Back to Journals » Drug Design, Development and Therapy » Volume 17
Profile of Linzagolix in the Management of Endometriosis, Including Design, Development and Potential Place in Therapy: A Narrative Review
Authors Donnez J, Cacciottola L , Squifflet JL, Dolmans MM
Received 20 September 2022
Accepted for publication 17 January 2023
Published 8 February 2023 Volume 2023:17 Pages 369—380
DOI https://doi.org/10.2147/DDDT.S269976
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Jacques Donnez,1,2 Luciana Cacciottola,3 Jean-Luc Squifflet,4 Marie-Madeleine Dolmans3,4
1Department of Gynaecology, Université Catholique de Louvain, Brussels, Belgium; 2Société de Recherche pour l’Infertilité (SRI), Brussels, Belgium; 3Gynecology Research Laboratory, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, Brussels, Belgium; 4Gynecology Department, Cliniques Universitaires St-Luc, Brussels, Belgium
Correspondence: Marie-Madeleine Dolmans, Gynecology Research Unit, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, Avenue Mounier 52, bte B1.52.02, Brussels, 1200, Belgium, Tel +32 02 764 5237, Fax +32 02 764 9507, Email [email protected]
Abstract: Estrogens play a critical role in the pathogenesis of endometriosis and it is logical to assume that lowering estradiol levels with oral gonadotropin-releasing hormone (GnRH) antagonists may prove effective, especially in women who fail to respond to progestogens. Indeed, due to progesterone resistance, oral contraceptives and progestogens work well in two-thirds of women suffering from endometriosis, but are ineffective in 33% of women. Oral GnRH antagonists have therefore been evaluated for management of premenopausal women with endometriosis-associated pelvic pain. The first publication on these drugs reported the efficacy of elagolix. The present paper is a narrative review of linzagolix, which is an orally administered GnRH receptor antagonist with low pharmacokinetic/pharmacodynamic variability. It binds to and blocks the GnRH receptor in the pituitary gland, resulting in a dose-dependent drop in luteinizing hormone (LH) and follicle-stimulating hormone (FSH) production. This reduction in LH and FSH levels in turn leads to a dose-dependent decline in estrogen. Phase 2 and 3 trials are reviewed and discussed here. There is a place for GnRH antagonists in the management of symptomatic endometriosis, and linzagolix with or without add-back therapy (ABT) is one option that can be used at low doses, avoiding the need for ABT, which is contraindicated in some patients.
Keywords: endometriosis, pelvic pain, dysmenorrhea, progesterone resistance, GnRH antagonist
Endometriosis is a common but enigmatic disease, known to be estrogen-dependent. Various factors can contribute to its development and progression,1–3 but it is characterized by the rise and fall of hormones, particularly estrogen, mainly in the form of estradiol whose ovarian secretion is under the control of gonadotropin-releasing hormone (GnRH). Finding appropriate medical therapy is challenging, as existing treatments have certain limitations.2–4
The symptoms of endometriosis include dysmenorrhea, chronic non-menstrual pelvic pain, dyspareunia, dyschezia and infertility, which can seriously impact quality of life. Endometriosis-related pain is typically cyclical in nature, reflecting the response to circulating reproductive hormones, principally estrogen. Endometriosis is also one of the leading causes of infertility and is often diagnosed when women seek treatment to conceive. The World Endometriosis Research Foundation estimated the aggregate annual cost of endometriosis to be in the region of $80 billion in the US and around $60 billion in the UK, Germany, France and Italy in 2012 based on exchange rates at the time.5,6
Drawbacks of Current Endometriosis Therapies
Existing treatment options for endometriosis are either medical or surgical.2 Medical therapy should ideally be contingent on the phenotype, severity of symptoms and extent of endometriosis, and aim to relieve pain or reduce lesion size.2–4 According to what is known about retrograde menstruation, blocking menstrual bleeding by means of hormone therapy to induce oligo- or amenorrhea may in theory modulate and control the symptoms of endometriosis.2–4 First-line medical therapies (oral contraceptives [OCPs] and progestogens) do work in two-thirds of women experiencing endometriosis-associated pain,7,8 but their long-term efficacy is somewhat limited. Many experts believe that progestogens should be considered the first choice,9 but the failure rate is estimated to be around 33% due to so-called “progesterone resistance”.2 In endometriotic lesions, a lack of secretory transformation during the luteal phase and varying patterns of estrogen receptors and progesterone receptors (PRs) in eutopic and ectopic endometrium strongly indicate that PRs, while present, are inactive in a biological sense. Indeed, the notion of progesterone resistance was first proposed back in 1997.10 Since then, numerous papers have advanced theories substantiating this hypothesis (see 2 for review). According to Bulun et al11,12 and Yilmaz and Bulun,13 the fact that endometriotic stromal cells are unable to produce progesterone-induced paracrine factors may be due to a lack of PR-B.14
Second-line treatments (injectable depot formulations of GnRH agonists), which are offered only if OCPs or progestogens fail, are associated with menopausal symptoms.15 GnRH agonists initially act to overstimulate GnRH receptors (flare-up effect), before subsequently desensitizing them, leading to lower levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and greatly reduced estrogen production. This results in chemical pseudo-menopause, where patients suffer menopausal symptoms like bone mineral density (BMD) loss and hot flushes.
Although GnRH agonists may be able to treat the symptoms of endometriosis, these drugs also have serious drawbacks,2,4,8,14,15 including:
- Delivery by injection. Various GnRH agonists like leuprolide acetate, goserelin and triptorelin need to be injected monthly or quarterly.
- The flare-up effect that delays the therapeutic impact and possibly worsens symptoms for the first few weeks.
- Full suppression of estradiol to postmenopausal levels of less than 20 pg/mL and multiple related troublesome side effects, including hot flushes, loss of libido, vaginal dryness and BMD loss after 6 months of GnRH agonist treatment.
- Requirement for add-back therapy (ABT) after six months of treatment to counterbalance the adverse effects of total estrogen suppression, despite hormonal ABT being contraindicated in some women.
- Unpredictable reversibility of treatment. After the end of therapy, it can take months for ovarian function to return to normal, obviously causing problems for women wishing to conceive after treatment or in case of treatment cessation due to drug intolerance.
Mechanism of Action of Linzagolix
Linzagolix is an oral GnRH receptor antagonist drug with low pharmacokinetic/pharmacodynamic (PK/PD) variability.16 It works by binding to and blocking the GnRH receptor in the pituitary gland, which causes a dose-dependent decline in production of LH and FSH. This drop in LH and FSH levels then results in a dose-dependent downturn in estrogen concentrations (Figure 1).
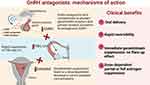 |
Figure 1 Mechanism of action of GnRH antagonists. |
At appropriate doses, linzagolix has been found to maintain estradiol values within the target range of 20–60 pg/mL, which is ideal to alleviate symptoms linked to endometriosis, while at the same time mitigating BMD loss and other unpleasant effects related to total estradiol suppression. At higher doses, linzagolix pushes estradiol levels below 20 pg/mL, considered to be full suppression2–4 (Figure 2).
 |
Figure 2 Estradiol levels up to week 24 in women given a placebo, 75 mg, 100 mg and 200 mg linzagolix. Patients taking a placorrectedcebo were switched to 100 mg linzagolix at week 12. E2: estradiol. Adapted from Donnez J, Dolmans MM. Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GnRH Antagonists: A Review. J Clin Med. 2021;10(5):1085 (https://creativecommons.org/licenses/by/4.0/)2. |
Linzagolix can potentially overcome some of the disadvantages of GnRH agonists, while advantages over GnRH agonists include:
- Ease of administration. Linzagolix can be given orally once a day, irrespective of the timing of meals.
- Rapid onset of therapeutic effects. Because it blocks the GnRH receptor, linzagolix curbs LH and FSH within hours, reducing estradiol concentrations very fast.
- Dose-dependent suppression. The tested once-daily 75 mg dose (EDELWEISS 1 phase 2b trial) maintains estradiol values between 20 and 60 pg/mL and can potentially be used as stand-alone therapy for sizable numbers of patients suffering from endometriosis-associated pain. The once-daily 200 mg dose of linzagolix that fully suppresses estradiol levels (below 20 pg/mL) requires ABT if administered long term to offset the side effects.17,18
- Rapid reversibility of effects. With a half-life of approximately 15 hours, ovarian activity may recover within days of treatment cessation.
Clinical Characteristics of Linzagolix
In July 2018, orally delivered GnRH antagonist elagolix (Orilissa®) was officially approved in the US to treat women with moderate-to-severe endometriosis-related pain (150 mg once daily up to 2 years and 400 mg [200 mg twice daily] up to 6 months).19 Relugolix, an oral GnRH receptor antagonist (40 mg daily), was also tested along with hormonal ABT for management of symptoms linked to endometriosis and uterine fibroids. These are the only doses under investigation for both indications. In September 2021, Myovant announced that the US Food and Drug Administration (FDA) agreed to look into another drug application for Myfembree® (40 mg relugolix, 1 mg estradiol, and 0.5 mg norethindrone acetate) for the treatment of moderate-to-severe endometriosis-associated pain.20
In our opinion, linzagolix has the best-in-class clinical profile thanks to:
- Optimal attributes for PK consistency. Linzagolix has been shown to maintain a steady PK profile with little variability by virtue of its high bioavailability and low volume of distribution.16
- Its half-life of 14–15 hours. This allows once-daily dosing across indications, optimizing patient compliance and limiting drug exposure.16,17
- Dosing flexibility. Consistent PK and PD profiles enable development of linzagolix doses both with and without hormonal ABT, depending on the need for partial or full suppression of estrogen. Different degrees of estrogen suppression may be applied according to the disease phenotype and the final goal of therapy.16,17
- Potential to avoid hormonal ABT. The possibility of partial suppression, not requiring hormonal ABT, could become first-line therapy for many patients. Linzagolix was indeed developed for symptoms associated with endometriosis as a treatment in itself (not needing hormonal ABT). Nevertheless, hormonal ABT may be contraindicated in some patients, while others could be at risk of or have tolerability issues, or simply favor clinical management of their endogenous estrogen concentrations without impacting BMD to a point that would necessitate hormone replacement therapy.16–18,21–23
- Compliance benefits. Linzagolix may have an edge in terms of patient compliance thanks to no known interactions with food, CYP3A4 or OATP1B1/B3 enzyme pathways, and because it can be taken in a single dose anytime during the day at no risk of diminished and/or variable exposure to the active substance.16,17
Linzagolix was recently granted approval by the European Commission for the management of myoma-related heavy menstrual bleeding, and has emerged as the only GnRH antagonist using a non-hormonal approach to meet the needs of women who cannot or do not wish to take hormones.21
Preclinical and Clinical Development of Linzagolix for Endometriosis-Associated Pain
Before in-licensing linzagolix, Kissei conducted preclinical testing, including a Phase 1 clinical trial in healthy females of Japanese and European descent, as well as three-phase 2a clinical trials in subjects of Japanese descent with endometriosis. One of them involved a subgroup of women affected by both endometriosis and uterine fibroids.22 These trials showed that linzagolix had a linear PK profile, suppressed estradiol in a predictable dose-dependent manner, and possessed a well-tolerated dose range that relieved symptoms. After in-licensing by Kissei, an investigational new drug application for linzagolix was submitted and accepted by the FDA in May 2016. In 2019, the EDELWEISS 1 phase 2b clinical trial was completed, prior to initiation of two pivotal Phase 3 clinical trials (EDELWEISS 2 and EDELWEISS 3).
Preclinical Studies and Phase 1 Clinical Trial
Preclinical studies found linzagolix to be a highly potent and selective antagonist of the GnRH receptor. Toxicology and pharmacological safety studies did not raise any tolerance or safety concerns, or drug–drug interaction issues. The phase 1 clinical trial showed linzagolix to have a favorable safety profile and high tolerance at once-daily doses up to 400 mg over seven days. In addition, its PK profile was linear, with a half-life of around 15 hours, and displayed no significant differences between Japanese and European women. Furthermore, linzagolix exhibited low distribution volume, so it remained in the blood rather than accumulating in fatty tissue. Finally, this phase 1 clinical trial ruled out any food interaction. Linzagolix induced dose-dependent reductions in LH and FSH over time, correlating with its ability to regulate estradiol levels according to dose. Based on its low PK variability and absence of dose overlap in the above clinical trial, it was expected that personalized doses of linzagolix would be able to control biological responses more tightly. Indeed, in 2016, a phase 1 trial assessed the potential impact of linzagolix on induction of CYP3A4, which governs most of the metabolism of hormonal ABT. This study did not reveal any meaningful CYP3A4 induction, probably indicating that linzagolix would not disrupt hormonal ABT and is at low risk of drug–drug interactions.
In 2017, a phase 1 PK and PD clinical trial was initiated to evaluate two different ABT doses in patients given 100 mg and 200 mg linzagolix over six weeks. The findings of this study, reported in June 2017, endorsed the ABT dose (1 mg estradiol/0.5 mg norethindrone acetate [NETA]) and use of linzagolix in clinical trials.
Completed Phase 2a Clinical Trials
In 2013 and 2014, Kissei completed three-phase 2a clinical trials on linzagolix in Japanese women suffering from endometriosis. The trials (KLH1201, KLH1202 and KLH1203), assessing doses of 50, 75, 100 and 200 mg linzagolix or a placebo, found that linzagolix reduced endometriosis-associated pain and suppressed estradiol according to dose.22,23 These studies provided the framework (design and dose selection) for the phase 2b EDELWEISS 1 trial.
Completed Phase 2b EDELWEISS 1 Clinical Trial Investigating Endometriosis-Associated Pain
In 2019, the phase 2b EDELWEISS 1 clinical trial was completed in patients with endometriosis, having recruited women with moderate-to-severe endometriosis-related pain from 64 gynecological centers across the US and Europe.18 The study included a screening period, followed by two consecutive 12-week treatment durations (part A and part B). There was then an optional 28-week treatment extension phase or, for those not participating further, a post-trial follow-up of 24 weeks or longer. Altogether, 328 subjects were randomized to 1 of 6 treatment groups, namely placebo, fixed-dose at 50 mg, 75 mg, 100 mg and 200 mg a day, and 75 mg titrated dose. In the first group, the placebo was administered over the course of 12 weeks (part A), after which all placebo subjects were switched to active treatment (100 mg daily) for another 12 weeks (part B). Patients in the titrated-dose arm all started on 75 mg a day for 12 weeks (part A), with their dose titrated up to 100 mg, down to 50 mg, or remaining the same (75 mg) for the next 12 weeks (part B) based on mean serum estradiol levels determined at weeks 4 and 8. Most (71%) patients completing the 24-week treatment course did opt for treatment extension and were given linzagolix for a further 28 weeks. Subjects in the 200 mg group received 100 mg linzagolix a day during the extension period. Women in all other groups maintained the same treatment they were given at the end of part B.
Menstrual dysmenorrhea (DYS) and non-menstrual pelvic pain (NMPP) were evaluated using a 4-point verbal rating scale (VRS) and an 11-point numeric rating scale (NRS). The primary endpoint of this EDELWEISS trial was a responder analysis, where satisfactory responses were defined as a drop of at least 30% in combined DYS and NMPP severity. These scores were logged daily and assessed through an electronic diary recording a VRS of 0 (no pain) through to 3 (severe pain) over the last 28 days of treatment. The main secondary safety endpoint was BMD after 24 weeks of treatment investigated by dual-energy x-ray absorptiometry (DXA).
The EDELWEISS clinical trial achieved its primary endpoint, yielding a statistically significant difference in the linzagolix response rate compared to the placebo after 12 weeks of therapy. Patient response rates were 34.5% with the placebo, 61.5% with 75 mg linzagolix, and 56.3% with 200 mg linzagolix18 (Figure 3).
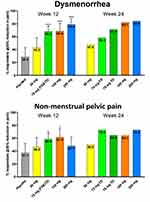 |
Figure 3 Responder rates for overall dysmenorrhea and non-menstrual pelvic pain at 12 and 24 weeks. Notes: Responders were defined as women with a 30% decrease in the mean 28-day pain score compared with baseline. The 75 mg fixed dose (FD) and titrated-dose (TD) groups were pooled for analysis at 12 weeks. Percentage responders at week 12 were compared to placebo (***p < 0.001; *p < 0.05). No statistical testing was performed at week 24 because there was no placebo control. Adapted from Fertil Steril, 114(1), Donnez J, Taylor HS, Taylor RN, et al, Treatment of endometriosis-associated pain with linzagolix, an oral gonadotropin-releasing hormone–antagonist: a randomized clinical trial, 44-55, Copyright 2020, with permission from Elsevier.18 |
With regard to DYS in the VRS ranking, subjects receiving 200 mg reported the best responder rate at 78.9% versus the placebo at just 28.5%. Responses to doses of 75 mg and above were statistically highly significant. In terms of the NMPP VRS endpoint, responder rates were statistically significant at doses of 75 and 100 mg, and both showed similar responder rates at 58.5% and 61.5%, respectively.
Moreover, doses of 75, 100 and 200 mg linzagolix were found to alleviate dyschezia and enhance overall patient well-being, as determined by the Endometriosis Health Profile-30 (EHP-30) score, Patient Global Impression of Change (PGIC) scale, Patient Global Impression of Severity (PGIS), perceived activity impairment, and the modified Biberoglu & Behrman score. DYS was also assuaged with all regimes, reaching statistical significance with administration of 200 mg of the drug.
On the whole, the impact of treatment observed at week 12 was maintained or had further improved with all linzagolix doses by week 24 and was typically sustained until week 52.18 Linzagolix therapy displayed clinical benefits over 52 weeks of continuous daily administration, providing relief from endometriosis-associated pain. The greatest benefits were reaped by patients given doses of 75 mg and above. Significant relief from pelvic pain was noted by week 12 and maintained or enhanced by weeks 24 and 52. This long-term linzagolix treatment yielded sustained respite from DYS, NMPP, dyspareunia and dyschezia, as well as improved quality of life and patient assessments of disease severity.
The critical safety endpoint for linzagolix is BMD loss due to estradiol suppression. With the 75 mg treatment protocol, mean BMD loss in the lumbar spine was −0.798% at 6 months, the lower limit of the 95% confidence interval for BMD decline from baseline to week 24 standing at −1.57%. We therefore feel that this regimen, with an acceptable benefit/risk ratio, could be applied chronically without the need for hormonal ABT. In the 200 mg linzagolix group, however, mean BMD in the lumbar spine dropped by more than −2.5% after 6 months of treatment, showing that high doses of linzagolix must be combined with hormonal ABT.
Long-term linzagolix administration was well tolerated for up to one year. Consistent with its therapeutic class and mechanism of action, the most commonly reported treatment-emergent adverse event was hot flushes, which was more often encountered at higher doses. Changes in BMD between baseline and week 52, evaluated by DXA scans, were in line with values recorded after 24 weeks of treatment. BMD loss in the 75 mg linzagolix group was within an acceptable range, while the decline with the 200/100 mg regimen was clinically relevant. For corroborative testing, the 200 mg dose was combined with estrogen/progestogen ABT (1 mg estradiol/0.5 mg NETA) to prevent significant BMD loss during continuous administration.
Ongoing Phase 3 EDELWEISS 3 Clinical Trial Investigating Endometriosis-Associated Pain
May 2019 saw the beginning of the phase 3 program, initially involving two clinical trials: EDELWEISS 2, with approximately 450 subjects (150 per arm) in the US and Puerto Rico, and EDELWEISS 3, also with around 450 subjects (150 per arm) across sites in the US, Canada, Europe and CIS countries. These two double-blind, placebo-controlled trials sought to assess two once-daily doses of linzagolix, namely 75 mg without hormonal ABT, and 200 mg with hormonal ABT (Figure 4). Patients chronicled their pain levels in an electronic diary every day.
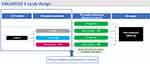 |
Figure 4 EDELWEISS 3 is a randomized, double-blind, placebo-controlled, multicenter phase 3 trial of linzagolix in women with moderate-to-severe endometriosis-associated pain. |
After 24 weeks of evaluation, subjects were able to opt for treatment extension. In this extended protocol, patients given a placebo were randomly assigned to either 75 mg linzagolix without hormonal ABT or 200 mg with hormonal ABT, while those on active linzagolix regimens continued with their respective doses. Co-primary endpoints were responder analyses of both DYS and NMPP after 12 weeks of therapy. Upon completion of treatment, all patients were followed for at least another 24 weeks.
In January 2021, ObsEva announced that they would be discontinuing EDELWEISS 2 and its clinical extension trial because of challenges in patient screening and recruitment, as well as difficulties relating to the ongoing coronavirus pandemic.
As stated above, the phase 3 EDELWEISS 3 trial in women with moderate-to-severe endometriosis-related pain tested two doses of linzagolix, a once-daily 200 mg dose in association with hormonal ABT, and a 75 mg dose without ABT.24,25 The 200 mg regimen met the co-primary objectives in terms of efficacy, demonstrating a decline in DYS and NMPP by 3 months of therapy. By 6 months, statistically significant and clinically meaningful improvements were noted in the first five secondary endpoints, namely DYS, NMPP, dyschezia, overall pelvic pain, and ability to conduct normal daily activities (Figure 5). The 75 mg regimen without hormonal ABT showed a statistically significant alleviation of DYS compared to the placebo after 3 months. However, while this dose afforded some improvement in NMPP by 3 months, it was not statistically significant versus the placebo so did not meet the co-primary efficacy objective. The co-primary efficacy endpoints for DYS and NMPP were dependent on patient-reported symptoms that were recorded daily in an electronic diary using a VRS from 0 (no pain) to 3 (severe pain). Responder thresholds for monthly pain ratings were a drop of at least 1.1 for DYS and 0.8 for NMPP. A p-value of <0.05 denotes a significant difference from the placebo. At 6 months, DYS responder rates were 80% with 200 mg linzagolix plus hormonal ABT (p < 0.001), 49.5% with 75 mg linzagolix (p < 0.001), and 23.5% with the placebo. NMPP responder rates were 57.1% with 200 mg linzagolix plus hormonal ABT (p = 0.003), 52.2% with 75 mg linzagolix (p = 0.036), and 38.5% with the placebo. By 52 weeks, both the 200 mg dose with ABT and the 75 mg dose yielded significant improvements in both co-primary efficacy endpoints (DYS and NMPP).
Secondary Endpoints at 6 Months
The 200 mg linzagolix dose plus hormonal ABT achieved statistical significance in terms of the first five secondary endpoints versus the placebo. By 6 months, improvements were observed in DYS, NMPP, dyschezia, overall pelvic pain, and the ability to complete everyday tasks. The 75 mg protocol also showed favorable results.24
Safety Results at 6 Months (Figure 6)
In phase 3 of the EDELWEISS 3 clinical trial, linzagolix was found to be well tolerated on the whole, with minimal BMD loss in the lumbar spine: 0.79% with 200 mg linzagolix plus hormonal ABT and 0.89% with 75 mg linzagolix (25). Adverse events were noted in just over 5% of subjects in any active treatment arm. They included headaches (10.5%, 8.1%, and 8.0%), hot flushes (6.8%, 7.5%, and 2.5%), and fatigue (6.8%, 3.8%, and 2.5%) with 200 mg linzagolix plus hormonal ABT, 75 mg linzagolix, and placebo administration, respectively.
Discussion and Conclusions
Endometriosis requires a life-long management plan, with a therapeutic approach making the most of medical therapy to avoid repeated surgical procedures.2,4 The notion of progesterone resistance should be considered an explanation for why 33% of patients do not respond to OCPs and progestogens.2 As stated earlier, there is a pressing need for further treatment options and a number of papers have reported results from clinical trials on three potentially effective oral GnRH antagonists (see 4 for review). As demonstrated in the present paper, linzagolix at different doses (75 mg and 200mg with or without ABT) suppresses ovarian function in a dose-dependent manner. It also modulates estradiol levels which, according to the threshold hypothesis,26 may provide relief from endometriosis-associated pain while mitigating side effects caused by extreme hypoestrogenism. It would, of course, be useful to have results from comparative studies with estro-progestogens or progestogens to ascertain whether GnRH antagonists actually have significant benefits over traditional first-line medications. Pragmatic trials investigating their impact on pain symptoms, quality of life, side effects, tolerability and treatment adherence need to be conducted. In case of peritoneal endometriosis-related symptoms, we fully support use of first-line therapy (OCPs or progestogens). However, if these treatments fail, GnRH antagonists could well step up to the task, but future studies should confirm the legitimacy of this approach.2,4
The debate around the best strategy in case of endometriomas has recently become a hot topic, and women with endometrioma-related infertility face a dilemma when choosing appropriate therapy: surgery or in vitro fertilization?.27,28 In fact, ovarian endometriomas respond poorly to medical therapy, essentially due to their anatomical structure.2,4 Frequent inflammation of the ovarian stroma surrounding endometriomas is responsible for depleting the ovarian reserve29,30 and may represent one avenue of research into a medical approach. Recent guidelines31 and reviews32 have reported lower recurrence rates when medical therapy is associated postoperatively, so this option needs to be investigated further in clinical trials. Comparisons should be made between use of progestogens and new strategies, like those involving GnRH antagonists.
Deep nodular endometriotic lesions should be defined as nodules measuring at least 2–3 cm fixed to the posterior part of the cervix33 and often related to severe DYS and dyspareunia. Since their progression is slow, it is difficult to establish exactly when they developed.4, 33–36 The response of deep endometriotic nodules to medical therapy (progestogens or OCPs) is a source of controversy, and a recent review by Reis et al37 suggested that deep lesions are more resistant to size regression upon medical treatment, essentially due to their widely reported and proven progesterone resistance.2 There is no doubt that surgery plays a key role in the management of symptomatic deep endometriosis.33
It is widely known that preoperative therapy with GnRH agonist is able to decrease the size of lesions and surrounding inflammation and infiltration, facilitating less aggressive surgery and avoiding bowel resection.33 Preliminary results from an ongoing study into the impact of GnRH antagonist now prove a reduction in the size of deep lesions, offering a possible alternative to aggressive surgery. This approach should be systematically adopted in case of pain recurrence after surgery, as repeat surgery is associated with significantly more complications and fewer benefits in terms of pain.33 Other causes, including neuropathic and nociplastic components or central sensitization, must also be considered, since it is sometimes difficult to attribute recurrence of pain directly to recurrence of disease. Further investigations into the benefits of long-term GnRH antagonist therapy in case of recurrence of severe pelvic pain are surely warranted.
In conclusion, appropriate counseling of endometriosis patients is fundamental and healthcare workers need to provide a comprehensive overview of the efficacy and side effects of all available therapies. On the other hand, costs linked to endometriosis are already estimated to be in the region of $69.4 billion per year,38,39 so it is essential to encourage and promote groundbreaking research into medical alternatives that can enhance the quality of life of all affected women. As stressed by both Buggio et al15 and our group,2,4 the costs of any long-term medical treatment need to be carefully balanced. Future studies should focus on comparative investigations with estro-progestogens or progestogens and be designed as superiority trials. Cost-effectiveness and the added value of GnRH antagonists compared to traditional first-line therapies should be addressed and scrutinized, as should their efficacy in women who are poor responders due to the well-documented phenomenon of progesterone resistance.
Acknowledgments
The authors thank Mira Hryniuk, BA, for reviewing the English language of the manuscript and Deborah Godefroidt for her administrative assistance.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the Fonds National de la Recherche Scientifique de Belgique (F.R.S.-FNRS/FRIA FC29657 awarded to L. Cacciottola, and grant 5/4/150/5 awarded to M-M. Dolmans) and the Fondation St Luc.
Disclosure
JD was a member of the SAB of ObsEva until July 31, 2022. The authors report no other conflicts of interest in this work.
References
1. Donnez J. The heterogeneity of endometriotic lesions could be explained by their progesterone resistance. Hum Reprod. 2021;36(9):2624–2625. doi:10.1093/humrep/deab151
2. Donnez J, Dolmans MM. Endometriosis and medical therapy: from progestogens to progesterone resistance to GnRH antagonists: a review. J Clin Med. 2021;10(5):1085. doi:10.3390/jcm10051085
3. Cacciottola L, Donnez J, Dolmans MM. Can endometriosis-related oxidative stress pave the way for new treatment targets? Int J Mol Sci. 2021;22(13):7138. doi:10.3390/ijms22137138
4. Donnez J, Dolmans MM. GnRH antagonists with or without add-back therapy: a new alternative in the management of endometriosis? Int J Mol Sci. 2021;22(21):11342. doi:10.3390/ijms222111342
5. Soliman AM, Rahal Y, Robert C, et al. Impact of endometriosis on fatigue and productivity impairment in a cross-sectional survey of Canadian women. J Obstet Gynaecol Can. 2021;43(1):10–18. doi:10.1016/j.jogc.2020.06.022
6. Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366–373.e8. doi:10.1016/j.fertnstert.2011.05.090
7. Vercellini P, Donati A, Ottolini F, et al. A stepped-care approach to symptomatic endometriosis management: a participatory research initiative. Fertil Steril. 2018;109(6):1086–1096. doi:10.1016/j.fertnstert.2018.01.037
8. Vercellini P, Buggio L, Frattaruolo MP, Borghi A, Dridi D, Somigliana E. Medical treatment of endometriosis-related pain. Best Pract Res Clin Obstet Gynaecol. 2018;51:68–91. doi:10.1016/j.bpobgyn.2018.01.015
9. Casper RF. Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil Steril. 2017;107(3):533–536. doi:10.1016/j.fertnstert.2017.01.003
10. Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68(4):585–596. doi:10.1016/S0015-0282(97)00191-X
11. Bulun SE, Yilmaz BD, Sison C, et al. Endometriosis. Endocr Rev. 2019;40(4):1048–1079. doi:10.1210/er.2018-00242
12. Bulun SE, Cheng YH, Pavone ME, et al. 17Beta-hydroxysteroid dehydrogenase-2 deficiency and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):44–50. doi:10.1055/s-0029-1242992
13. Yilmaz BD, Bulun SE. Endometriosis and nuclear receptors. Hum Reprod Update. 2019;25(4):473–485. doi:10.1093/humupd/dmz005
14. Donnez J, Cacciottola L. Endometriosis: an inflammatory disease that requires new therapeutic options. Int J Mol Sci. 2022;23(3):1518. doi:10.3390/ijms23031518
15. Barbara G, Buggio L, Facchin F, Vercellini P. Medical treatment for endometriosis: tolerability, quality of life and adherence. Front Glob Womens Health. 2021;2:729601. doi:10.3389/fgwh.2021.729601
16. Pohl O, Baron K, Riggs M, French J, Garcia R, Gotteland JP. A model-based analysis to guide gonadotropin-releasing hormone receptor antagonist use for management of endometriosis. Br J Clin Pharmacol. 2022;88(5):2359–2371. doi:10.1111/bcp.15171
17. Donnez J, Taylor RN, Taylor HS. Partial suppression of estradiol: a new strategy in endometriosis management? Fertil Steril. 2017;107(3):568–570. doi:10.1016/j.fertnstert.2017.01.013
18. Donnez J, Taylor HS, Taylor RN, et al. Treatment of endometriosis-associated pain with linzagolix, an oral gonadotropin-releasing hormone-antagonist: a randomized clinical trial. Fertil Steril. 2020;114(1):44–55. doi:10.1016/j.fertnstert.2020.02.114
19. Lamb YN. Elagolix: first global approval. Drugs. 2018;78(14):1501–1508. doi:10.1007/s40265-018-0977-4
20. Markham A. Relugolix: first global approval. Drugs. 2019;79(6):675–679. doi:10.1007/s40265-019-01105-0
21. Yselty. European medicines agency (Europa.eu); 2012.
22. Pohl O, Marchand L, Bell D, Gotteland JP. Effects of combined GnRH receptor antagonist linzagolix and hormonal add-back therapy on vaginal bleeding-delayed add-back onset does not improve bleeding pattern. Reprod Sci. 2020;27(4):988–995. doi:10.1007/s43032-020-00172-z
23. Tezuka M, Tamai Y, Kuramochi Y, Kobayashi K, Fushimi N, Kiguchi S. Pharmacological characterization of linzagolix, a novel, orally active, non-peptide antagonist of gonadotropin-releasing hormone receptors. Clin Exp Pharmacol Physiol. 2022;49(10):1082–1093. doi:10.1111/1440-1681.13688
24. Taylor H, Gemzell-Danielsson K. Linzagolix for endometriosis-associated pain: efficacy results from Edelweiss 3, a phase 3, randomized, double blind, placebo-controlled trial
25. Donnez J, Taylor H, Gemzell-Danielsson H, et al. O-306 Linzagolix for endometriosis-associated pain: safety results from Edelweiss 3, a phase 3, randomized, double-blind, placebo-controlled trial. Hum Reprod. 2022;37(Supplement_1):deac105. doi:10.1093/humrep/deac105.103
26. Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166(2):740–745. doi:10.1016/0002-9378(92)91706-G
27. Lessey BA, Gordts S, Donnez O, et al. Ovarian endometriosis and infertility: in vitro fertilization (IVF) or surgery as the first approach? Fertil Steril. 2018;110(7):1218–1226. doi:10.1016/j.fertnstert.2018.10.003
28. Donnez J. Women with endometrioma-related infertility face a dilemma when choosing the appropriate therapy: surgery or in vitro fertilization. Fertil Steril. 2018;110(7):1216–1217. doi:10.1016/j.fertnstert.2018.10.002
29. Kitajima M, Defrère S, Dolmans MM, et al. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil Steril. 2011;96(3):685–691. doi:10.1016/j.fertnstert.2011.06.064
30. Kitajima M, Dolmans MM, Donnez O, Masuzaki H, Soares M, Donnez J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil Steril. 2014;101(4):1031–1037. doi:10.1016/j.fertnstert.2013.12.049
31. Becker CM, Bokor A, Heikinheimo O, et al. ESHRE guideline: endometriosis. Hum Reprod Open. 2022;2022(2):hoac009. doi:10.1093/hropen/hoac009
32. Capezzuoli T, Vannuccini S, Mautone D, et al. Long-term hormonal treatment reduces repetitive surgery for endometriosis recurrence. Reprod Biomed Online. 2021;42(2):451–456. doi:10.1016/j.rbmo.2020.09.018
33. Donnez O, Donnez J. Deep endometriosis: the place of laparoscopic shaving. Best Pract Res Clin Obstet Gynaecol. 2021;71:100–113. doi:10.1016/j.bpobgyn.2020.05.006
34. Koninckx PR, Ussia A, Adamyan L, Wattiez A, Gomel V, Martin DC. Pathogenesis of endometriosis: the genetic/epigenetic theory. Fertil Steril. 2019;111(2):327–340. doi:10.1016/j.fertnstert.2018.10.013
35. Donnez O, Orellana R, Van Kerk O, Dehoux JP, Donnez J, Dolmans MM. Invasion process of induced deep nodular endometriosis in an experimental baboon model: similarities with collective cell migration? Fertil Steril. 2015;104(2):491–7.e2. doi:10.1016/j.fertnstert.2015.05.011
36. Koninckx PR, Ussia A, Adamyan L, Wattiez A, Donnez J. Deep endometriosis: definition, diagnosis, and treatment. Fertil Steril. 2012;98(3):564–571. doi:10.1016/j.fertnstert.2012.07.1061
37. Reis FM, Coutinho LM, Vannuccini S, Batteux F, Chapron C, Petraglia F. Progesterone receptor ligands for the treatment of endometriosis: the mechanisms behind therapeutic success and failure. Hum Reprod Update. 2020;26(4):565–585. doi:10.1093/humupd/dmaa009
38. Soliman AM, Yang H, Du EX, Kelley C, Winkel C. The direct and indirect costs associated with endometriosis: a systematic literature review. Hum Reprod. 2016;31(4):712–722. doi:10.1093/humrep/dev335
39. Soliman AM, Surrey E, Bonafede M, Nelson JK, Castelli-Haley J. Real-world evaluation of direct and indirect economic burden among endometriosis patients in the United States. Adv Ther. 2018;35(3):408–423. doi:10.1007/s12325-018-0667-3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


