Back to Journals » Drug Design, Development and Therapy » Volume 17
Profile of Relugolix in the Management of Advanced Hormone-Sensitive Prostate Cancer: Design, Development, and Place in Therapy
Authors Tatenuma T, Miyamoto H
Received 18 April 2023
Accepted for publication 1 August 2023
Published 4 August 2023 Volume 2023:17 Pages 2325—2333
DOI https://doi.org/10.2147/DDDT.S373546
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Tomoyuki Tatenuma,1,2 Hiroshi Miyamoto1,3
1Department of Pathology & Laboratory Medicine, University of Rochester Medical Center, Rochester, NY, USA; 2Department of Urology, Yokohama City University Graduate School of Medicine, Yokohama, Japan; 3Department of Urology, University of Rochester Medical Center, Rochester, NY, USA
Correspondence: Hiroshi Miyamoto, Department of Pathology & Laboratory Medicine, University of Rochester Medical Center, 601 Elmwood Avenue, Box 626, Rochester, NY, 14642, USA, Tel +1 585 275 8748, Fax +1 585 273 3637, Email [email protected]
Abstract: Androgen deprivation therapy, primarily via a gonadotropin-releasing hormone receptor agonist or antagonist together with or without an androgen receptor antagonist, remains the mainstay of medical treatment for advanced prostate cancer. Meanwhile, relugolix has been developed as the first orally active, non-peptide, selective antagonist for the gonadotropin-releasing hormone receptor. Previous randomized studies involving patients with prostate cancer have demonstrated comparable efficacy in androgen suppression between relugolix vs other gonadotropin-releasing hormone antagonists or agonists. This review summarizes available data on the design and development of relugolix and its therapeutic application, and discusses if relugolix represents a promising oral alternative to injectable androgen deprivation therapy. Based on current published evidence, further investigation is likely required to determine the actual clinical benefits of relugolix therapy against prostate cancer.
Keywords: androgen deprivation therapy, gonadotropin-releasing hormone antagonist, prostate cancer, relugolix
Introduction
Prostate cancer remains one of the most commonly diagnosed malignancies among men.1,2 Moreover, the number of cancer-related deaths throughout the world has increased from 307,500 in 20121 to 375,304 in 2020.2 Although definitive therapy, such as radical prostatectomy and irradiation, offers excellent oncologic outcomes in most men with localized disease, a considerable number of these patients develop disease recurrence for which adjuvant therapy is often required.3,4
Since its discovery over 80 years ago,5 androgen deprivation therapy (ADT) has been the mainstay of the treatment for advanced prostate cancer.6,7 It has also been utilized in neoadjuvant and adjuvant settings. Gonadotropin-releasing hormone (GnRH) receptor agonists, such as leuprorelin and goserelin, together with or without androgen receptor (AR) antagonists, have thus been commonly employed as a form of chemical castration. Constant stimulation with GnRH analogues induces the desensitization of the GnRH receptor in the pituitary gland and thereby reduces the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which subsequently leads to shutdown of androgen production in the testis. However, a transient increase in the levels of serum testosterone, known as testosterone surge or flare up, after the initiation of GnRH agonist treatment, as well as microsurges on repeat administration, may result in serious adverse events, including temporal worsening of prostate cancer.8,9
GnRH receptor antagonists that physically block the binding of GnRH to the receptor have then been approved for clinical use in men with prostate cancer.10,11 Unlike agonists, GnRH antagonists do not usually induce testosterone surge or clinical flare.11–13 Moreover, of various ADT-associated side effects, the incidence of a cardiac event or death has been shown to be considerably lower in prostate cancer patients with a GnRH antagonist degarelix than in those with GnRH agonists.13,14 Meanwhile, a meta-analysis of five prospective randomized clinical trials demonstrated significant improvement of not only overall survival to which reduction of cardiac death had contributed but also progression-free survival in the degarelix group, compared with prostate cancer patients treated with GnRH agonists.13 Despite these favorable data for degarelix, however, GnRH agonists remain the preferred approach in actual practices, presumably due to the need for monthly injections and the high rate (eg, 40%)10 of injection site reactions associated with degarelix therapy, as well as the long-term experience of GnRH agonist therapy. More recently, as detailed below, relugolix has been developed as an orally active, selective antagonist for the GnRH receptor.
We performed a computerized bibliographic search of the PubMed database, using the following keywords variably combined: “androgen deprivation”; “antiandrogen”; “GnRH agonist”; “GnRH antagonist”; “GnRH receptor”; “medical castration”; “prostate”; “prostate cancer”; and “relugolix”. We then selected only studies published in peer-reviewed journals (plus some articles found in their reference lists). In this article, we thus review available data on the design and development of relugolix and its therapeutic application for prostate cancer.
Drug Development and Pharmacodynamics/Pharmacokinetics
Cho et al15 in Takeda Chemical Industries Ltd. sought to develop potent and orally active non-peptide antagonists of the human LH-releasing hormone (LHRH) receptor via focusing on a type II β-turn16 as a dominant structure for the binding of LH to the receptor. They described the design and synthesis of 3-(N-benzyl-N-methylaminomethyl)-7-(2,6-difluorobenzyl)-4,7-dihydro-2-(4-isobutyrylaminophenyl)-4-oxothieno[2,3-b]pyridine-5-carboxylate hydrochloride (T-98475), as well as its biological properties. T-98475 was found to have a 50-fold higher binding affinity than LHRH and inhibited LHRH-mediated LH release in cultured cynomolgus pituitary cells while showing no agonist activity at the highest dose (ie, 500 nM) examined. In castrated male cynomolgus monkeys, oral administration of T-98475 (60 mg/Kg) resulted in considerable (eg, approximately 70%) reduction in plasma LH levels.
To identify the derivatives of T-98475 exhibiting higher efficacy, a subsequent study was performed by the same group via two strategies: 1) chemical modification of the ester moiety; and 2) replacement of the thienopyridin-4-one scaffold with other heterocyclic surrogates.17 Of those containing a biaryl moiety, as well as thienopyrimidine-2,4-dione core as a scaffold, 5-(N-benzyl-N-methylaminomethyl)-1-(2,6-difluorobenzyl)-6-[4-(3-methoxyureido)phenyl]-3-phenylthieno[2,3-d]pyrimidine-2,4(1H,3H)-dione (TAK-013) was further investigated. The receptor binding affinity of TAK-013 (IC50 in CHO cells expressing the human LHRH receptor: 0.1 nM) was higher than that of T-98475 (0.2 nM). Similarly, the IC50 for the antagonistic effect (ie, inhibition of LHRH-induced arachidonic acid release from CHO cells expressing the human LHRH receptor) of TAK-013 (0.06 nM) was lower than that of T-98475 (0.6 nM). Indeed, oral administration of TAK-013 (30 mg/Kg) in castrated male monkeys achieved stronger (eg, 89%) suppression of plasma LH levels.
It has been well known that the inhibition of cytochrome P450 3A4 (CYP3A4) could induce drug–drug interactions.18 To reduce the CYP3A4 inhibition in thienopyrimidine-2,4-dione derivatives including TAK-013, further optimization of this scaffold was investigated via focusing on their chemical modification at the 5 and 3 positions based on computational modeling, resulting in the identification of 1-{4-[1-(2,6-difluorobenzyl)-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl]phenyl}-3-methoxyurea (TAK-385, Relugolix; Figure 1A).19 The IC50s of relugolix for the binding affinity to the LHRH receptor and the inhibition of arachidonic acid release in CHO cells cultured in the absence of fetal bovine serum were 0.08 nM and 0.33 nM, respectively, while relugolix at 3 mg/Kg maintained the inhibitory effects on plasma LH levels in monkeys which lasted for at least 48 hours. Importantly, relugolix was found to completely lack the CYP3A4 inhibition.
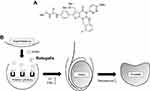 |
Figure 1 Chemical structure (A) and mechanism of action (B) of relugolix. |
The effects of relugolix showing low affinity for rodent GnRH receptors were further assessed in knock-in mice possessing the human GnRH receptor.20 Oral administration of relugolix (30 mg/Kg, twice daily for 4 weeks) in these knock-in males significantly reduced the weights of the testis and ventral prostate, as well as serum levels of testosterone similar to those in orchiectomized males. The reduction in these was then completely restored 4 weeks after the discontinuation of relugolix treatment. These findings indicated that orally administered relugolix could function as an LHRH antagonist and inhibited the hypothalamus-pituitary-gonadal axis potently and reversibly in vivo (Figure 1B).
In men (80 mg)21 and women (40 mg),22 daily oral doses of relugolix have been shown to induce reduction in serum testosterone and estradiol to castrated and postmenopausal levels, respectively. In addition, Cmax of a single 40-mg dose of relugolix was 20.8 ng/mL in healthy men, and steady-state levels were reached within 11–14 days with an approximate 2-fold accumulation after repeated daily dosing.21 Similarly, the elimination half-life of relugolix was 35 hours (180 mg) to 64 hours (20 mg). Relugolix was excreted mainly in feces and to a small degree in urine.
Phase I Studies
In two phase I studies,21,23 the efficacy of relugolix, as well as its tolerability and safety, has been explored.
A three-part, randomized, double-blind, placebo-controlled, dose-escalating, phase I trial involving 176 UK healthy male volunteers with the age range of 40–75 years, which is most commonly associated with prostate cancer, was first conducted21 to evaluate pharmacokinetics and pharmacodynamics of relugolix after single and multiple doses, as described above. In addition, a loading dose of 360 mg on day 1 with or without 240 mg on day 2 was found to facilitate the achievement of castration levels of testosterone from ≥7 days to <3 days. After the cessation of relugolix on day 28, testosterone levels were normalized in most of the subjects in additional ≤28 days. Common side effects included bradycardia, headache, and hot flush all in mild-to-moderate severity, while bradycardia and headache were similarly occurred in the placebo group. Meanwhile, food intake, particularly a high-fat meal, diminished relugolix oral bioavailability by up to 52%.
Primarily to determine the optimal dose of relugolix, a two-part phase I trial was then performed in Japanese men with non-metastatic hormone-naïve prostate cancer [baseline serum testosterone level >1.5 ng/mL, prostate-specific antigen (PSA) >4.0 ng/mL in untreated patients or >0.2 ng/mL in those who had undergone radical prostatectomy or high-intensity focused ultrasound therapy and/or radiotherapy].23 In dose-escalation cohorts, a total of 13 patients received relugolix at 320 mg (as a loading dose on day 1)/80 mg (as a maintenance dose on days 2–28), 320/120 mg, 320/160 mg, or 360/120 mg. In expansion cohorts with randomization, 30 patients received relugolix at 320 mg on day 1 and either 80 mg or 120 mg daily for up to 96 weeks. Irrespective of dosages in all of these patients, serum testosterone was reduced to castration levels during the first week of the treatment, and PSA levels were steadily declined throughout the study. Meanwhile, 10 (77%) of the dose-escalation cohorts and all of the expansion cohorts had adverse event(s), most commonly with hot flush, viral upper respiratory tract infection, and/or diarrhea. Although dose-limiting toxicities were not seen, the findings suggested minimum initial and maintenance doses of 320 mg and 80 mg, respectively, for clinical effects. A notable limitation of this study included the small number of participants, although the observed effects of relugolix on the reduction of testosterone/PSA levels were comparable to those in earlier studies in Western population.21,24
Phase II Studies (See Table 1)
The interim results from at least two randomized phase II trials with relugolix treatment have been reported in abstract forms.24–26
 |
Table 1 Efficacy of Relugolix in Clinical Trials |
In the first study (NCT02135445),24 men with intermediate-risk localized prostate cancer eligible for 6-month ADT (with baseline testosterone >150.0 ng/dL and PSA >2.0 ng/mL) and subsequent external beam radiation therapy were received with either relugolix (320 mg on day 1 and 120 mg daily thereafter; n = 65) or degarelix (240 mg on day 1 and 80 mg every 4 weeks thereafter; n = 38) for 24 weeks. Median levels of testosterone in those with relugolix vs degarelix were 53.0 vs 49.0 ng/dL after 24 hours and 7.8 vs 10.1 ng/dL after 12 weeks. Castration levels of testosterone after 24 weeks were achieved in 95% of the relugolix patients and 89% of degarelix patients. Prostate size and PSA at 8–12 weeks were similarly reduced in the two groups. These findings suggested that oral relugolix and injectable degarelix had similar clinical effects. Hot flush was the most common side effect (57% with relugolix and 61% with degarelix). Of note, after the discontinuation of the GnRH treatment, testosterone levels were more rapidly recovered in the relugolix arm than in the degarelix arm.
In the second study (NCT02083185),25,26 men with prostate cancer appropriate for first-line ADT (with baseline testosterone >150.0 ng/dL and PSA >2.0 ng/mL) were received with relugolix (80 mg daily, n = 39 or 120 mg daily, n = 36) or leuprorelin (22.5 mg every 12 weeks, n = 20) for 48 weeks. Median levels of testosterone in those with relugolix vs leuprorelin were 36.9 vs 648.1 ng/dL after 3 days, 10.6 vs 13.0 ng/dL after 4 weeks, and 8.9 vs 11.5 ng/dL after 24 weeks, while sustained castration rates at 5–24 weeks were 92% vs 95%. After 24 weeks, PSA reduction of 97.3% to a median of 0.1 ng/mL with relugolix or 92.4% to a median of 0.2 ng/mL with leuprorelin was seen. Thus, the clinical efficacy of oral GnRH antagonist relugolix was shown to be comparable to that of injectable GnRH agonist leuprorelin. Meanwhile, all grade adverse events, including hot flush (59% vs 60%), were seen in 91% of the relugolix patients vs 95% of the leuprorelin patients. In addition, the reduction in the quality-of-life (QoL) scores from the baseline scores was similar between those with relugolix (81.4 minus 6.9 points) vs leuprorelin (79.6 minus 7.4 points).
Data from the NCT02135445 trial, for which the preliminary findings24 are described above, were then finalized.27 The characteristics of the 103 patients and their baseline data, as well as the details of radiotherapy, were similar between the relugolix and degarelix groups. The castration rates for the threshold of 50 ng/mL or 20 ng/dL were 95% or 82% with relugolix (median: 4 days) and 89% or 68% with degarelix (median: 3 days), respectively. In both groups (relugolix 26%, degarelix 29%), the prostate volume was reduced from baseline after 8–12 weeks of treatment. Similarly, at 24 weeks, ≥50% PSA reduction (relugolix 98%, degarelix 100%) and ≥90% PSA reduction (relugolix 95%, degarelix 92%) were detected in most of the patients. By 12 weeks after the discontinuation of the treatment, recovery of testosterone levels to baseline or 280 ng/mL occurred in 52% of the relugolix patients vs 16% of the degarelix patients. In addition, changes in the QoL scores during 24-week treatment, as well as sexual activity or ADT-related symptoms, were similar between the two arms. Most of the patients (relugolix 86%, degarelix 97%) had at least one adverse event, while severe (grade ≥3) events were rare (relugolix 2%, degarelix 11%) and none discontinued the treatment due to the side effects. These data indicated the comparable efficacy of relugolix and degarelix in achieving androgen/PSA reduction in men with prostate cancer. However, long-term suppression of GnRH by relugolix was not tested. Meanwhile, a potentially major limitation was the lack of blinding in this trial, which might have affected the assessments of safety and QoL.
More recently, a single-arm, open-label, multicenter trial (Apa-RP study; NCT04523207) was conducted to assess the effect of relugolix (loading dose of 360 mg commenced within 90 days post-surgery, 120 mg/day for 6 weeks) in combination with a non-steroidal AR antagonist apalutamide (for the latter 4 weeks) on postoperative biochemical recurrence in 12 men with high-risk prostate cancer undergoing radical prostatectomy.28 All these patients achieved castration levels of testosterone by initial 2-week relugolix monotherapy, which was maintained until the end of coadministration (except one with no measurement). Adverse events (all grade 1 or 2) occurred in 9 (75%) patients during relugolix monotherapy and 8 (67%) patients during combination therapy, and the most common event was grade 1 hot flush (n = 6 during monotherapy, n = 4 during combination therapy). In addition, although the relugolix label recommended avoiding coadministration with P-glycoprotein/CYP3A4 inducers (eg, apalutamide) or, if unavoidable, doubling the daily dose of relugolix (ie, 240 mg), the standard dose of relugolix, as chemical castration, combined with apalutamide was shown to be efficacious. Limitations of this trial included its open-label design and small sample size.
Phase III Study (See Table 1)
A phase III study (HERO trial)29 was conducted to assess the efficacy and safety of relugolix, by comparison with those of a GnRH agonist, in men with advanced prostate cancer. Patients enrolled at 155 centers in North or South America, Europe, or Asia-Pacific region had one of the following: biochemical or clinical relapse after definitive therapy; newly diagnosed hormone-sensitive metastatic disease; and locally advanced disease unlikely to be cured by local primary intervention. They were randomly assigned to receive either relugolix (360 mg orally on day 1, 120 mg/day thereafter; n = 622) or leuprorelin (injection every 3 months; n = 308) for 48 weeks.
The rates of testosterone suppression to castration levels (ie, <50 ng/dL) on days 4 and 15 were 56.0% and 98.7% in the relugolix group and 0% and 12.0% in the leuprorelin group, respectively. Sustained castration through 48 weeks was achieved in 96.7% of the relugolix patients and 88.8% of the leuprorelin patients. Thus, testosterone data at all points examined showed significant (P<0.001) superiority of relugolix over leuprorelin. Additionally, in a subgroup analysis, the proportion of patients with testosterone recovery to the lower limit of the normal range (ie, ≥280 ng/dL) 90 days after treatment discontinuation was significantly (P=0.002) higher in the relugolix arm (54%; n = 137) than in the leuprorelin arm (3%; n = 47). Moreover, in the entire cohorts, PSA response (ie, >50% decrease) at day 15 followed by confirmation at day 29 was significantly (P<0.001) more often seen in the relugolix patients (79.4%) than in the leuprorelin patients (19.8%).
Regarding safety, most of the participants (92.9% with relugolix, 93.5% with leuprorelin) experienced adverse events, including grade 3 or 4 events (18.0% with relugolix, 20.5% with leuprorelin). As seen in earlier studies, hot flush was the most common event (54.3% with relugolix, 51.6% with leuprorelin). Thus, the incidence rates of adverse events were similar between the two groups, except that of mild/moderate diarrhea which was slightly higher in the relugolix arm (12.2% vs 6.8%) where no patients were withdrawn due to diarrhea. However, major cardiovascular events, such as non-fatal myocardial infarction and stroke, were less often in the relugolix arm of the entire patients (2.9% vs 6.2%) or a subgroup of patients with a known history of these events (3.6% vs 17.8%).
These data from the HERO study indicated that relugolix could induce rapid and sustained suppression of androgens, as well as androgen recovery, both of which were superior to leuprorelin. Relugolix was also found to be associated with a considerably lower risk of major adverse cardiovascular events, compared with leuprorelin, while cardiovascular disease often as a pre-existing condition is the leading cause of death in men with prostate cancer.30 The androgen recovery within 90 days after discontinuation of relugolix might particularly indicate its suitability for use in patients undergoing intermittent ADT, an option associated with improved QoL,31 or short-term ADT in combination with definitive therapy, as well as in those withdrawing ADT due to, for example, serious complications.
Meta-Analysis of Randomized Trials
The results of a network meta-analysis to compare the efficacy (ie, castration rate with testosterone ≤50 ng/dL) and safety (ie, adverse events) of relugolix vs degarelix (3 doses) in advanced prostate cancer have recently been documented.32 Data were extracted from four phase III randomized studies involving 2059 patients, including the HERO study29 and others primarily assessing those of degarelix. Compared with GnRH antagonists, there were no significant differences in the risk ratio (RR) of sustained castration at 12 months for relugolix (1.09) or degarelix (0.98 for all doses, 1.02 for 80 mg, 1.03 for 160 mg, 0.46 for 480 mg), although relugolix had the highest rank. Similarly, compared with GnRH agonists, relugolix (RR = 0.99/0.72/0.44) and degarelix (RR = 1.01/1.05/0.74) were not significantly associated with the risk of adverse events (all/serious/cardiovascular). These data suggested that the efficacy and safety were comparable among relugolix, degarelix, and GnRH agonists. However, comparisons of long-term (>12 months) follow-up data on oncologic outcomes, as well as adverse events such as cardiac disease, metabolic syndrome, and cognitive disorders often seen after extended ADT,33,34 could not be made. Moreover, subgroup analysis based on various stages of prostate cancer (eg, biochemical recurrence after definitive therapy, locally advanced disease, hormone naïve metastatic disease) was not feasible.
Population pharmacokinetic and semimechanistic population pharmacokinetic/pharmacodynamic analyses of five clinical trials (ie, two Phase I, two Phase II, one phase III) have also been performed to characterize the systematic relugolix exposure and its effect on testosterone concentrations.35 Age, body weight, and race, as well as health status (ie, healthy men vs prostate cancer patients), showed no apparent impact on these. In addition, simulation with this model demonstrated sustained castration levels in 97.3% and 85.5% of the participants after temporary interruption for 7 and 14 days, respectively.
Drug Approval and Controversies
Based on the findings from the clinical trials, particularly the HERO study,29 described above, relugolix was approved for the treatment of prostate cancer by the US Food and Drug Administration in 2020 and the European Medicines Agency in 2022. Additionally, since 2019, relugolix has been prescribed in women with uterine fibroids in Japan. Of note, however, there are no available data on the long-term oncologic prognosis following relugolix therapy in patients with prostate cancer.
Recently, the HERO study has been criticized,36 which may indicate the requirement of new post-marketing trials to further determine the actual benefits of relugolix in men with prostate cancer. In this opinion article, five issues were listed. First, the control arm with leuprorelin might not have been optimal and instead degarelix with the identical mechanisms of action might represent a more adequate control. Second, the primary endpoint served the trial rather than the participants and measured androgen levels from day 29 at when disease flare induced by the LHRH agonist might have been considerably affected. Third, no evidence to prolong the life of the participants or prevent the disease progression and metastasis was shown, while the incidence of diarrhea was higher in the relugolix patients. Fourth, the exclusion criteria, including the anticipation of undergoing chemotherapy or surgery within two months of initiating ADT, might have introduced a selection bias. Fifth, the trial design which prohibited various medications that could affect cardiovascular comorbidities might have limited the enrolment of patients (with age-related cardiovascular risk). Additional criticisms of the HERO study include only a small subset of patients receiving concomitant therapy with anti-AR agents such as enzalutamide (n = 17; 2.7%) or other anti-cancer agents such as docetaxel (n < 10; <1.3%)37 to assess the safety of relugolix which may often be used in combination with these agents in real-world practice. As a result, the current NCCN guidelines for prostate cancer do not recommend the use of relugolix combined with other therapies.
A concern for treatment adherence (ie, percentage of expected doses actually taken) to oral therapy is unavoidable. Adherence with oral relugolix was >99% in the HERO study,29 which was comparable to that of injectable leuprorelin. However, real-world adherence rates for oral ADT agents, such as abiraterone acetate, were reported to be 92–96% in patients with castration-resistant prostate cancer.38,39
Cost-effectiveness of relugolix treatment for prostate cancer has also been explored. In a recent US study,40 The monthly cost of ADT with relugolix was estimated to be $2866.94, which was almost five times higher than that with leuprorelin ($602.99). Further analysis showed the incremental effect of 0.46 quality-adjusted life-year (QALY) and the incremental cost-effectiveness ratio of $49,571.10/QALY for relugolix. The relative rates of progression-free survival/overall survival at 5 years for relugolix and leuprorelin used as the first-line therapy were 72.7%/86.0% and 61.0%/85.9%, respectively. Although the impact of adverse events was not considered in these analyses, ADT with relugolix might thus not be a cost-effective alternative for the management of advanced prostate cancer, compared to ADT with leuprorelin.
Conclusions
Relugolix is the first orally active non-peptide GnRH receptor antagonist. As such, there are no injection site reactions that are often associated with degarelix or GnRH agonists. Importantly, previous randomized trials have indicated that relugolix has comparable or even better effects, compared with degarelix or GnRH agonists, on rapid and sustained androgen suppression, as well as adverse events, in patients with prostate cancer. Relugolix thus represents an oral alternative to injectable ADT. However, it remains to be seen whether previous observations on relugolix can readily translate into clinically relevant outcomes of prostate cancer. Remarkably, none of the studies described in this article have reported long-term oncologic outcomes of patients who received relugolix therapy. Furthermore, while the development of castration-resistant disease following ADT remains a major challenge in patients with advanced prostate cancer, the specific implication of relugolix in castration-resistant prostate cancer cannot thus be discussed. Additional issues include considerable reduction in the absorption of oral relugolix by food intake and potential interactions between relugolix and P-glycoprotein/CYP3A4 inducers such as apalutamide. Accordingly, further accumulation of data on the actual clinical benefits of relugolix therapy for prostate cancer is warranted.
Data Sharing Statement
All data provided in the manuscript are from cited published studies.
Funding
There is no funding to report.
Disclosure
HM has received research funding from Astellas Scientific and Medical Affairs, Ferring Research Institute, and Bristol-Myers Squibb. The authors report no other conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistic, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistic 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Lancee M, Tikkinen KAO, de Reijke TM, Kataja VV, Aben KKH, Vernooij RWM. Guideline of guidelines: primary monotherapies for localised or locally advanced prostate cancer. BJU Int. 2018;122(4):535–548. doi:10.1111/bju.14237
4. Van den Broeck T, van den Bergh RCN, Briers E, et al. Biochemical recurrence in prostate cancer: the European Association of Urology prostate cancer guidelines panel recommendations. Eur Urol Focus. 2020;6(2):231–234. doi:10.1016/j.euf.2019.06.004
5. Huggins C. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Arch Surg. 1941;43(2):209–223. doi:10.1001/archsurg.1941.01210140043004
6. Singer EA, Golijanin DJ, Miyamoto H, Messing EM. Androgen deprivation therapy for prostate cancer. Expert Opin Pharmacother. 2008;9(2):211–228. doi:10.1517/14656566.9.2.211
7. Harris AE, Metzler VM, Lothion-Roy J, et al. Exploring anti-androgen therapies in hormone dependent prostate cancer and new therapeutic routes for castration resistant prostate cancer. Front Endocrinol. 2022;13:1006101. doi:10.3389/fendo.2022.1006101
8. Zinner NR, Bidair M, Centeno A, Tomera K. Similar frequency of testosterone surge after repeat injections of goserelin (Zoladex) 3.6 mg and 10.8 mg: results of a randomized open-label trial. Urology. 2004;64(6):1177–1181. doi:10.1016/j.urology.2004.07.033
9. van Poppel H, Nilsson S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology. 2008;71(6):1001–1006. doi:10.1016/j.urology.2007.12.070
10. Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102(11):1531–1538. doi:10.1111/j.1464-410X.2008.08183.x
11. Tombal B, Miller K, Boccon-Gibod L, et al. Additional analysis of the secondary end point of biochemical recurrence rate in a Phase 3 trial (CS21) comparing degarelix 80 mg versus leuprolide in prostate cancer patients segmented by baseline characteristics. Eur Urol. 2010;57(5):836–842. doi:10.1016/j.eururo.2009.11.029
12. Van Poppel A, Abrahamsson P-A. Considerations for the use of gonadotropin-releasing hormone agonists and antagonists in patients with prostate cancer. Int J Urol. 2020;27(10):830–837. doi:10.1111/iju.14303
13. Klotz L, Miller K, Crawford ED, et al. Disease control outcomes from analysis of pooled individual patient data from five comparative randomised clinical trials of degarelix versus luteinising hormone-releasing hormone agonists. Eur Urol. 2014;66(6):1101–1108. doi:10.1016/j.eururo.2013.12.063
14. Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, Nilsson J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65(3):565–573. doi:10.1016/j.eururo.2013.10.032
15. Cho N, Harada M, Imaeda T, et al. Discovery of a novel, potent, and orally active nonpeptide antagonist of the human luteinizing hormone-releasing hormone (LHRH) receptor. J Med Chem. 1998;41(22):4190–4195. doi:10.1021/jm9803673
16. Karten MJ, Rivier JE. Gonadotropin-releasing hormone analog design. Structure-function studies toward the development of agonists and antagonists: rationale and perspective. Endocr Rev. 1986;7(1):44–66. doi:10.1210/edrv-7-1-44
17. Sasaki S, Cho N, Nara Y, et al. Discovery of a thieno[2,3-d]pyrimidine-2,4-dione bearing a p-methoxyureidophenyl moiety at the 6-position: a highly potent and orally bioavailable non-peptide antagonist for the human luteinizing hormone-releasing hormone receptor. J Med Chem. 2003;46(1):113–124. doi:10.1021/jm020180i
18. Pal D, Mitra AK. MDR- and CYP3A4-mediated drug-drug interactions. J Neuroimmune Pharmacol. 2006;1(3):323–329. doi:10.1007/s11481-006-9034-2
19. Miwa K, Hitaka T, Imada T, et al. Discovery of 1-{4-[1-(2,6-difluorobenzyl)-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl]phenyl}-3-methoxyurea (TAK-385) as a potent, orally active, non-peptide antagonist of the human gonadotropin-releasing hormone receptor. J Med Chem. 2011;54(14):4998–5012. doi:10.1021/jm200216q
20. Nakata D, Masaki T, Tanaka A, et al. Suppression of the hypothalamic-pituitary-gonadal axis by TAK-385 (relugolix), a novel, investigational, orally active, small molecule gonadotropin-releasing hormone (GnRH) antagonist: studies in human GnRH receptor knock-in mice. Eur J Pharmacol. 2014;723:167–174. doi:10.1016/j.ejphar.2013.12.001
21. MacLean DB, Shi H, Faessel HM, Saad F. Medical castration using the investigational oral GnRH antagonist TAK-385 (relugolix): Phase 1 study in healthy males. J Clin Endocrinol Metab. 2015;100(12):4579–4587. doi:10.1210/jc.2015-2770
22. Rocca ML, Palumbo AR, Lico D, et al. Relugolix for the treatment of uterine fibroids. Expert Opin Pharmacother. 2020;21(14):1667–1674. doi:10.1080/14656566.2020.1787988
23. Suzuki H, Uemura H, Mizokami A, et al. Phase I trial of TAK-385 in hormone treatment-naïve Japanese patients with nonmetastatic prostate cancer. Cancer Med. 2019;8(13):5891–5902. doi:10.1002/cam4.2442
24. Dearnaley D, Saltzstein DR, Sylvester JE, et al. Noe/adjuvant ADT to EBRT: randomized Phase 2 trial of the oral GnRH antagonist, TAK-385 (relugolix, RGX) and degarelix (DGX) in patients (pts) with prostate cancer (PC). Ann Oncol. 2016;27(Suppl6):vi243. doi:10.1093/annonc/mdw372.18
25. Saad F, Bailen JL, Pieczonka CM, et al. Second interim analysis (IA2) results from a phase II trial of TAK-385, an oral GnRH antagonist, in prostate cancer patients (pts). J Clin Oncol. 2016;34(Suppl2):200. doi:10.1200/jco.2016.34.2_suppl.200
26. Shore ND, Beach M, Bailen JL, et al. Testosterone lowering, PSA response and quality of life in patients with advanced hormone sensitive prostate cancer receiving TAK-385, an oral GnRH antagonist: phase 2 interim analysis. J Urol. 2016;195(Suppl4):e654. doi:10.1016/j.juro.2016.02.388
27. Dearnaley D, Saltzstein DR, Sylvester JE, et al. The oral gonadotropin-releasing hormone receptor antagonist relugolix as neoadjuvant/adjuvant androgen deprivation therapy to external beam radiotherapy in patients with localised intermediate-risk prostate cancer: a randomized, open-label, parallel-group phase 2 trial. Eur Urol. 2020;78(2):184–192. doi:10.1016/j.eururo.2020.03.001
28. Brown G, Belkoff L, Hafron JM, et al. Coadministration of apalutamide and relugolix in patients with localized prostate cancer at high risk for metastasis. Target Oncol. 2023;18(1):95–103. doi:10.1007/s11523-022-00932-8
29. Shore ND, Saad F, Cookson MS, et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382(23):2187–2196. doi:10.1056/NEJMoa2004325
30. Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–3897. doi:10.1093/eurheartj/ehz766
31. Mohler JL, Antonarakis ES. NCCN guidelines updates: management of prostate cancer. J Natl Compr Canc Netw. 2019;17(5.5):583–586. doi:10.6004/jnccn.2019.5011
32. Sari Motlagh R, Abufaraj M, Mori K, et al. The efficacy and safety of relugolix compared with degarelix in advanced prostate cancer patients: a network meta-analysis of randomized trials. Eur Urol Oncol. 2022;5(2):138–145. doi:10.1016/j.euo.2021.07.002
33. Abufaraj M, Iwata T, Kimura S, et al. Differential impact of gonadotropin-releasing hormone antagonist versus agonist on clinical safety and oncologic outcomes on patients with metastatic prostate cancer: a meta-analysis of randomized controlled trials. Eur Urol. 2021;79(1):44–53. doi:10.1016/j.eururo.2020.06.002
34. Sari Motlagh R, Quhal F, Mori K, et al. The risk of new onset dementia and/or Alzheimer disease among patients with prostate cancer treated with androgen deprivation therapy: a systematic review and meta-analysis. J Urol. 2021;205(4):60–67. doi:10.1097/JU.0000000000001341
35. Lee TY, Pierrillas PB, Lin YW, et al. Population PK and semimechanistic PK/PD modeling and simulation of relugolix effects on testosterone suppression in men with prostate cancer. Clin Pharmacol Ther. 2023;113(1):124–134. doi:10.1002/cpt.2743
36. Powell K, Burns MC, Prasad V. Relugolix: five reasons why the US food and drug administration should have exercised restraint. Eur Urol. 2023;83(2):101–102. doi:10.1016/j.eururo.2022.08.029
37. Sahu KK, Tripathi N, Agarwal N, Swami U. Relugolix in the management of prostate cancer. Expert Rev Anticancer Ther. 2022;22(9):891–902. doi:10.1080/14737140.2022.2105209
38. Lafeuille MH, Grittner AM, Lefebvre P, et al. Adherence patterns for Abiraterone acetate and concomitant prednisone use in patients with prostate cancer. J Manag Care Spec Pharm. 2014;20(5):477–484. doi:10.18553/jmcp.2014.20.5.477
39. Behl AS, Ellis LA, Pilon D, Xiao Y, Lefebvre P. Medication adherence, treatment patterns, and dose reduction in patients with metastatic castration-resistant prostate cancer receiving Abiraterone acetate or enzalutamide. Am Health Drug Benefits. 2017;10(6):296–303.
40. Adekunle OA, Seoane-Vazquez E, Brown LM. Cost-effectiveness analysis of androgen deprivation therapy with relugolix for the treatment of advanced prostate cancer. J Am Pharm Assoc. 2023;63(3):817–824.e3. doi:10.1016/j.japh.2022.12.019
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
