Back to Journals » Drug Design, Development and Therapy » Volume 17
Profile of Lasmiditan in the Acute Treatment of Migraine in Adults: Design, Development, and Place in Therapy
Authors Anderson CC, VanderPluym JH
Received 6 April 2023
Accepted for publication 26 June 2023
Published 3 July 2023 Volume 2023:17 Pages 1979—1993
DOI https://doi.org/10.2147/DDDT.S380440
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Christopher C Anderson,* Juliana H VanderPluym*
Department of Neurology, Mayo Clinic, Scottsdale, AZ, USA
*These authors contributed equally to this work
Correspondence: Juliana H VanderPluym, Department of Neurology, Mayo Clinic, 13400 E Shea Blvd, Scottsdale, AZ, 85259, USA, Tel +1 480-301-8000, Email [email protected]
Abstract: Migraine is a common neurological disorder that is present in a large proportion of the global population. It is estimated to occur in around 20.7% of women and 10.7% of men in the United States. The pathophysiology of migraine is a major focus of research, and medications have been developed to interrupt the processes that generate headache and other bothersome symptoms of migraine attacks. The triptan class of medications acts as a direct agonist at the 5-HT1B/D receptor but its use is limited by contraindications for those with coronary or cerebrovascular disease. Lasmiditan is a first-in-class agonist at the 5-HT1F serotonin receptor that does not appear to generate vasoconstriction. This article reviews the design, development, and place in therapy for lasmiditan. A narrative review of the literature using the Ovid MEDLINE database was performed. The rationale behind the development of lasmiditan and pre-clinical, proof-of-concept, Phase II, pivotal, Phase III trials and post-hoc data is covered. Additionally, the efficacy and safety of lasmiditan when compared to other acute treatments in migraine is described, including lasmiditan’s side effect profile and status as a Schedule V substance. Further, head-to-head studies of lasmiditan compared with other acute treatments are required.
Keywords: lasmiditan, ditan, 5-HT1F agonist, migraine, rescue therapy, acute treatment
Introduction
Migraine is a common neurological disorder that is present in a large proportion of the global population (~958.8 million).1 Based on epidemiologic data, the prevalence of migraine is about 20.7% in women and 10.7% in men in the United States.2
The International Classification of Headache Disorders-3 defines migraine as a recurrent headache disorder with acute attacks lasting between 4 and 72 hours with at least two of the following symptoms: unilateral location, pulsating quality, moderate or severe pain intensity, and aggravation by or causing avoidance of routine physical activity. Additionally, migraine should be accompanied by either nausea and/or vomiting as well as photophobia and phonophobia.3
The pathophysiology of migraine has been an important topic of investigation. The now outdated vascular theory of migraine pathophysiology proposed that migraine pain was generated by the vasodilation of intra- and extracranial vessels. As a result, migraine was often referred to by the term “vascular” headache. Acute treatment of migraine was, therefore, tailored to counteract this abnormality by inducing vasoconstriction. The quintessential agent used to promote vasoconstriction was ergotamine, which has alpha-adrenergic and serotonergic agonism at therapeutic doses but also has activity at several other receptors.3 The triptan class of medication was produced with the intention of reducing the pain and disability associated with “vascular” headaches but also by being more selective for and providing direct agonism to the 5-HT1 (5-hydroxytryptamine) class of serotonin receptors, particularly 5-HT 1B/1D receptors. In 1992, sumatriptan was the first triptan medication approved by the FDA for use in acute treatment of migraine with or without aura. Per formulary instructions, sumatriptan is contraindicated in those with coronary artery disease, coronary artery vasospasm, Wolff-Parkinson-White or other cardiac accessory conduction pathway disorders, history of stroke, transient ischemic attack, hemiplegic or basilar migraine, peripheral vascular disease, ischemic bowel disease, uncontrolled hypertension, concurrent use of ergotamine derivatives or MAO-inhibitors, allergy to sumatriptan or its metabolites, and severe liver disease that would interfere with the drug’s metabolism.4 Additional triptans were approved including zolmitriptan (1997), rizatriptan (1998), naratriptan (1998), almotriptan (2001), frovatriptan (2001), and eletriptan (2002), all of which have similar contraindications for vascular diseases.
The vascular theory of migraine pathophysiology has been challenged after studies determined that vascular changes are more likely a product of the migraine disease process rather than a feature that generates pain and that the changes are not always present in those with migraine-related headache pain.5,6 Instead, it appears more likely that migraine is a primary disorder of neuronal sensory processing that involves a genetic predisposition to neuronal hyperexcitability, which reduces the threshold by which allostatic load (physiologic stress) on certain hypothalamic and trigeminal centers generate paroxysmal cranial pain. This then results in subsequent sensitization of the trigeminocervical complex (TCC) due to sterile neurogenic neuroinflammatory changes at both the central and peripheral portions of the trigeminovascular system that can lead to “chronification” of acute pain in susceptible individuals.7–9
Concern for causing harm limits the use of triptans and ergots for those with vascular disease. One study of 230,000 patients with migraine found that at least 20% of commercially insured US patients with migraine have cardiovascular disease that likely contraindicates the use of triptans.10 As a result, there has been an ongoing need for rescue therapies that do not have a risk of vasoconstriction.
Lasmiditan is the first of the “ditan” class of medications, which may be an opportunity to fulfill the need for rescue therapies for patients with vascular contraindications. The current article is a narrative review to examine the design, development, and current place in therapy for lasmiditan.
Literature Search Strategy
This narrative review was informed by a systematic search of the literature carried out in the Ovid MEDLINE database on 12/8/2022. The following search terms were included: “Reyvow”, “Lasmiditan”, “Ditans”, “5-HT1F agonist”, “migraine”, “pain management”, and “quality of life”. Hand-search of references within the accepted papers was performed from study inception until 2/28/2023. Only studies published in English were eligible for inclusion in the review. One author (CA) screened the results for inclusion.
Lasmiditan: Design and Development
Chemical Properties
Lasmiditan hemisuccinate’s chemical name is 2,4,6-trifluoro-N-[6-(1-methylpiperidine-4-carbonyl)pyridine-2-yl]benzamide hemisuccinate (Figure 1).11 This compound exhibits a pyridinoyl-piperidine scaffold, which is not found in other anti-migraine rescue therapies and is thought to contribute to its unique properties.12 The structure confers a lipophilic nature to the compound and allows it to cross the blood–brain barrier to a larger degree than triptans and likely plays a role in the drug’s intended and unintended effects on the central nervous system.13
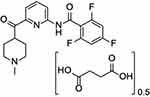 |
Figure 1 Biochemical structure of Lasmiditan. This compound exhibits a pyridinoyl-piperidine scaffold, which is not found in other anti-migraine rescue therapies and is thought to contribute to its unique properties.11 |
Lasmiditan hemisuccinate is a white, crystalline powder which is sparingly soluble in water, slightly soluble in ethanol, and soluble in methanol. Lasmiditan tablets contain the active ingredient as well as several inactive ingredients including croscarmellose sodium, magnesium stearate, microcrystalline cellulose, pregelatinized starch, sodium lauryl sulfate.
Mechanism of Action
Lasmiditan is a selective 5-HT1F direct agonist. 5-HT1F is distributed throughout the nervous system with high concentrations within the meninges, trigeminal ganglion, trigeminal nucleus caudalis, hypothalamus, thalamus, and cortex as well as other brain sites.14 While the precise mechanism of action is unknown, the medication’s 5-HT1F agonism is thought to contribute most of its clinical effect. It is thought that presynaptic binding of lasmiditan to nerve terminals at peripheral trigeminovascular sites leads to a reduction in calcitonin gene-related peptide (CGRP) release. CGRP appears to play a role in migraine generation. Centrally, lasmiditan likely acts at trigeminal, thalamic, and cortical sites to reduce glutamatergic tone via stimulation of inhibitory 5-HT1F receptors on glutamatergic neurons that are thought to induce central sensitization.14
5-HT1F receptor subtype was isolated in 1992 using a collection of degenerate polymerase chain reaction primers from the 5-HT1A, 5-HT1C, 5-HT2, and 5-HTD receptors and was found to have chemical properties similar to other 5-HT receptors, including the ability to inhibit adenylate cyclase and thus reduce cAMP concentration intracellularly. However, the receptor demonstrated a unique profile when it came to affinity for commonly used serotonergic ligands and received a designated subtype.15 The 5-HT1F receptor was noted to be localized only to the brain, uterus, and myometrium but not within the heart, testis, pancreas, liver, kidney, or spleen. To further define central nervous system 5-HT1F location, Castro et al utilized radiolabeled sumatriptan in the presence of 5-carboxamidotryptamine to identify 5-HT1F receptors within the human brain. Results indicated that 5-HT1F receptors accounted for 67.5% of 5-HT binding within Layer V of the cerebral cortex as well as 53.4% of 5-HT binding within the substantia gelatinosa of the upper cervical spinal cord and 39.5% of binding in the trigeminal nucleus (pars caudalis). Lesser degrees of binding were also present within the tractus solitarius, basal nuclei, and periaqueductal gray regions.16
In 1996, work from Bouchelet et al identified 5-HT1F present in both the trigeminal ganglia, distal vessels of the basal circulation, and pial vasculature in the human brain.17 When the authors later investigated the localization of specific 5-HT subtypes within the microvascular bed, they found that 5-HT1F was not found within human brain endothelial or smooth muscle cultures but rather was found in human astrocyte culture. The authors concluded that non-neuronal 5-HT1F receptors in the human cerebral cortex microvasculature are glial rather than vascular in origin.18 Additionally, researchers were able to determine that human cerebral arteries do not exhibit dose-dependent vasoconstriction in response to the selective 5-HT1F receptor agonist LY344864 when compared to sumatriptan. Within the same publication, the authors noted that 5-HT1F mRNA was detected within human coronary arteries but to a much weaker degree compared to 5-HT1B and was even absent in some cases. The authors concluded that while this finding could potentially be explained by post-mortem delay and RNAse activity, they suspected that intersubject variability in receptor expression was a more likely explanation.19
Given the lack of evidence that 5-HT1F receptor activation produces vasoconstriction, this target was pursued for therapeutic use in those with contraindications to triptans and ergots. Several compounds were created, but only lasmiditan was considered safe for use.
In 2010, Nelson et al demonstrated that lasmiditan (COL-144, LY573144) was highly selective for the 5-HT1F serotonin receptor compared to 5-HT1B and 5-HT1D serotonin receptors with a selectivity ratio of greater than 480-fold.12 They also demonstrated that lasmiditan had a higher selectivity for the 5-HT1F receptor compared to prior compounds (eg, LY344864). Additionally, they reported that lasmiditan did not vasoconstrict rabbit saphenous vein (surrogate for human coronary arteries) and also that the compound prevented the release of inflammatory markers associated with trigeminal ganglion stimulation in a rodent model.12
Pharmacokinetic Properties
Lasmiditan is absorbed rapidly with a median Tmax of 1.8 hours. When co-administered with a high-fat meal, the mean lasmiditan Cmax and AUC values increased by 22% and 19%, respectively, and delayed the median Tmax by 1 hour yet this was not expected to impact the clinical effect of the medication. Within the body, lasmiditan is moderately protein bound at between 55% and 60% and is independent of concentration between 15 and 500 ng/mL. The drug is eliminated geometrically with a mean half-life value of 5.7 hours. There is no observed accumulation of lasmiditan with daily dosing. Lasmiditan is eliminated primarily by metabolism to inactive metabolites by both hepatic and extrahepatic mechanisms. In particular, lasmiditan is metabolized to M7 and M18 (M7 + M8 pathways). There is currently no evidence that lasmiditan is metabolized by MAO-A, MAO-B, flavin monooxygenase 3, CYP450 reductase, xanthine oxidase, alcohol dehydrogenase, aldehyde dehydrogenase, or aldo-keto reductase. Renal excretion is a minor route of lasmiditan elimination, and very low amounts of lasmiditan are recovered unchanged in urine (3% of the dose). The inactive metabolites, however, are recovered in the urine, of which S-M8 represents 66% of the dose in urine within 48 hours post-administration of the drug.11,20
Efficacy Data
Efficacy of lasmiditan has been determined based on findings from multiple studies, including pre-clinical, Phase II and phase III clinical trials (Table 1).
 |
Table 1 Efficacy of Lasmiditan Clinical Studies in Acute Migraine |
In 2010, Ferrari et al reported results from a randomized, placebo-controlled, double-blind, proof-of-concept and dose-finding study. This study enrolled 130 subjects and was randomized to receive either intravenous lasmiditan or placebo. Doses ranged from 2.5 mg to 45 mg. The authors reported that doses 20 mg or higher were effective for the treatment of migraine at 2 hours post-dose. The investigators reported that adverse events occurred in 65% of subjects receiving IV lasmiditan vs 43% who received placebo with dizziness, paresthesias, and limb heaviness as most common.21 They noted that further studies were needed to determine the safety and efficacy of oral lasmiditan.
Phase II Studies
The first phase II trial was performed by Farkkila et al in 2012. Their study was a double-blind, dose-ranging study across 43 headache centers across Europe. In their study, 391 total subjects received treatment with 86 patients receiving placebo and 305 receiving lasmiditan. Subjects were randomized to receive 50 mg, 100 mg, 200 mg, and 400 mg in the treatment arm. The investigators reported that every lasmiditan dose resulted in a significant improvement in headache pain at 2 hours compared to placebo with a linear association: 50 mg induced a 17.9% difference, 100 mg induced 38.2% difference, 200 mg induced a 28.8% difference, and 400 mg induced a 38.7% difference. However, higher doses were associated with an increased proportion of adverse events: (53/82 [65%] for 50 mg, 59/82 [72%] for 100 mg, 61/71 [86%] for 200 mg, and 59/70 [84%] for 400 mg dose vs 19/86 [22%] with placebo). The most common adverse events were dizziness, fatigue, vertigo, paresthesias, and somnolence. More severe adverse events were noted at higher doses: 16/82 [20%] at 50 mg, 23/82 [28%] at 100 mg, 28/71 [39%] at 200 mg, and 31/70 [44%] at 400 mg compared with 5/86 [6%] with placebo.22 The authors concluded that the 50 mg and 100 mg oral doses appeared to provide adequate headache relief at 2 hours without significantly heightening the risk of severe adverse effects, making those doses good candidates for future study.
The MONONOFU Trial
In 2020, Sakai et al performed a second, major phase II trial that was performed in Japan. This study was called RandoMized, DOuble-bliNd, PlacebO-coNtrolled Trial Of Lasmiditan in a Single Migraine Attack in Japanese Patients SuFfering From Migraine With or WithoUt Aura or MONONOFU Trial (NCT03962738). This was a multicenter, randomized, double-blind, placebo-controlled trial that sought to enroll adults diagnosed with migraine with and without aura and test the efficacy and safety of lasmiditan. The primary endpoint was pain freedom at 2 hours post-dose. This trial included 846 participants who were randomized to receive lasmiditan 50 mg, 100 mg, 200 mg, or placebo in a 7:3:7:6 ratio. Subjects were asked to rate the treatment response within 4 hours of dosing for treatment of their single moderate-to-severe attack.
In their study, the authors found that subjects receiving lasmiditan 200 mg and 100 mg doses exhibited statistically significant pain freedom at 2 hours compared with placebo (200 mg: 40.8%, 73/179; OR 3.46, 95% CI 2.17–5.54, p < 0.001; 100 mg: 32.4%, 67/207, OR 2.41, 95% CI 1.51–3.83, p < 0.001; placebo: 16.6%, 35/211). Those who received the 50 mg dose did not have a statistically significant response compared with placebo. For secondary endpoints, pain relief at 2 hours was significant for the 200 mg (p < 0.001), 100 mg (p < 0.001), and 50 mg doses (p = 0.037). Additionally, relief from most bothersome symptom was significant for the 200 mg (p = 0.023) and 100 mg (p = 0.04) doses but not the 50 mg dose. Similarly, sustained pain-freedom was only statistically significant for the 200 mg and 100 mg doses at 24- and 48 hours post-dose. Adverse events included dizziness (39.4%; 188/477), somnolence (19.3%, 92/477), and malaise (10.5%, 50/477). Adverse events were more commonly experienced by subjects receiving lasmiditan compared with placebo (23.4%), and those who received the 200 mg dose (80.8%), and 100 mg dose (70.7%) experienced a higher likelihood of adverse events compared to those having received the 50 mg dose (50.6%). There were no major adverse cardiovascular events during this trial.26 Post-hoc analysis demonstrated that there were no significant differences in patient characteristics or migraine characteristics with regard to efficacy of the 50 mg, 100 mg, 200 mg doses and that the 200 mg dose appeared to be the most efficacious in this study population.27
Pivotal Trials
The SAMURAI Trial
In 2018, results from A Study of Two Doses of LAsMiditan (100 mg and 200 mg) Compared to Placebo in the AcUte Treatment of MigRAIne: A Randomized, Double-blind, Placebo-controlled Parallel Group Study or SAMURAI trial (NCT02439320) were published by Kuca et al. In their pivotal study, the investigators enrolled 1866 to participate from 99 centers across the United States who were then randomized to receive either lasmiditan 200 mg, 100 mg, or placebo. Subjects were also randomized to receive a second dose of either lasmiditan vs placebo. All subjects were given instructions to treat a moderate-to-severe headache that did not appear to be improving the study drug vs placebo within 4 hours of onset. The primary endpoint of the study was the proportion of subjects obtaining pain freedom at 2 hours following the administration of the first 200 mg oral dose. Secondary endpoints included the proportion of subjects obtaining pain freedom at 2 hours following the 100 mg dose, and the proportions of subjects who were free of their most bothersome symptom at 2 hours following either the 200 mg or the 100 mg dose.
In this study, the investigators found that subjects receiving the 200 mg and 100 mg doses experienced pain freedom at 2 hours in 32.2% and 28.2% of cases compared with 15.3% for placebo (200 mg: OR 2.6, 95% CI 2.0–3.6, p < 0.001; 100 mg: OR 2.2, 95% CI 1.6–3.0, p < 0.001). For most bothersome symptom relief at 2 hours, the authors found that 40.7% and 40.9% of subjects achieved relief compared with 29.5% for placebo (200 mg: OR 1.6, 95% CI 1.3–2.1, p < 0.001; 100 mg: OR 1.7, 95% CI, 1.3–2.2, p < 0.001). Most common adverse events included dizziness, fatigue, lethargy, nausea, paresthesias, and somnolence, and at least one adverse event occurred in 42.7% of subjects receiving the lasmiditan 200 mg dose and 36.3% of those receiving the 100 mg dose vs 16.4% for placebo.23
The SPARTAN Trial
In 2019, results from the A Study of Three Doses of Lasmiditan (50 mg, 100 mg and 200 mg) Compared to Placebo in the Acute TReaTment of MigrAiNe: A Randomized, Double-blind, Placebo-controlled Parallel Group Study or SPARTAN (NCT02605174) trial were published by Goadsby et al. In this second pivotal study, the investigators sought to demonstrate the safety and efficacy of three doses of lasmiditan: 50 mg, 100 mg, and 200 mg for the acute treatment of migraine. In total, 2869 subjects were recruited from 125 headache centers across the US, UK, and Germany and were considered eligible for participation, of whom 2583 received either study drug vs placebo. Importantly, subjects with cardiovascular risk factors were enrolled in the SPARTAN trial unlike in the SAMURAI trial where these subjects were excluded.
Subjects were randomized in a 1:1:1:1 fashion to receive lasmiditan 50 mg, 100 mg, 200 mg, or placebo. Similar to the SAMURAI trial, subjects were also randomized to receive a second dose of either lasmiditan vs placebo. Subjects were instructed to treat their next moderate-to-severe headache within 4 hours and were allowed to take a repeat dose of lasmiditan vs placebo up to 24 hours as long as no other rescue medications had been used during that time. Primary endpoint was pain freedom and most bothersome symptom-freedom at 2 hours from administration of any dose. Secondary endpoints included the proportions of subjects who experienced pain improvement (from moderate/severe to mild/none or mild to none); who achieved pain-freedom at 24 hours and 48 hours post-first dose; who had freedom from their most bothersome symptom and headache pain at other time points; who utilized a second dose of the study drug for rescue; and subject global impression of change and level of disability.
In this study, the investigators found that subjects receiving lasmiditan experienced a higher percentage of pain freedom at 2 hours compared with placebo (200 mg: 38.8% OR 2.3, 95% CI 1.8–3.1, p < 0.001; 100 mg: 31.4%, OR 1.7, 95% CI 1.3–2.2, p < 0.001; 50 mg: 28.6%, OR 1.5, 95% CI 1.1–1.9, P = 0.003; placebo: 21.3%). All doses of lasmiditan were also associated with freedom from subjects’ most bothersome symptom at 2 hours (200 mg: 48.7%, OR 1.9, 95% CI 1.4–2.4, p < 0.001; 100 mg: 44.2%, OR 1.6, 95% CI 1.2–2.0, p < 0.001; 50 mg: 40.8%, OR 1.4, 95% CI 1.1–1.8, p = 0.009; placebo: 33.5%). The most common adverse events included dizziness, somnolence, and paresthesias and were present in 253/649 (39.0%), 229/635 (36.1%), and 166/654 (25.4%) of patients receiving lasmiditan 200 mg, 100 mg, and 50 mg, respectively, v 75/645 (11.6%) receiving placebo.24
Phase III Randomized Control Trials
The CENTURION Trial
In 2021, Ashina et al performed a Phase III multicenter, randomized, placebo-controlled trial (NCT03670810) to further investigate first attack efficacy of lasmiditan across four attacks. Subjects were eligible for inclusion if they were 18 years old, met diagnostic ICHD3 criteria for episodic migraine, experienced 3–8 headache days per month, and had a MIDAS score 11. These patients were also required to remain stable on their preventive medication for 3 months. In this study, subjects were randomized to receive lasmiditan 200 mg, 100 mg, or placebo + lasmiditan for their 3rd or 4th attack.
The authors enrolled 1613 subjects with 1471 receiving at least one dose of the study drug. For both the 200 mg and 100 mg doses, therapeutic gain (% difference, 2-hour pain freedom vs placebo) was significant: 2 hours (200 mg vs placebo: 20.9% gain or 29.3% vs 8.4%, OR 4.6, 95% CI 3.1–6.8, p < 0.001; 100 mg vs placebo: 17.4% gain or 25.8% vs 8.4%, OR 3.8, 95% CI 2.6–5.7, p < 0.001). Lasmiditan was found to have a maximum benefit within 4 to 6 hours with separation vs placebo for the 200 mg dose starting as early as 30 minutes post-dose. Both doses demonstrated consistent relief at 24 hours and 48 hours. For other secondary outcomes, lasmiditan was found to be superior to placebo at relieving most bothersome symptom as well as functional disability at 2 hours. The authors also found that those receiving lasmiditan had lower pain recurrence at both the 24- and 48-hour timepoints. Regarding the consistency of response per patient across multiple doses, results indicated that both doses of lasmiditan were superior to placebo at generating both pain relief and pain freedom within 2 hours across 2 of 3 attacks. Finally, for those patients receiving a 50 mg dose of lasmiditan for their 3rd or 4th attack, the investigators found that lasmiditan 50 mg was superior to placebo for pain freedom at 2 hours (50 mg: 19.1% vs 12.0%; p = 0.015) but not pain relief at 2 hours (50 mg: 45.8% vs 39.6%, p = 0.11).25
Long-Term Studies
The GLADIATOR Trial
The GLADIATOR Trial (NCT02565186, An Open-label, LonG-term, Safety Study of LAsmiDItan in the Acute Treatment Of MigRaine) was a Phase III, prospective, randomized, open-label study that was performed to assess the long-term safety and efficacy of lasmiditan. This study analyzed results from 2037 subjects who had completed either one of the previous pivotal trials (SAMURAI or SPARTAN). Subjects were randomized 1:1 to receive either 100 mg or 200 mg of lasmiditan to treat multiple migraine attacks over 1 year. Subjects had a diagnosis of migraine with or without aura based on the ICHD-2 criteria and were required to experience 3–8 attacks per month. Because subjects were recruited from SPARTAN in addition to the SAMURAI, those with cardiovascular risk factors were eligible for participation in GLADIATOR. In this trial, subjects were asked to treat their moderate-to-severe headaches with lasmiditan 100 or 200 mg within 4 hours of pain onset. They were allowed to repeat a dose one time after two hours. While subjects were allowed to use their own rescue medications, triptans, opioids, barbiturates, and ergots were not permitted. Subjects recorded their responses to the medication in an electronic diary at 0 (predose), 0.5, 1, 2, 4, 24, and 48 hours post-dose. Additionally, disability related to migraine was also assessed via MIDAS scoring at 1, 3-, 6-, 9-, and 12-month clinic visits.
Interim results from the GLADIATOR Trial were reported in 2019 and 2020, and full results were published in 2020.28–30 The authors reported that the subjects were followed for a median of 288 days. Prior to the initiation of the trial, subjects scored 29.3 (100 mg) and 28.9 (200 mg) on the MIDAS (severe disability >20) and at the 3-, 6-, 9-, and 12-month timepoints, subjects demonstrated significantly lower levels of disability compared to baseline for both groups. After 1 year, the authors reported that MIDAS scores dropped 12.5 and 12.2 points for the 100 mg and 200 mg doses, respectively, indicating a 49% and 53% response. For secondary measures, the authors reported that there were statistically significant reductions in work/school absenteeism, monthly headache days, and mean headache intensity. Interestingly, there were no significant differences between the 100 mg and 200 mg doses.
Overall, 19,879 migraine attacks were treated with lasmiditan in the GLADIATOR trial analysis. The most common adverse events included dizziness (18.5%), somnolence (8.5%), and paresthesias (6.8%). The authors reported that adverse events were less likely to occur with subsequent attacks. For the 2h post-dose time-point, subjects reported that they were pain free 26.7% and 32.2% of the time for the 100 mg and 200 mg doses, respectively. The authors reported that 52.2% of subjects dropped out of the study and speculated that the cause was likely the burden of e-diary reporting and the 12-hour driving restriction that is required for lasmiditan use. Ultimately, the investigators concluded that lasmiditan was well tolerated and appeared safe and efficacious at the 1-year time point.30
Post-Hoc Studies
Several post-hoc studies have been published based on data from the SAMURAI, SPARTAN, CENTURION, MONONOFU, and GLADIATOR Trials.
Efficacy and Timing
In 2019, an integrated analysis was published from the SAMURAI and SPARTAN data demonstrated that those subjects receiving lasmiditan 100 mg or 200 mg reported statistically significant pain and most bothersome symptom relief as early as 30 minutes post-dose compared with placebo though relief from nausea in particular was not statistically significant.31 Based on further post-hoc analyses from the SAMURAI and SPARTAN trials, it appeared that both pain and most bothersome symptom relief were persistent up to 24–48 hours post-dose with lasmiditan 50 mg, 100 mg, and 200 mg.32 Additionally, to identify predictive response to lasmiditan, Tepper et al analyzed subgroups of patients from the SAMURAI and SPARTAN trials and found no individual patient characteristics, migraine disease characteristics, or migraine attack characteristics accurately predicted headache pain freedom or most bothersome symptom freedom at 2 hours. However, regarding efficacy, the investigators analyzed responses from a specific subgroup of difficult to treat patients (based on headache frequency, duration, MIDAS, BMI, and history of psychiatric disorders) and found that those patients experienced statistically significant outcomes compared to placebo when receiving the 100 mg or 200 mg dose of lasmiditan. Of interest, difficult to treat migraine attacks (severe pain, nausea, delayed treatment >2 hours) also responded to 100 mg and 200 mg of lasmiditan significantly more than placebo with those headaches that had been present >4 prior to treatment responding only to the 200 mg dose (32.4% vs 15.9%, OR 2.7, 95% CI 1.17–6.07, P = 0.018). The only negative predictor was the presence of a “needs complete bed rest” level of disability, which demonstrated no difference between the lasmiditan 200 mg vs placebo groups (25.9% vs 18.5%, OR 1.56, 95% CI 0.96–2.53, P = 0.07).33 Conversely, subgroup analysis focused on headache pain severity found that subjects with mild headache at the time of treatment experienced significantly higher rates of pain freedom at 2 hours, most bothersome symptom freedom at 2 hours, and sustained pain freedom at 24 hours compared with those experiencing moderate-to-severe pain.34
For special populations, MacGregor et al in 2022 analyzed subjects from the MONONOFU and CENTURION trials and found that subjects receiving lasmiditan 200 mg for perimenstrual migraine exhibited statistically significant improvement in pain freedom at all timepoints compared with placebo (200 mg: 37/110, 33.6% vs 6/79, 7.6%, OR 6.04, 95% CI 2.39–15.25, P < 0.001); however, 50 mg and 100 mg doses did not achieve statistical significance. The authors cited small sample size as a probable reason for the lack of significance at lower doses, particularly the 50 mg dose (n = 24).35
Repeat Dose at 2 Hours
With regard to a repeat dose of lasmiditan after 2 hours post-first dose, Loo et al reported that a lower percentage of patients administered a repeat dose when their first dose was lasmiditan compared with placebo. Interestingly, the authors reported that those taking lasmiditan for their first dose had similar pain outcomes and adverse events at 2 hours following a second dose of lasmiditan vs placebo. The authors concluded that there was no clear benefit of repeating a dose of lasmiditan after 2 hours based on patient outcomes from the SAMURAI and SPARTAN trials.36
However, data published by Clemow et al in 2020 suggested that there was a small, but statistically significant increase in response when performing dose escalation with a repeat dose of lasmiditan, ie, taking 50 mg for the first dose and 100 mg or 200 mg for the second dose. Conversely, subjects who started with a higher dose experienced a reduction in pain outcomes when switching to a lower dose. Importantly, the authors reported that there was a positive balance in efficacy vs adverse events when increasing from 50 mg to 100 mg despite an increase in adverse events.37
Predictive Response
In 2020, Knievel et al used data from the SAMURAI and SPARTAN trials to determine whether prior response to triptans could predict efficacy of lasmiditan. The authors separated subjects into “Good” vs “Insufficient” triptan response groups and compared their responses to lasmiditan 50 mg, 100mg, and 200 mg vs placebo. The authors found that there was no statistically significant difference between those assigned to the “Good” vs “Insufficient” groups with regard to efficacy of lasmiditan at producing headache pain freedom, most bothersome symptom freedom, or headache pain relief. Additionally, the investigators found that a sub-population of triptan naive participants also experienced statistically significant benefits, ie, pain freedom, most bothersome symptom freedom, and headache pain relief. The authors concluded that lasmiditan efficacy was similar regardless of prior response or exposure to triptans.38
In 2022, Reuter et al published a post-hoc analysis from the CENTURION trial regarding previous response to triptans. The investigators found that triptan poor-responders experienced significant improvements in pain outcomes compared with placebo as early as 30 minutes (200 mg) and 1 hour (100 mg) post-dose (p < 0.05). The authors reported improvements in this subgroup with regard to pain freedom at 2 hours, 24 hours, for the 100 mg and 200 mg doses (p < 0.001), though freedom from most bothersome symptom at 2 hours was only significant for the 100 mg dose and not the 200 mg dose.39
Safety Studies
Post-Hoc Analyses (SAMURAI, SPARTAN, CENTURION, and MONONOFU Trials)
In 2019, safety results were published from the SAMURAI and SPARTAN trials. The authors reported that their safety cohort included 3177 subjects who received lasmiditan (50 mg: 654, 100 mg: 1265, 200 mg: 1258) and 1262 subjects who received placebo. The investigators reported no deaths during either of the two trials but reported treatment emergent serious adverse events in 7 patients (200 mg: 3/1258, 0.2%, 100 mg: 1/1265, 0.1%, 50 mg: 1/654, 0.2%, placebo: 2/1262, 0.2%). Subjects reporting ≥1 adverse event were significantly higher in those receiving lasmiditan compared to placebo (Pooled lasmiditan: 1134/3177, 35.9%; Placebo: 174/1262, 13.8%; P < 0.001). Similar to previous studies, the most common adverse events were dizziness, paresthesias, somnolence, and fatigue.40
For the CENTURION Trial, Tassorelli et al reported that the incidence of serious adverse events was low overall for lasmiditan and similar to placebo (200 mg: 0.4%; 100 mg: 0.2%; placebo: 0.4%). The authors reported that adverse events were more likely with the first dose compared with subsequent doses (less than 45% of subjects experienced the same adverse event with subsequent doses). Additionally, subjects reported that the time of onset for adverse events was between 40 min to 60 minutes. Of interest, serotonin syndrome was reported in 2 subjects after receiving lasmiditan with only 1 subject meeting criteria for serotonin syndrome based on the presence of myoclonus, ataxia, hyperreflexia as well as diaphoresis and elevated temperature. There were no deaths or major cardiovascular events reported.41
Following the publication of the MONONOFU Trial in 2021, Doty et al combined results from the SAMURAI, SPARTAN, CENTURIAN, and MONONOFU trials to assess the relationship between the incidence of treatment-related adverse events and efficacy after lasmiditan treatment. In this study, the authors reported that those who had achieved pain freedom or mild pain at 2 hours post-dose (200 mg) experienced a statistically significant increase in the incidence of ≥1 adverse events compared with those who continued to experience moderate-to-severe pain (48.2% vs 28.7%), which was also the case for CNS-related adverse events (dizziness, somnolence, or paresthesias). The authors posited that the incidence of adverse events may be related to high levels of central penetration of lasmiditan.42
Dizziness
Given the high incidence of dizziness with lasmiditan dosing, Tepper et al published a study in 2019 to further characterize this adverse event in greater detail. The authors reported that the incidence of dizziness increased based on dose with 8.6%, 14.9%, 16.8% of subjects experiencing this symptom for 50 mg, 100 mg, and 200 mg doses, respectively, vs 2.9% for placebo. Risk factors for developing dizziness included higher lasmiditan dose, non-Hispanic ethnicity, mild or moderate severity of migraine attack, and low BMI. Subjects reported a mean onset of dizziness at 30 to 40 minutes and a mean duration of 1.5 to 2 hours post-dose. However, dizziness did not appear to generate statistically significant changes in daily activity or drug efficacy. Separately, the authors reported that 21 subjects reported vertigo (20 with lasmiditan vs 1 with placebo). Vertigo was considered moderate in the majority of cases (12/20) and severe in a minority of cases (4/20).43
Cardiovascular Risk Factors
Utilizing data from the SAMURAI and SPARTAN Trials, Shapiro et al found that subjects with at least one cardiovascular risk factor receiving lasmiditan did not experience a statistically significant increase in overall cardiovascular-related adverse events compared to placebo (30/3177, 0.9% vs 5/1262, 0.4%, OR 2.46, 95% CI 0.95–6.39, p = 0.06). However, there was a significant increase in subjects who experienced cardiac dysrhythmia in general (12/3177, 0.9% vs 3/1262, 0.2%, OR 3.59, 95% CI 1.09–11.79, p = 0.02) though each individual symptom was not statistically significant.44
Older Population
In 2021, Martin et al published results of post-hoc analyses from data abstracted from the SAMURAI, SPARTAN, and GLADIATOR trials that focused on patients ≥65 years old. This subgroup included 132 (4.2%, 132/3177) subjects who received lasmiditan and 54 (4.3%, 54/1262) subjects who received placebo. In this subgroup, the investigators found non-significant differences in the incidence of at least 1 adverse event occurring in 35% of older patients compared with 36% of younger patients based on data from the SAMURAI and SPARTAN trials (vs 13.9% non-elderly and 11.1% elderly who received placebo). For subjects in the GLADIATOR trial, there were higher rates of adverse events related to lasmiditan treatment for younger patients compared with older patients (960/1945, 49.4% vs 32/85, 37.6%), but this finding was reported as not statistically significant (p-value not reported).45
Driving
Due to the high incidence of CNS-related adverse events post-dose, two randomized, placebo-controlled trials were performed to determine the safety of operating a motor vehicle while under the effect of lasmiditan. Pearlman et al carried out the studies utilizing the Cognitive Research Corporation Driving Simulator (CRCDS) and compared results between those receiving lasmiditan, alprazolam, and placebo. For Study 1, subjects received a single oral dose of either 50 mg, 100 mg, or 200 mg and were compared against those receiving alprazolam 1 mg or placebo. Driving assessments were performed at 1.5 hours following the dose. Results indicated that subjects receiving lasmiditan experienced an increased number of virtual collisions (defined as collisions with other vehicles, off-road crashes, and number of lane deviations exceeding 4ft) compared with placebo with a higher number of collisions occurring with higher doses of lasmiditan (absolute numbers not reported). Those receiving lasmiditan also experienced a higher number of lane exceedances and with longer duration compared with placebo (p < 0.001). For Study 1, subjects reported higher levels of sleepiness with lasmiditan prior to the initiation of the driving test compared with placebo, but levels of sleepiness were less than those reported for alprazolam (p < 0.001). Peak dose of lasmiditan was noted at 1.5 hours post-dose compared with later time-points. For Study 2, subjects received lasmiditan 100 mg or 200 mg and were compared to 50 mg of diphenhydramine or placebo. Driving assessments were performed at 8 hours, 12 hours, and 24 hours post-dose. In Study 2, there was no statistically significant increase in the number of lane exceedances at 8 hours for the 100 mg dose, but there was a higher number for the 200 mg dose. The authors reported that there were significant differences at 12 hours for the 100 mg dose only and no differences at 24 hours for either dose. The investigators further reported that there was no statistically significant difference in the lane exceedance maximum (maximum lateral deviation from center) or duration of lane exceedance (time to make corrections back to center) for either dose at 8, 12, or 24 hours compared with placebo. Of interest, most subjects reported that they were safe to drive at 1.5 hours post-dose (50 mg: 80%, 100 mg: 67.9%, 200 mg: 55.3% vs alprazolam 2 mg: 43.5%), and all subjects reported that they felt safe to drive at 8 hours. Based on these results, the authors concluded that there appeared to be clinically meaningful impairment in driving performance at 1.5 hours post-dose but cautioned against operating a motor vehicle within 8 hours of receiving a dose of lasmiditan.46
Lasmiditan and Acute Treatment of Migraine: Place in Therapy
Because of lasmiditan’s unique mechanism of action, headache providers have been eager to define its role in the care of patients with migraine. Several meta-analyses have been published based on data from the previously mentioned clinical trials which have demonstrated that lasmiditan is effective at relieving acute migraine pain compared with placebo47–49 with several demonstrating that the 200 mg dose is more effective than the 100 mg dose.50–52 When compared to triptans, lasmiditan has shown lower odds ratios for pain freedom at 2 hours compared with most triptans (OR 1.72, 95% CI 1.06–2.80 to OR 3.40 95% CI 2.12–5.44) as well as pain relief at 2 hours (OR 1.46, 95% CI 1.09–1.96 to OR 3.31, 95% CI 2.41–4.55). Lasmiditan has also been found to have non-significant differences in odds ratios compared with small molecule anti-CGRP gepant rescue medications such as ubrogepant and rimegepant for both pain freedom and pain relief. Additionally, lasmiditan has also been found to have the highest incidence of adverse events compared with triptans and gepants.53 For a more detailed review of clinical data from trials comparing doses of lasmiditan vs placebo and from trials of other acute migraine treatments, please see the meta-analyses published in 2021 by Yang et al and VanderPluym et al.49,53 In 2022, Johnston et al compared lasmiditan, ubrogepant, and rimegepant using the number needed to treat (NNT) and the number needed to harm (NNH). The authors reported NNT values for pain freedom at 2 hours as 15 (95% CI: 9–37), 10 (95% CI: 7–16), and 7 (95% CI: 5–9) for lasmiditan 50 mg, 100 mg, and 200 mg, respectively. NNT for most bothersome symptom freedom at 2 hours was reported as 15 (95% CI: 8–59), 9 (95% CI: 7–16), and 8 (95% CI: 6–12) for lasmiditan 50 mg, 100 mg, and 200 mg, respectively. Additionally, NNT for pain relief at 2 hours was 9 (95% CI: 6–16), 6 (95% CI: 5–8), and 6 (95% CI: 5–8). For comparison, rimegepant 75 mg exhibited the following results: pain freedom at 2 hours: 8 (95% CI: 5–14), most bothersome symptom freedom at 2 hours 12 (95% CI: 7–30), and pain relief at 2 hours 6 (95% CI: 5–9). Ubrogepant 100 mg exhibited the following results: pain freedom at 2 hours: 9 (95% CI: 6–20), most bothersome symptom freedom at 2 hours 10 (95% CI: 6–22), and pain relief at 2 hours 8 (95% CI: 5–14). Regarding NNH for dizziness, all lasmiditan doses exhibited lower NNH compared with gepants with lasmiditan 200 mg exhibiting a NNH of 9 (95% CI: 6–15) and 100 mg exhibiting a NNH of 11 (95% CI: 7–17) vs −84 and −81 for ubrogepant 50 mg and rimegepant 75 mg, respectively. NNH for nausea was infrequent for all therapies and was lowest for rimegepant 24 (95% CI: 4–224) vs lasmiditan 200 mg 49 (95% CI: 21–144).
To further characterize the efficacy of lasmiditan vs gepants, Polavieja et al performed a meta-analysis that demonstrated that lasmiditan 100 mg and 200 mg exhibited higher odds of achieving both pain freedom/relief 2 hours compared with either rimegepant 75 mg or ubrogepant 50 mg. They also reported that lasmiditan 50 mg demonstrated no significant difference in pain outcomes vs gepants. However, as with the previous studies described, this study did not report on outcomes from head-to-head trials and relied only on comparison of the results from separate clinical trials.54
Lasmiditan has been investigated across racial and ethnic groups based on data from the SAMURAI and SPARTAN trials. Results from post-hoc analysis indicated that pain outcomes were not significantly different between Black vs White participants as well as Hispanic/Latinx vs non-Hispanic/Latinx ethnic backgrounds.
Cost and access to any new medication must be considered. For example, the cost of lasmiditan on the GoodRX site as of June 2023 is over $700 for eight 100mg tablets.55 Insurance coverage of lasmiditan varies and often patients are required to try and fail or have contraindication to standard acute migraine therapies, such as triptans. In general, Medicare does not cover lasmiditan. This is surprising and frustrating given that an older population would be more likely to have a history of cardiovascular and cerebrovascular disease or risk factors and as such would be most in need of a medication that lacks vasoactive properties.
Drug Use Special Considerations
Lasmiditan is currently listed as a Schedule V controlled substance, indicating a low potential of abuse relative to higher tiers. Per prescribing information, a human abuse potential study was performed in recreational polydrug uses. Subjects received single oral doses of 100 mg, 200 mg, and 400 mg and responses were compared to alprazolam (2 mg) and placebo. Subjects in this abuse potential study reported significantly higher “drug liking” scores compared to placebo, but lower scores compared to alprazolam. Additionally, subjects reported euphoric mood with 200 mg, 400 mg doses around 43–49% of the time, like alprazolam 2 mg. The authors interpreted this response as positive for abuse potential. Further reports from Phase 2 and 3 studies demonstrated that euphoria and hallucination occurred at a low frequency (1% of subjects) but were still higher than placebo.
Per prescribing information, physical withdrawal was not observed in healthy subjects following cessation after seven once daily doses of lasmiditan 200 mg or 400 mg.11,20
Conclusion
Lasmiditan was approved by the FDA for the acute treatment of migraine in 2019 at 50 mg, 100 mg, and 200 mg doses. Unlike triptans, lasmiditan can only be taken once in a 24-hour period and should be taken early in the headache attack for maximum effectiveness. It is the first and only agent within the ditan class of medications that is available for clinical use. Lasmiditan is highly selective for 5-HT1F serotonin receptors where it acts as a direct agonist within the central nervous system. Based on both animal and human studies, activation of this receptor is not associated with dose-dependent vasoconstriction like the triptan or ergot class of medications. Lasmiditan is a Schedule V controlled-substance and carries an 8-hour driving restriction.
The efficacy of lasmiditan was established by several clinical studies, including the SAMURAI, SPARTAN, CENTURION, MONONOFU, and GLADIATOR Trials as well as numerous post-hoc analyses. Overall, lasmiditan 50, 100 mg, and 200 mg show lower efficacy at improving pain outcomes at 2 hours compared with the triptan class of medications and similar efficacy compared with gepants. Higher doses of lasmiditan appear to be associated with higher rates of pain freedom, most bothersome symptom freedom, and pain relief at 2 hours compared with lower doses. Though lasmiditan has the highest incidence of treatment related adverse events compared with lower doses, triptans, and gepants, these adverse events are mostly mild in intensity and may even be associated with good treatment response. Lasmiditan appears safe for routine clinical use in those with cardiovascular disease, and elderly patients have not been shown to experience higher rates of adverse events compared with younger patients.
Abbreviations
CGRP, calcitonin gene-related peptide; 5-HT, 5-hydroxytryptamine.
Funding
There is no funding to report.
Disclosure
Christopher C. Anderson has no conflicts to disclose for this work. Juliana H. VanderPluym reports research grant from Amgen and Agency for Healthcare Research and Quality, Contract No. 290-2015-00013-I, Investigator, 2020. She acts as Current Neurology and Neuroscience Reports, Headache Section Co-editor, 2020-Present.
References
1. Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16(11):877–897. doi:10.1016/S1474-4422(17)30299-5
2. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: updated age, sex, and socioeconomic-specific estimates from government health surveys. Headache. 2021;61(1):60–68. doi:10.1111/head.14024
3. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi:10.1177/0333102417738202
4. GlaxoSmithKline. IMITREX highlights of prescribing information. Available from: https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Imitrex_Tablets/pdf/IMITREX-TABLETS-PI-PIL.PDF.
5. Charles A. Vasodilation out of the picture as a cause of migraine headache. Lancet Neurol. 2013;12(5):419–420. doi:10.1016/S1474-4422(13)70051-6
6. Amin FM, Asghar MS, Hougaard A, et al. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross-sectional study. Lancet Neurol. 2013;12(5):454–461. doi:10.1016/S1474-4422(13)70067-X
7. Edvinsson L, Haanes KA, Warfvinge K. Does inflammation have a role in migraine? Nat Rev Neurol. 2019;15(8):483–490. doi:10.1038/s41582-019-0216-y
8. Dodick DW. A phase-by-phase review of migraine pathophysiology. Headache. 2018;58(Suppl 1):4–16. doi:10.1111/head.13300
9. Borsook D, Maleki N, Becerra L, McEwen B. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron. 2012;73(2):219–234. doi:10.1016/j.neuron.2012.01.001
10. Dodick DW, Shewale AS, Lipton RB, et al. Migraine patients with cardiovascular disease and contraindications: an analysis of real-world claims data. J Prim Care Community Health. 2020;11:2150132720963680. doi:10.1177/2150132720963680
11. Reyvow. Lasmiditan prescribing info. Available from: https://www.reyvow.com/hcp/savings-support?gclid=EAIaIQobChMIsvCqkujq_QIVxvbjBx13mwAGEAAYASAAEgJQW_D_BwE.
12. Nelson DL, Phebus LA, Johnson KW, et al. Preclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditan. Cephalalgia. 2010;30(10):1159–1169. doi:10.1177/0333102410370873
13. Kovalchin J, Ghiglieri A, Zanelli E, Ings R, Mathers T. Lasmiditan acts specifically on the 5-HT1F receptor in the central nervous system. Cephalalgia. 2016;36(1 Suppl):103. doi:10.1177/0333102415584311
14. Clemow DB, Johnson KW, Hochstetler HM, Ossipov MH, Hake AM, Blumenfeld AM. Lasmiditan mechanism of action - review of a selective 5-HT(1F) agonist. J Headache Pain. 2020;21(1):71. doi:10.1186/s10194-020-01132-3
15. Adham N, Kao HT, Schecter LE, et al. Cloning of another human serotonin receptor (5-HT1F): a fifth 5-HT1 receptor subtype coupled to the inhibition of adenylate cyclase. Proc Natl Acad Sci USA. 1993;90(2):408–412. doi:10.1073/pnas.90.2.408
16. Castro ME, Pascual J, Romon T, Del Arco C, Del Olmo E, Pazos A. Differential distribution of [3H]sumatriptan binding sites (5-HT1B, 5-HT1D and 5-HT1F receptors) in human brain: focus on brainstem and spinal cord. Neuropharmacology. 1997;36(4–5):535–542. doi:10.1016/s0028-3908(97)00061-0
17. Bouchelet I, Cohen Z, Case B, Seguela P, Hamel E. Differential expression of sumatriptan-sensitive 5-hydroxytryptamine receptors in human trigeminal ganglia and cerebral blood vessels. Mol Pharmacol. 1996;50(2):219–223.
18. Cohen Z, Bouchelet I, Olivier A, et al. Multiple microvascular and astroglial 5-hydroxytryptamine receptor subtypes in human brain: molecular and pharmacologic characterization. J Cereb Blood Flow Metab. 1999;19(8):908–917. doi:10.1097/00004647-199908000-00010
19. Bouchelet I, Case B, Olivier A, Hamel E. No contractile effect for 5-HT1D and 5-HT1F receptor agonists in human and bovine cerebral arteries: similarity with human coronary artery. Br J Pharmacol. 2000;129(3):501–508. doi:10.1038/sj.bjp.0703081
20. Wilbraham D, Berg PH, Tsai M, et al. Abuse potential of lasmiditan: a phase 1 randomized, placebo- and alprazolam-controlled crossover study. J Clin Pharmacol. 2020;60(4):495–504. doi:10.1002/jcph.1543
21. Ferrari MD, Farkkila M, Reuter U, et al. Acute treatment of migraine with the selective 5-HT1F receptor agonist lasmiditan--a randomised proof-of-concept trial. Cephalalgia. 2010;30(10):1170–1178. doi:10.1177/0333102410375512
22. Farkkila M, Diener HC, Geraud G, et al. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11(5):405–413. doi:10.1016/S1474-4422(12)70047-9
23. Kuca B, Silberstein SD, Wietecha L, et al. Lasmiditan is an effective acute treatment for migraine: a Phase 3 randomized study. Neurology. 2018;91(24):e2222–e2232. doi:10.1212/WNL.0000000000006641
24. Goadsby PJ, Wietecha LA, Dennehy EB, et al. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain. 2019;142(7):1894–1904. doi:10.1093/brain/awz134
25. Ashina M, Reuter U, Smith T, et al. Randomized, controlled trial of lasmiditan over four migraine attacks: findings from the CENTURION study. Cephalalgia. 2021;41(3):294–304. doi:10.1177/0333102421989232
26. Sakai F, Takeshima T, Homma G, Tanji Y, Katagiri H, Komori M. Phase 2 randomized placebo-controlled study of lasmiditan for the acute treatment of migraine in Japanese patients. Headache. 2021;61(5):755–765. doi:10.1111/head.14122
27. Takeshima T, Komori M, Tanji Y, Ozeki A, Tatsuoka Y. Efficacy of lasmiditan across patient and migraine characteristics in Japanese patients with migraine: a secondary analysis of the MONONOFU trial. Adv Ther. 2022;39(11):5274–5288. doi:10.1007/s12325-022-02304-0
28. Brandes JL, Klise S, Krege JH, et al. Interim results of a prospective, randomized, open-label, Phase 3 study of the long-term safety and efficacy of lasmiditan for acute treatment of migraine (the GLADIATOR study). Cephalalgia. 2019;39(11):1343–1357. doi:10.1177/0333102419864132
29. Lipton RB, Lombard L, Ruff DD, et al. Trajectory of migraine-related disability following long-term treatment with lasmiditan: results of the GLADIATOR study. J Headache Pain. 2020;21(1):20. doi:10.1186/s10194-020-01088-4
30. Brandes JL, Klise S, Krege JH, et al. Long-term safety and efficacy of lasmiditan for acute treatment of migraine: final results of the GLADIATOR study. Cephalalgia Rep. 2020;3:251581632095817. doi:10.1177/2515816320958176
31. Ashina M, Vasudeva R, Jin L, et al. Onset of efficacy following oral treatment with lasmiditan for the acute treatment of migraine: integrated results from 2 randomized double-blind placebo-controlled phase 3 clinical studies. Headache. 2019;59(10):1788–1801. doi:10.1111/head.13636
32. Doty EG, Krege JH, Jin L, Raskin J, Halker Singh RB, Kalidas K. Sustained responses to lasmiditan: results from post-hoc analyses of two Phase 3 randomized clinical trials for acute treatment of migraine. Cephalalgia. 2019;39(12):1569–1576. doi:10.1177/0333102419859313
33. Tepper SJ, Vasudeva R, Krege JH, et al. Evaluation of 2-hour post-dose efficacy of lasmiditan for the acute treatment of difficult-to-treat migraine attacks. Headache. 2020;60(8):1601–1615. doi:10.1111/head.13897
34. Peres MFP, Vasudeva R, Baygani SK, Dennehy EB, Vincent M, Friedman DI. Lasmiditan efficacy in migraine attacks with mild vs. moderate or severe pain. Curr Med Res Opin. 2021;37(6):1031–1038. doi:10.1080/03007995.2021.1903846
35. MacGregor EA, Komori M, Krege JH, et al. Efficacy of lasmiditan for the acute treatment of perimenstrual migraine. Cephalalgia. 2022;42(14):1467–1475. doi:10.1177/03331024221118929
36. Loo LS, Plato BM, Turner IM, et al. Effect of a rescue or recurrence dose of lasmiditan on efficacy and safety in the acute treatment of migraine: findings from the phase 3 trials (SAMURAI and SPARTAN). BMC Neurol. 2019;19(1):191. doi:10.1186/s12883-019-1420-5
37. Clemow DB, Hochstetler HM, Dong Y, Hauck P, Peres MFP, Ailani J. Effect of a change in lasmiditan dose on efficacy and safety in patients with migraine. Postgrad Med. 2021;133(4):449–459. doi:10.1080/00325481.2020.1860619
38. Knievel K, Buchanan AS, Lombard L, et al. Lasmiditan for the acute treatment of migraine: subgroup analyses by prior response to triptans. Cephalalgia. 2020;40(1):19–27. doi:10.1177/0333102419889350
39. Reuter U, Krege JH, Lombard L, et al. Lasmiditan efficacy in the acute treatment of migraine was independent of prior response to triptans: findings from the CENTURION study. Cephalalgia. 2022;42(1):20–30. doi:10.1177/03331024211048507
40. Krege JH, Rizzoli PB, Liffick E, et al. Safety findings from Phase 3 lasmiditan studies for acute treatment of migraine: results from SAMURAI and SPARTAN. Cephalalgia. 2019;39(8):957–966. doi:10.1177/0333102419855080
41. Tassorelli C, Bragg S, Krege JH, et al. Safety findings from CENTURION, a phase 3 consistency study of lasmiditan for the acute treatment of migraine. J Headache Pain. 2021;22(1):132. doi:10.1186/s10194-021-01343-2
42. Doty EG, Hauck PM, Krege JH, et al. The association between the occurrence of common treatment-emergent adverse events and efficacy outcomes after lasmiditan treatment of a single migraine attack: secondary analyses from four pooled randomized clinical trials. CNS Drugs. 2022;36(7):771–783. doi:10.1007/s40263-022-00928-y
43. Tepper SJ, Krege JH, Lombard L, et al. Characterization of dizziness after lasmiditan usage: findings from the SAMURAI and SPARTAN acute migraine treatment randomized trials. Headache. 2019;59(7):1052–1062. doi:10.1111/head.13544
44. Shapiro RE, Hochstetler HM, Dennehy EB, et al. Lasmiditan for acute treatment of migraine in patients with cardiovascular risk factors: post-hoc analysis of pooled results from 2 randomized, double-blind, placebo-controlled, phase 3 trials. J Headache Pain. 2019;20(1):90. doi:10.1186/s10194-019-1044-6
45. Martin VT, Ahmed Z, Hochstetler HM, et al. Tolerability and safety of lasmiditan treatment in elderly patients with migraine: post hoc analyses from randomized studies. Clin Ther. 2021;43(6):1066–1078. doi:10.1016/j.clinthera.2021.04.004
46. Pearlman EM, Wilbraham D, Dennehy EB, et al. Effects of lasmiditan on simulated driving performance: results of two randomized, blinded, crossover studies with placebo and active controls. Hum Psychopharmacol. 2020;35(5):e2732. doi:10.1002/hup.2732
47. Hou M, Xing H, Li C, et al. Short-term efficacy and safety of lasmiditan, a novel 5-HT(1F) receptor agonist, for the acute treatment of migraine: a systematic review and meta-analysis. J Headache Pain. 2020;21(1):66. doi:10.1186/s10194-020-01138-x
48. Maiti R, Mishra A, Puliappadamb HM, Jena M, Srinivasan A. Efficacy and safety of lasmiditan for acute treatment of migraine in adults: a meta-analysis. J Clin Pharmacol. 2021;61(12):1534–1544. doi:10.1002/jcph.1962
49. VanderPluym JH, Halker Singh RB, Urtecho M, et al. Acute treatments for episodic migraine in adults: a systematic review and meta-analysis. JAMA. 2021;325(23):2357–2369. doi:10.1001/jama.2021.7939
50. Yang Y, Sun Y, Gao B, Wang Z, Chen Z, Wang Z. Lasmiditan for acute treatment of migraine in adults: a systematic review and meta-analysis of randomized controlled trials. CNS Drugs. 2020;34(10):1015–1024. doi:10.1007/s40263-020-00753-1
51. Zhu H, Tang Y, Zhou T, Song J. The efficacy of lasmiditan for the treatment of migraine: a meta-analysis of randomized controlled studies. Clin Neuropharmacol. 2020;43(6):191–195. doi:10.1097/WNF.0000000000000417
52. Gu P, Chen C, Wu Q, et al. The effect and safety of 5-HT(1F) receptor agonist lasmiditan on migraine: a systematic review and meta-analysis. Biomed Res Int. 2021;2021:6663591. doi:10.1155/2021/6663591
53. Yang CP, Liang CS, Chang CM, et al. Comparison of new pharmacologic agents with triptans for treatment of migraine: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(10):e2128544. doi:10.1001/jamanetworkopen.2021.28544
54. Polavieja P, Belger M, Venkata SK, Wilhelm S, Johansson E. Relative efficacy of lasmiditan versus rimegepant and ubrogepant as acute treatments for migraine: network meta-analysis findings. J Headache Pain. 2022;23(1):76. doi:10.1186/s10194-022-01440-w
55. GoodRx. Reyvow. Available from: https://www.goodrx.com/reyvow/medicare-coverage#:~:text=In%20general%2C%20Medicare%20prescription%20drug%20plans%20%28Part%20D%29,may%20be%20covered%20under%20Medical%20Insurance%20%28Part%20B%29.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
