be taken into account. Furthermore, a notable disparity in the sensitization to birch allergens and Bet v 1 was observed between individuals with or without PFAS and apple allergy. Measuring sIgE levels of allergen components can be an effective tool for managing birch pollen allergy with PFAS.
Ethics Approval and Informed Consent
All the patients signed a written informed consent before they were recruited. This study was approved by the ethics committee of Beijing shijitan hospital, capital medical university. The study complied with the Declaration of Helsinki.
Acknowledgments
We thank professor Liu Lei (Office of Academic Affairs, Beijing Shijitan hospital, capital medical university) for his outstanding statistical support.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This study was supported by fundings as following: Capital’s Funds for Health Improvement and Research (2022-2-2082), Beijing High-level Public Health Technical Talents Construction Project (02-36), China Railway Group Technology Project (J2021Z603), Beijing Medical Management Center Cultivation Program (PX2022030), Haidian District Health Commission Cultivation Program (HP2022-03-506001).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Wallace DV, Dykewicz MS. Seasonal allergic rhinitis: a focused systematic review and practice parameter update. Curr Opin Allergy Clin Immunol. 2017;17(4):286–294. doi:10.1097/ACI.0000000000000375
2. D’Amato G, Spieksma FT, Liccardi G, et al. Pollen-related allergy in Europe. Allergy. 1998;53(6):567–578. doi:10.1111/j.1398-9995.1998.tb03932.x
3. Biedermann T, Winther L, Till SJ, et al. Birch pollen allergy in Europe. Allergy. 2019;74(7):1237–1248. doi:10.1111/all.13758
4. Raith M, Swoboda I. Birch pollen-The unpleasant herald of spring. Front Allergy. 2023;4:1181675. doi:10.3389/falgy.2023.1181675
5. Lappe BL, Ebelt S, D’Souza RR, et al. Pollen and asthma morbidity in Atlanta: a 26-year time-series study. Environ Int. 2023;177:107998. doi:10.1016/j.envint.2023.107998
6. Wang XY, Ma TT, Wang XY, et al. Prevalence of pollen-induced allergic rhinitis with high pollen exposure in grasslands of northern China. Allergy. 2018;73(6):1232–1243. doi:10.1111/all.13388
7. Loraud C, de Menonville CT, Bourgoin-Heck M, et al. Emergence of pollen food allergy syndrome in asthmatic children in Paris. Pediatr Allergy Immunol. 2021;32(4):702–708. doi:10.1111/pai.13435
8. Muluk NB, Cingi C. Oral allergy syndrome. Am J Rhinol Allergy. 2018;32(1):27–30. doi:10.2500/ajra.2018.32.4489
9. Lyons SA, Clausen M, Knulst AC, et al. Prevalence of food sensitization and food allergy in children across Europe. J Allergy Clin Immunol Pract. 2020;8(8):2736–2746. doi:10.1016/j.jaip.2020.04.020
10. Valenta R, Kraft D. Type 1 allergic reactions to plant-derived food: a consequence of primary sensitization to pollen allergens. J Allergy Clin Immunol. 1996;97(4):893–895. doi:10.1016/s0091-6749(96)80062-5
11. Kim MA, Kim DK, Yang HJ, et al. Pollen-food allergy syndrome in Korean pollinosis patients: a nationwide survey. Allergy Asthma Immunol Res. 2018;10(6):648–661. doi:10.4168/aair.2018.10.6.648
12. Skypala IJ, Hunter H, Krishna MT, et al. BSACI guideline for the diagnosis and management of pollen food syndrome in the UK. Clin Exp Allergy. 2022;52(9):1018–1034. doi:10.1111/cea.14208
13. Siekierzynska A, Piasecka-Kwiatkowska D, Myszka A, et al. Apple allergy: causes and factors influencing fruits allergenic properties-Review. Clin Transl Allergy. 2021;11(4):e12032. doi:10.1002/clt2.12032
14. Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, et al. EAACI molecular allergology user’s guide. Pediatr Allergy Immunol. 2016;27(23):1–250. doi:10.1111/pai.12563
15. Gronlund H, Gafvelin G. Recombinant Bet v 1 vaccine for treatment of allergy to birch pollen. Hum Vaccin. 2010;6(12):970–977. doi:10.4161/hv.6.12.13348
16. Alessandri C, Ferrara R, Bernardi ML, et al. Molecular approach to a patient’s tailored diagnosis of the oral allergy syndrome. Clin Transl Allergy. 2020;10:22. doi:10.1186/s13601-020-00329-8
17. Wu L, Hou X, Luo W, et al. Three patterns of sensitization to mugwort, timothy, birch and their major allergen components revealed by Latent class analysis. Mol Immunol. 2022;145:59–66. doi:10.1016/j.molimm.2022.03.009
18. Hao GD, Zheng YW, Wang ZX, et al. High correlation of specific IgE sensitization between birch pollen, soy and apple allergens indicates pollen-food allergy syndrome among birch pollen allergic patients in northern China. J Zhejiang Univ Sci B. 2016;17(5):399–404. doi:10.1631/jzus.B1500279
19. Li JD, Du ZR, Liu J, et al. Characteristics of pollen-related food allergy based on individual pollen allergy profiles in the Chinese population. World Allergy Organ J. 2020;13(5):100120. doi:10.1016/j.waojou.2020.100120
20. Lipp T, Acar SA, Aggelidis X, et al. Heterogeneity of pollen food allergy syndrome in seven Southern European countries: the @IT.2020 multicenter study. Allergy. 2021;76(10):3041–3052. doi:10.1111/all.14742
21. WHO/IUIS allergen nomenclature sub-committee. Available from: http://www.allergen.org/.
22. Breiteneder H, Kraft D. The history and science of the major birch pollen allergen Bet v 1. Biomolecules. 2023;13(7):1151. doi:10.3390/biom13071151
23. Ebo DG, Bridts CH, Verweij MM, et al. Sensitization profiles in birch pollen-allergic patients with and without oral allergy syndrome to apple: lessons from multiplexed component-resolved allergy diagnosis. Clin Exp Allergy. 2010;40(2):339–347. doi:10.1111/j.1365-2222.2009.03345.x
24. Ciprandi G, Comite P, Mussap M, et al. Profiles of Birch Sensitization (Bet v 1, Bet v 2, and Bet v 4) and Oral Allergy Syndrome Across Italy. J Invest Allergol Clin Immunol. 2016;26(4):244–248. doi:10.18176/jiaci.0041
25. Shirasaki H, Yamamoto T, Koyanagi Y, et al. Detection of specific IgE antibodies in sera of Japanese birch-allergic patients using recombinant allergens Bet v 1, Bet v 2 and Bet v 4. Allergol Int. 2008;57(1):93–96. doi:10.2332/allergolint.O-07-502
26. Sekerkova A, Polackova M. Detection of Bet v1, Bet v2 and Bet v4 specific IgE antibodies in the sera of children and adult patients allergic to birch pollen: evaluation of different IgE reactivity profiles depending on age and local sensitization. Int Arch Allergy Immunol. 2011;154(4):278–285. doi:10.1159/000321819
27. Odongo L, Mulyowa G, Goebeler M, et al. Bet v 1- and Bet v 2-associated plant food sensitization in Uganda and Germany: differences and similarities. Int Arch Allergy Immunol. 2015;167(4):264–269. doi:10.1159/000439533
28. Moverare R, Westritschnig K, Svensson M, et al. Different IgE reactivity profiles in birch pollen-sensitive patients from six European populations revealed by recombinant allergens: an imprint of local sensitization. Int Arch Allergy Immunol. 2002;128(4):325–335. doi:10.1159/000063855
29. Li L, Chang C, Guan K. Birch Pollen Allergens. Curr Protein Pept Sci. 2022;23(11):731–743. doi:10.2174/1389203723666220815095725
30. Baek HS, Jeong JW, Lee HB, et al. Molecular sensitization patterns in birch pollen-sensitized Korean children according to the presence of oral allergy syndrome. Medicine. 2020;99(10):e19469. doi:10.1097/MD.0000000000019469
31. Elisyutina O, Fedenko E, Campana R, et al. Bet v 1‐specific IgE levels and PR‐10 reactivity discriminate silent sensitization from phenotypes of birch allergy. Allergy. 2019;74(12):2525–2528. doi:10.1111/all.13931
32. Nelson HS. Allergen immunotherapy (AIT) for the multiple-pollen sensitive patient. Expert Rev Clin Pharmacol. 2016;9(11):1443–1451. doi:10.1080/17512433.2016.1237874
33. Xu L, Luo W, Lu Y, et al. A comprehensive analysis of the components of common weed pollen and related allergens in patients with allergic diseases in southern China. Mol Immunol. 2022;147:180–186. doi:10.1016/j.molimm.2022.05.005
34. Kato M, Miyamoto M, Takayanagi F, et al. Pollen food allergy syndrome in Japanese children and adolescents: risk factors and pollen sensitisation. J Immunol Res. 2023;2023:4075264. doi:10.1155/2023/4075264
35. Hwang Y, Motomura C, Fukuda H, et al. Relationship among airborne pollen, sensitization, and pollen food allergy syndrome in Asian allergic children. PeerJ. 2022;10:e14243. doi:10.7717/peerj.14243
36. Ciprandi G, Comite P, Ferrero F, et al. Birch allergy and oral allergy syndrome: the practical relevance of serum immunoglobulin E to Bet v 1. Allergy Asthma Proc. 2016;37(1):43–49. doi:10.2500/aap.2016.37.3914
37. Costa J, Mafra I, Lopes M, Paiva-Martins F. A biochemical perspective on the fate of virgin olive oil phenolic compounds in vivo. Crit Rev Food Sci Nutr. 2022;1–38. doi:10.1080/10408398.2022.2045897
38. Gomez F, Aranda A, Campo P, et al. High prevalence of lipid transfer protein sensitization in apple allergic patients with systemic symptoms. PLoS One. 2014;9(9):e107304. doi:10.1371/journal.pone.0107304
39. Cudowska B, Kaczmarski M, Restani P. Lipid transfer protein in diagnosis of birch-apple syndrome in children. Immunobiology. 2008;213(2):89–96. doi:10.1016/j.imbio.2007.07.006
40. Le TM, van Hoffen E, Lebens AF, et al. Anaphylactic versus mild reactions to hazelnut and apple in a birch-endemic area: different sensitization profiles? Int Arch Allergy Immunol. 2013;160(1):56–62. doi:10.1159/000339244
41. Deng S, Yin J. Mugwort pollen-related food allergy: lipid transfer protein sensitization and correlation with the severity of allergic reactions in a Chinese population. Allergy Asthma Immunol Res. 2019;11(1):116–128. doi:10.4168/aair.2019.11.1.116
42. Gao Z, Fu WY, Sun Y, et al. Artemisia pollen allergy in China: component-resolved diagnosis reveals allergic asthma patients have significant multiple allergen sensitization. Allergy. 2019;74(2):284–293. doi:10.1111/all.13597
43. Wolbing F, Kunz J, Kempf WE, et al. The clinical relevance of birch pollen profilin cross-reactivity in sensitized patients. Allergy. 2017;72(4):562–569. doi:10.1111/all.13040
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



