Back to Journals » Infection and Drug Resistance » Volume 16
Profile and Frequency of Mutations Conferring Drug-Resistant Tuberculosis in the Central, Southeastern and Eastern Ethiopia
Authors Agonafir M, Belay G , Feleke A , Maningi N, Girmachew F, Reta M , Fourie PB
Received 1 March 2023
Accepted for publication 5 May 2023
Published 12 May 2023 Volume 2023:16 Pages 2953—2961
DOI https://doi.org/10.2147/IDR.S408567
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Mulualem Agonafir,1 Gurja Belay,1 Adey Feleke,1 Nontuthuko Maningi,2 Feven Girmachew,3 Melese Reta,2,4 P Bernard Fourie2
1Department of Microbial, Cellular and Molecular Biology, College of Natural and Computational Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 2Department of Medical Microbiology, Faculty of Health Sciences, University of Pretoria, Pretoria, South Africa; 3Ethiopian Public Health Institute, Addis Ababa, Ethiopia; 4Department of Medical Laboratory Sciences, College of Health Sciences, Woldia University, Woldia, Ethiopia
Correspondence: Mulualem Agonafir, Department of Microbial, Cellular and Molecular Biology, College of Natural and Computational Sciences, Addis Ababa University, P.O. Box 34738, Addis Ababa, Ethiopia, Tel +251911446959, Email [email protected]
Purpose: Advances in molecular tools that assess genes harboring drug resistance mutations have greatly improved the detection and treatment of drug-resistant tuberculosis (DR-TB). This study was conducted to determine the frequency and type of mutations that are responsible for resistance to rifampicin (RIF), isoniazid (INH), fluoroquinolones (FLQs) and second-line injectable drugs (SLIDs) in Mycobacterium tuberculosis (MTB) isolates obtained from culture-positive pulmonary tuberculosis (TB) patients in the central, southeastern and eastern Ethiopia.
Patients and Methods: In total, 224 stored culture-positive MTB isolates from pulmonary TB patients referred to Adama and Harar regional TB laboratories between August 2018 and January 2019 were assessed for mutations conferring RIF, INH, FLQs and SLIDs resistance using GenoType®MTBDRplus (MTBDRplus) and GenoType®MTBDRsl (MTBDRsl).
Results: RIF, INH, FLQs and SLIDs resistance-conferring mutations were identified in 88/224 (39.3%), 85/224 (38.0%), 7/77 (9.1%), and 3/77% (3.9%) of MTB isolates, respectively. Mutation codons rpoB S531L (59.1%) for RIF, katG S315T (96.5%) for INH, gyrA A90V (42.1%) for FLQs and WT1 rrs (100%) for SLIDs were observed in the majority of the isolates tested. Over a 10th of rpoB mutations detected in the current study were unknown.
Conclusion: In this study, the most common mutations conferring drug resistance to RIF, INH, FLQs were identified. However, a significant proportion of RIF-resistant isolates manifested unknown rpoB mutations. Similarly, although few in number, all SLID-resistant isolates had unknown rrs mutations. To further elucidate the entire spectrum of mutations, tool such as whole-genome sequencing is imperative. Furthermore, the expansion of molecular drug susceptibility testing services is critical for tailoring patient treatment and preventing disease transmission.
Keywords: drug resistance, Ethiopia, line probe assay, mutation, tuberculosis
Introduction
Though curable and preventable, with an estimated 9.9 million new cases and 1.3 million deaths in 2020, TB is still one of the main causes of death globally. DR-TB especially resistant to INH and RIF, termed as multidrug-resistant TB (MDR-TB), poses a great threat to the public. Worldwide, 132,222 individuals were reported to have MDR-TB and rifampicin-resistant tuberculosis (RR-TB) in 2020.1 Although Ethiopia achieved the End TB Strategy milestone of a 20% reduction in the TB incidence rate from 2015 to 2020, it is still 1 of the 30 high burden countries heavily affected by TB and TB/HIV.1 Furthermore, despite the fact that Ethiopia is one of the countries that has transitioned out of the 30 high MDR/RR-TB countries,1 DR-TB is still a problem where 2.8% of the new and 18.6% of previously treated cases were reported to have MDR-TB.2
Significant progress has been made in the diagnosis of DR-TB since the introduction of novel molecular diagnostic technologies, such as Xpert MTB/RIF and line probe assays (LPAs), in recent years. As a result, the proportion of patients diagnosed with MDR/RR-TB and placed on treatment has increased significantly.3 Prior to the availability of these tools, TB/DR-TB diagnosis took weeks to months due to both culture-based detection and drug susceptibility testing (DST),4 which had repercussions on patient care and the overall TB control program. These include provision of inappropriate treatment to patients, amplification of resistance and continued spread of drug-resistant strains.5
Improved knowledge on molecular mechanisms of resistance to anti-TB drugs in the last 20 years has led to the development of commercial genotypic assays for the rapid detection of drug resistance.6,7 Among these assays, the World Health Organization (WHO) approved the use of LPAs for the detection of Mycobacterium tuberculosis complex (MTBC) and common mutations associated with drug resistance to INH, RIF as well as FLQs and SLIDs.5,8
Ethiopia is one of the countries that has benefited from the utilization of these technologies. LPAs from the Hain LifeSciences, Nehren, Germany: MTBDRplus and MTBDRsl assays were incorporated into the DR-TB diagnostic algorithm of the national TB control program of Ethiopia.2,9 In line with this, the national and regional TB reference laboratories have been mandated to perform these assays.
It is well established that a few key mutations are responsible for most of the resistance in anti-TB drugs.10–12 Besides, the frequency of these mutations differs geographically and this variability affects the efficacy of rapid diagnostic tools in identifying drug resistance.11,12 Hence, the pool of information on the frequency and pattern of mutations related to drug resistance against key anti-TB drugs from different geographical locations is vital in supporting an evidence-based and patient-centered care, ultimately contributing to the overall TB control effort of the country. However, information on DR-TB, particularly on variants of mutations conferring drug resistance to key anti-TB drugs, is scarce in Ethiopia. Therefore, the current study aimed to determine the frequency and type of mutations conferring drug resistance to INH, RIF, FLQs and SLIDs in isolates obtained from culture-positive pulmonary TB patients from the central, eastern and southeastern Ethiopia.
Materials and Methods
Study Population and Setting
A cross-sectional study was conducted using 224 culture-positive MTB isolates obtained from pulmonary TB patients whose specimens were referred to the regional TB laboratories in Adama and Harar between August 2018 and January 2019, for culture and DST. Adama TB Regional TB Reference Laboratory (ATRRL) serves patients mostly coming from the central and parts of south and eastern regions of Ethiopia that include Amhara, Oromia and Afar. Whereas, Harar Regional TB Reference Laboratory (HRTRL) serves the eastern regions, including Harar, Diredawa, Somali and east Oromia.
Pertinent clinical and sociodemographic information to the study such as treatment history, age, sex and address of patients were collected.
Culture and Mycobacterium tuberculosis Complex Isolates
Sputum specimens were processed following standard procedures and inoculated onto the BACTEC™ MGIT™ 960 broth culture system (BD, Sparks, MD, USA). The growth of MTBC was confirmed by Capilia TB-Neo (Tauns Laboratories, Japan) and AFB smear staining.13 The isolates confirmed as MTBC were then subcultured onto Lowenstein Jensen media and harvested within 3–4 weeks. Two cryo-vials containing 1mL 7H9 liquid medium were prepared and two loopful of colonies of MTBC culture were transferred to each vial, which was later transported to the University of Pretoria’s Medical Microbiology Research Department for further testing.
DNA Extraction
DNA extraction was performed for each isolate using the PrimeXtractTM kit (Longhorn Vaccines and Diagnostics, San Antonio, TX, USA). Briefly, 200μL of the culture together with 200μL of 100% ethanol and 200μL lysis buffer were transferred to a 1.5-mL microcentrifuge tube and vortexed. The mixture was then centrifuged and incubated for 5 minutes at room temperature. The entire content was then transferred to an extraction column and centrifuged at 13,000rpm for 60 seconds. After removing the extraction column, the eluate was discarded. The extraction column was then filled with 500μL of wash buffer, centrifuged, and the eluate was removed. This step was repeated twice followed by washing of the filter with additional 500μL wash buffer and centrifugation of the extraction column to remove trace wash buffer. Finally, the nucleic acid was eluted by 1min of centrifugation at 13,000 rpm using 50μL of preheated (60–70°C) elution solution. The extracted DNA was stored at −20°C for further use.
Genotypic Drug Susceptibility Testing
MTBDRplus v2.0. was performed on 224 MTBC culture-positive isolates. MDR/RR-TB isolates were subjected to MTBDRsl v2.0. All the procedures that included master mix preparation, amplification and hybridization were performed following the manufacturer's instruction (Hain Lifescience, Nehren, Germany).14,15 We were not able to perform the test for eight isolates due to reagent shortage.
Interpretation of Results
The evaluation sheet provided with the kit was used to paste the developed strips and determine the resistance status according to the manufacturer’s instruction. Four control zones on each strip ensured that the test proceeded smoothly and the reagents performed well. These include conjugate control (CC), which demonstrates the efficiency of the conjugate binding and substrate reaction; amplification control (AC), which excludes mistakes during extraction and amplification and the carry-over of amplification inhibitors; M. tuberculosis complex control (TUB), which hybridizes with amplicons derived from all members of the Mycobacterium tuberculosis complex (MTBC); and locus controls (rpoB, katG, and inhA (MTBDRplus); gyrA, gyrB, rrs and eis (MTBDRsl)) which detect a gene region specific for the respective locus.
In each strip, there are wild-type (WT) probes and mutant (MUT) probes corresponding to each gene studied. When at least one of the WT bands was missing and the corresponding MUT band appeared, this indicated the presence of a known mutation in the gene of the tested strain, suggesting drug resistance. Additionally, the absence of a WT band without the corresponding MUT band was interpreted as resistance due to unknown mutations. The presence of all WT bands and the absence of all MUT bands were interpreted as isolates susceptible to the drugs tested. The presence of all WT bands together with MUT bands was defined as heteroresistant.
Quality Control
For each run of MTBDRplus and MTBDRsl assays, molecular grade water and reference strain H37Rv susceptible to all drugs tested were used as negative and positive controls, respectively.
Statistical Analysis
Data were first entered into Excel spreadsheet; cleared, and analyzed using the SPSS statistical software package, V20 (SPSS Inc., Chicago, IL, USA). Frequencies and percentages were used to describe clinical and sociodemographic characteristics as well as drug resistance conferring mutations. Tables and figures were used to present the results.
Ethical Approval
The study was ethically approved by the Ethical Review Board of Natural and Computational Sciences at Addis Ababa University and permission to transfer isolates to South Africa was obtained from the Ethiopian Food, Medicine and Health Care Administration and Control Authority (now known as the Ethiopian Food and Drug Administration) and the Health Department of South Africa. The study utilized isolates routinely obtained from patients for diagnostic and therapeutic purposes. No personal information of patients was collected.
Results
Characteristics of the Study Population
In the current study, a total of 224 culture-positive isolates were included. The median age of the patients from which isolates were collected was 28 years (±SD=12.19, range 9–69 years) and the majority (n=132, 58.9%) were male. The majority (n=181, 80.8%) of the patients were from the central (Arsi, East/North and South West Shoa zones) and eastern (Diredawa, Jigjiga, Harar, East and West Hararghe zones) regions of Ethiopia.
Drug Susceptibility Testing
GenoType MTBDRplus
In this study, we performed genotypic DST using MTBDRplus v2.0. on 224 isolates. Of these, 88 (39.3%), 85 (38.0%) were resistant to RIF and INH, respectively. Of those resistant to RIF, 82 (93.2%) were also resistant to INH and 6 (6.8%) were monoresistant. More than a third of the isolates (n=82, 36.6%) were MDR-TB. The majority (n=66, 80.5%) were from previously treated, males (n=51, 62.2%) and those aged between 15 and 34 years (n=50, 60.9%). Furthermore, 37 (45.1%) of those isolates identified as MDR-TB were from eastern Ethiopia, followed by central Ethiopia (n=26, 31.7%). There were 3 (1.3%) INH monoresistant isolates (Figure 1 and Table 1).
 |
Table 1 Comparison of Patient Characteristics and Drug Resistance Profile |
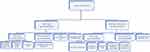 |
Figure 1 Study population and study flow diagram. |
GenoType MTBDRsl
The MTBDRsl v2.0. was performed on 73 MDR-TB and 6 RR isolates. Of these, 77 (MDR=71, RR=6) had an interpretable result and were included in the current study. Further resistance to FLQs and SLIDs was observed only in MDR-TB isolates. Accordingly, 7 (9.1%) were resistant to FLQs and 3 (3.9%) were resistant to SLIDs. All of the FLQ-resistant isolates were from patients’ previously treated patients with first-line anti-TB drugs. However, 2 (66.7%) of the SLID-resistant isolates were from new MDR-TB patients. Furthermore, all of the SLIDs and 4 (57.1%) of the FLQ-resistant isolates were from eastern Ethiopia (Figure 1 and Table 1).
Frequency of Mutations Conferring Drug Resistance to INH, RIF, FLQs and SLIDs
Mutations in the rpoB
The majority of isolates (n=52, 59.1%) with RIF resistance had mutation at codon S531L followed by D516V (n=13, 14.8%), H26Y (n=8, 9.1%) and H526D (n=3, 3.4%). The remaining (13.6%) had missing WT band without the corresponding MUT band and were reported as unknown mutations. These included WT7 mutation (n=8, 9.1%) and WT8 mutation (n=4, 4.5%) (Table 2).
 |
Table 2 Mutations Conferring Drug Resistance to RIF and INH |
Mutations in the katG and inhA
Of the 85 isolates with INH resistance, the majority (n=82, 96.5%) had mutation at codon 315 of the katG gene (n=78, 91.8% S315T1 and n=4, 4.7% S315T2), indicating high-level resistance. Mutations in the promoter region of inhA gene (which indicate low-level resistance) was observed in the INH monoresistant isolates (n=3, 100%) collected from drug-naïve patients. All had mutation at codon −15 (C-15t). No isolate had a co-mutation at katG and inhA genes (Table 2).
Mutations in the gyrA and rrs
Seven isolates (9.1%) showed mutations that conferred drug resistance to FLQs. The gyrA mutation A90V was observed in 3 (42.9%) isolates, whereas mutations D94A, D94G, D94N/Y were each observed in 1 isolate (14.3%). One isolate was heteroresistant where it had all the WT probe together with MUT probe (A90V and D94N/Y) (Table 3). No gyrB mutations were observed in the present study. Additionally, three MDR-TB isolates had missing WT bands and without the corresponding MUT band at the rrs gene.
 |
Table 3 Mutations Conferring Drug Resistance to FLQs and SLIDs |
Discussion
For anti-TB drugs to be effective, early diagnosis and effective drugs against the infecting MTB isolate are essential to improve the cure rate of the patients and hinder further transmission of the TB disease. Furthermore, identifying the mutations associated with anti-TB drug resistance is essential for the proper management of DR-TB patients. LPAs come at the forefront in undertaking these tasks, especially in developing countries such as Ethiopia, for some key anti-TB drugs used in the treatment of drug susceptible and DR-TB.
Mutations in the 81-bp region (codons 507–533) of the rpoB gene harbor over 95% of RIF resistance in MTB isolates and high-level RIF resistance is usually associated with point mutations in 531, 526 and 516 codons.16 In the current study, among 88 RIF-resistant MTB isolates, the most common gene mutation (59.1%) associated with RIF resistance was at codon S531L. This mutation was reported as a predominant mutation of the rpoB gene causing RIF resistance in various studies previously conducted in Ethiopia, which include Jigjiga town (80%),17 Amhara region (73%),18 Ethiopia (74.2%),19 St. Peter’s hospital, Addis Ababa, Ethiopia (81.3%),20 Southwest Ethiopia (82.4%)21 and Tigray region (70%).22 In agreement with our finding, higher frequency of mutation at codon S531L was reported in other countries such as Sudan (64.1%), India (62.3%), Iran (66%), Pakistan (64%), and China (58.2%).23–27
Although in less frequency, the second most common mutation D516V (14.8%) in this study was previously reported in Ethiopia,19,20 India28 and Sudan.23 However, higher or similar proportion to our findings was reported in Angola (17.2%),29 India (17.7%),24 China (10.1%)27 and Ecuador (28.6%).30 Eleven isolates (12.5%) showed mutations at codon 526 in which 9.1% were at H526Y and 3.4% at H526D. Comparable findings were reported in Ethiopia31 and India.24
In the current study, 13.6% of the isolates were classified as RIF resistant based on only the lack of WT probe hybridization. This proportion of isolates with unknown mutations is similar to other studies from St. Peter’s TB Specialized Hospital, Addis Ababa, Ethiopia (15.8%)31 and Southwest Ethiopia (14.7%)32 and a multicenter study in India, South Africa and Moldova (13%).33
INH resistance is mainly caused by mutations in the katG and inhA genes; with 50–95% of the INH-resistant isolates having katG S315 mutations34 depending on geographical distribution.35 Similarly, most of the INH-resistant isolates (96.5%) in our study had mutations in the katG gene (S315T1/T2) while the remaining 3.5% had mutations in inhA (c15T) promoter region. In agreement with our findings, a meta-analyses study that examined INH conferring mutations19 reported a prevalence of 95.8% for katG315 mutation and 5.9% for inhA promoter region mutation. High-level INH resistance causing mutation (S315T1)36 was the most frequent (91.8%) in our study and other studies conducted in Ethiopia.17,18,20,32,37 Furthermore, the katG 315 mutations reported to be frequent in MDR-TB patients36,38 were exclusively found in MDR-TB isolates in the current study.
Mutations in the inhA promoter region which are associated with low-level INH resistance are usually less frequent when compared with katG mutations.34 In this study, we found only three INH-resistant isolates (all monoresistant) with mutations at codon C15T of the inhA promoter gene. In earlier studies from Ethiopia, mutations in inhA promoter region were mostly in INH monoresistant isolates.20,32,38 Other studies also reported no or low proportion of mutation in the inhA promotor region.18,20,31,37,39
In Ethiopia, the indiscriminate use of FLQs for various indications might have led to the development of drug resistance against these key drugs.40–42 Mutations in the gyrA and gyrB gyrase genes especially at codons 90, 91 and 94 of gyrA (termed quinoline resistance-determining region, QRDR) are responsible for FQLs resistance in MTB isolates.43 In this study, gyrA mutation at codon A90V was the most common (42.9%) among the FLQ-resistant isolates, which is in agreement with a laboratory-based surveillance study40 in Ethiopia and a report from Morocco.44 Supporting our findings, D94N/D94Y was recently reported to be the second most common gyrA mutation in Ethiopia.22,40 Various investigations have indicated that D94G mutation is predominant across the corners of the globe.43,45–49 In our study, no mutation related to gyrB was observed, which is in concordance with previous studies in Ethiopia.22,40
Of note, one isolate had a WT probe hybridization and A90V and D94N/Y mutations indicating heteroresistance (coexistence of susceptible and resistant strain in a single specimen).50 Superinfection or reinfection by a second strain, mixed infection or within host evolution of strains could result in heteroresistance.51 As an intermediate stage of full resistance, the detection of heteroresistant mutations is important in guiding the provision of proper treatment regimen.52,53
Resistance to SLIDs is mostly associated with rrs A1401G mutation.54 Similarly, the A1401G mutation was reported as the most frequent in Ethiopia.40 However, in the current study, three isolates had unknown rrs mutation that results in cross-resistance to kanamycin, capreomycin and viomycin.15 It is well known that kanamycin and capreomycin are no more used in the treatment of DR-TB.55
Limitations
Our study is not without limitations. The number of isolates included in our study is relatively small and were obtained from patients referred to TB culture/DST laboratories. Furthermore, although proven effective, the sole use of LPAs will not be enough to describe the spectrum of mutations in the country. Hence, the findings of this study might not accurately represent the overall situation in Ethiopia.
Conclusion
The finding of our study showed that canonical drug resistance conferring mutations at rpoB, katG, gyrA were the most frequent in RIF-, INH- and FLQ-resistant isolates, respectively. INH monoresistant isolates had mutations exclusively in the inhA promoter region. However, a significant proportion of isolates with RIF resistance had unknown mutations that could affect the decision-making in patient management. Hence, the use of better tools such as whole-genome sequencing involving large number of isolates is vital to further elucidate such mutations and predict drug resistance.
Data Sharing Statement
Relevant data pertaining to this study will be provided upon a reasonable request to the corresponding author.
Acknowledgments
The authors would like to thank the Medical Microbiology Department at the University of Pretoria for their assistance with the laboratory work and appreciate the comprehensive assistance provided by the Department of Microbial, Cellular and Molecular Biology of Addis Ababa University. The authors would also like to thank the regional TB reference laboratories in Adama and Harar.
Funding
The laboratory work was supported by University of Pretoria, Medical Microbiology Department.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global Tuberculosis Report 2021. World Health Organization; 2021. Available from: https://apps.who.int/iris/handle/10665/346387.
2. Federal Ministry of Health of Ethiopia. Guidelines on Programmatic Management of Drug Resistance TB in Ethiopia.
3. World Health Organization. Global Tuberculosis Report 2020. World Health Organization; 2020. Available from: https://apps.who.int/iris/handle/10665/336069.
4. Jacobson KR, Theron D, Kendall EA, et al. Implementation of GENOTYPE MTBDRplus reduces time to multidrug-resistant tuberculosis therapy initiation in South Africa. Clin Infect Dis. 2013;56(4):503–508. doi:10.1093/cid/cis920
5. World Health Organization. The Use of Molecular Line Probe Assay for the Detection of Resistance to Isoniazid and Rifampicin: Policy Update. World Health Organization; 2016. Available from: https://apps.who.int/iris/handle/10665/250586.
6. World Health Organization. Line Probe Assays for Detection of Drug-Resistant Tuberculosis: Interpretation and Reporting Manual for Laboratory Staff and Clinicians. World Health Organization; 2022. Available from: https://apps.who.int/iris/handle/10665/354240.
7. Li G, Guo Q, Liu H, et al. Detection of resistance to fluoroquinolones and second-line injectable drugs among mycobacterium tuberculosis by a reverse dot blot hybridization assay. Infect Drug Resist. 2020;13:4091–4104. doi:10.2147/IDR.S270209
8. World Health Organization. The Use of Molecular Line Probe Assays for the Detection of Resistance to Second-Line Anti-Tuberculosis Drugs: Policy Guidance. World Health Organization; 2016. Available from: https://apps.who.int/iris/handle/10665/246131.
9. Federal Ministry of Health of Ethiopia. Guideline for the program and clinical management of drug resistant tuberculosis first; 2011.
10. World Health Organization. Catalogue of Mutations in Mycobacterium Tuberculosis Complex and Their Association with Drug Resistance. World Health Organization; 2021. Available from: https://apps.who.int/iris/handle/10665/341981.
11. Campbell PJ, Morlock GP, Sikes RD, et al. Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2011;55(5):2032–2041. doi:10.1128/AAC.01550-10
12. Seifert M, Catanzaro D, Catanzaro A, Rodwell TC. Genetic mutations associated with isoniazid resistance in Mycobacterium tuberculosis: a systematic review. PLoS One. 2015;10(3):e0119628. doi:10.1371/journal.pone.0119628
13. Shen GH, Chen CH, Hung CH, et al. Combining the Capilia TB assay with smear morphology for the identification of Mycobacterium tuberculosis complex. Int J Tuberc Lung Dis. 2009;13(3):371–376.
14. HAIN Lifescience. GenoType MTBDRplus v.2.0, Molecular genetic assay for identification of the M. tuberculosis complex and its resistance to rifampicin and isoniazid from clinical specimens and cultivated samples; 2015.
15. HAIN Lifescience. GenoType MTBDRsl VER 2.0, Molecular Genetic Assay for Identification of the M. tuberculosis Complex and its Resistance to Fluoroquinolones and Aminoglycosides/Cyclic Peptides from Sputum Specimens or Cultivated Samples; 2017.
16. Hirani N, Joshi A, Anand S, et al. Detection of a novel mutation in the rpoB gene in a multidrug resistant Mycobacterium tuberculosis isolate using whole genome next generation sequencing. J Glob Antimicrob Resist. 2020;22:270–274. doi:10.1016/j.jgar.2020.03.004
17. Brhane M, Kebede A, Petros Y. Molecular detection of multidrug-resistant tuberculosis among smear-positive pulmonary tuberculosis patients in Jigjiga town, Ethiopia. Infect Drug Resist. 2017;10:75–83. doi:10.2147/IDR.S127903
18. Tessema B, Beer J, Emmrich F, Sack U, Rodloff AC. Analysis of gene mutations associated with isoniazid, rifampicin and ethambutol resistance among Mycobacterium tuberculosis isolates from Ethiopia. BMC Infect Dis. 2012;12(1):37. doi:10.1186/1471-2334-12-37
19. Reta MA, Alemnew B, Abate BB, Fourie PB. Prevalence of drug resistance-conferring mutations associated with isoniazid- and rifampicin-resistant Mycobacterium tuberculosis in Ethiopia: a systematic review and meta-analysis. J Glob Antimicrob Resist. 2021;26:207–218. doi:10.1016/j.jgar.2021.06.009
20. Damena D, Tolosa S, Hailemariam M, et al. Genetic diversity and drug susceptibility profiles of Mycobacterium tuberculosis obtained from Saint Peter’s TB specialized Hospital, Ethiopia. PLoS One. 2019;14(6):e0218545. doi:10.1371/journal.pone.0218545
21. Tadesse M, Abebe G, Bekele A, et al. The predominance of Ethiopian specific Mycobacterium tuberculosis families and minimal contribution of Mycobacterium bovis in tuberculous lymphadenitis patients in Southwest Ethiopia. Infect Genet Evol. 2017;55:251–259. doi:10.1016/j.meegid.2017.09.016
22. Welekidan LN, Skjerve E, Dejene TA, et al. Frequency and patterns of first- and second-line drug resistance-conferring mutations in Mycobacterium tuberculosis isolated from pulmonary tuberculosis patients in a cross-sectional study in Tigray Region, Ethiopia. J Glob Antimicrob Resist. 2021;24:6–13. doi:10.1016/j.jgar.2020.11.017
23. Elbir H, Ibrahim NY. Frequency of mutations in the rpoB gene of multidrug-resistant Mycobacterium tuberculosis clinical isolates from Sudan. J Infect Dev Ctries. 2014;8(06):796–798. doi:10.3855/jidc.4496
24. Maurya A, Singh A, Kant S, et al. Use of GenoType® MTBDRplus assay to assess drug resistance and mutation patterns of multidrug-resistant tuberculosis isolates in northern India. Indian J Med Microbiol. 2013;31(3):230–236. doi:10.4103/0255-0857.115625
25. Hamed Z, Mohajeri P, Farahani A, et al. The frequency of point mutations associated with resistance to isoniazid and rifampin among clinical isolates of multidrug-resistant Mycobacterium tuberculosis in the west of Iran. Gene Rep. 2021;22:100981. doi:10.1016/j.genrep.2020.100981
26. Farooqi JQ, Khan E, Alam SMZ, Ali A, Hasan Z. Line probe assay for detection of rifampicin and isoniazid resistant tuberculosis in Pakistan. J Pak Med Assoc. 2012;62:767.
27. Jian J, Yang X, Yang J, Chen L. Evaluation of the GenoType MTBDRplus and MTBDRsl for the detection of drug-resistant Mycobacterium tuberculosis on isolates from Beijing, China. Infect Drug Resist. 2018;11:1627–1634. doi:10.2147/IDR.S176609
28. Alvarez-Uria G, Reddy R. Differences in rpoB, katG and inhA mutations between new and previously treated tuberculosis cases using the GenoType MTBDR plus assay. Infect Genet Evol. 2018;59:48–50. doi:10.1016/j.meegid.2018.01.022
29. Rando-Segura A, Aznar ML, Moreno MM, et al. Molecular characterization of rpoB gene mutations in isolates from tuberculosis patients in Cubal, Republic of Angola. BMC Infect Dis. 2021;21. doi:10.1186/s12879-021-06763-8
30. Franco-Sotomayor G, Garzon-Chavez D, Leon-Benitez M, de Waard JH, Garcia-Bereguiain MA, First A. Insight into the katG and rpoB gene mutations of multidrug-resistant Mycobacterium tuberculosis strains from Ecuador. Microb Drug Resist. 2019;25(4):524–527. doi:10.1089/mdr.2018.0203
31. Abate D, Tedla Y, Meressa D, Ameni G. Isoniazid and rifampicin resistance mutations and their effect on second-line anti-tuberculosis treatment. Int J Tuberc Lung Dis. 2014;18(8):946–951. doi:10.5588/ijtld.13.0926
32. Tadesse M, Aragaw D, Dimah B, et al. Drug resistance-conferring mutations in Mycobacterium tuberculosis from pulmonary tuberculosis patients in Southwest Ethiopia. Int J Mycobacteriol. 2016;5(2):185–191. doi:10.1016/j.ijmyco.2016.02.009
33. Seifert M, Georghiou SB, Catanzaro D, et al. MTBDR plus and MTBDR sl assays: absence of wild-type probe hybridization and implications for detection of drug-resistant tuberculosis. J Clin Microbiol. 2016;54(4):912–918. doi:10.1128/JCM.02505-15
34. Zhang Y, Yew WW. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis off J Int Union Tuberc Lung Dis. 2009;13(11):1320–1330.
35. Bostanabad S, Titov L, Bahrmand A, Nojoumi S. Detection of mutation in isoniazid-resistant mycobacterium tuberculosis isolates from tuberculosis patients in Belarus. Indian J Med Microbiol. 2008;26(2):143–147. doi:10.1016/S0255-0857(21)01930-7
36. van Soolingen D, de Haas PEW, van Doorn HR, Kuijper E, Rinder H, Borgdorff MW. Mutations at amino acid position 315 of the katG Gene are associated with high‐level resistance to isoniazid, other drug resistance, and successful transmission of Mycobacterium tuberculosis in The Netherlands. J Infect Dis. 2000;182(6):1788–1790. doi:10.1086/317598
37. Biadglegne F, Tessema B, Rodloff AC, Sack U. Magnitude of gene mutations conferring drug resistance in Mycobacterium tuberculosis isolates from lymph node aspirates in Ethiopia. Int J Med Sci. 2013;10(11):1589–1594. doi:10.7150/ijms.6806
38. Tilahun M, Shimelis E, Wogayehu T, et al. Molecular detection of multidrug resistance pattern and associated gene mutations in M. tuberculosis isolates from newly diagnosed pulmonary tuberculosis patients in Addis Ababa, Ethiopia. PLoS One. 2020;15(8):e0236054. doi:10.1371/journal.pone.0236054
39. Alelign A, Petros B, Ameni G. Smear positive tuberculosis and genetic diversity of M. tuberculosis isolates in individuals visiting health facilities in South Gondar Zone, northwest Ethiopia. PLoS One. 2019;14(8):e0216437. doi:10.1371/journal.pone.0216437
40. Diriba G, Alemu A, Tola HH, et al. Pre-extensively drug-resistant tuberculosis among multidrug-resistant tuberculosis patients in Ethiopia: a laboratory-based surveillance study. IJID Reg. 2022;5:39–43. doi:10.1016/j.ijregi.2022.08.012
41. Agonafir M, Lemma E, Wolde-Meskel D, et al. Phenotypic and genotypic analysis of multidrug-resistant tuberculosis in Ethiopia. Int J Tuberc Lung Dis. 2010;14(10):1259–1265.
42. Shibabaw A, Gelaw B, Gebreyes W, Robinson R, Wang SH, Tessema B. The burden of pre-extensively and extensively drug-resistant tuberculosis among MDR-TB patients in the Amhara region, Ethiopia. PLoS One. 2020;15(2):e0229040. doi:10.1371/journal.pone.0229040
43. Avalos E, Catanzaro D, Catanzaro A, et al. Frequency and geographic distribution of gyrA and gyrB mutations associated with fluoroquinolone resistance in clinical Mycobacterium tuberculosis isolates: a systematic review. PLoS One. 2015;10(3):e0120470. doi:10.1371/journal.pone.0120470
44. Chaoui I, Oudghiri A, El Mzibri M. Characterization of gyrA and gyrB mutations associated with fluoroquinolone resistance in Mycobacterium tuberculosis isolates from Morocco. J Glob Antimicrob Resist. 2018;12:171–174. doi:10.1016/j.jgar.2017.10.003
45. Jou R, Lee WT, Kulagina EV, et al. Redefining MDR-TB: comparison of Mycobacterium tuberculosis clinical isolates from Russia and Taiwan. Infect Genet Evol. 2019;72:141–146. doi:10.1016/j.meegid.2018.12.031
46. Singh PK, Singh U, Jain A. Emergence of specific gyr A mutations associated high-level fluoroquinolone-resistant Mycobacterium tuberculosis among multidrug-resistant tuberculosis cases in North India. Microb Drug Resist. 2021;27(5):647–651. doi:10.1089/mdr.2020.0240
47. Kabir S, Tahir Z, Mukhtar N, Sohail M, Saqalein M, Rehman A. Fluoroquinolone resistance and mutational profile of gyrA in pulmonary MDR tuberculosis patients. BMC Pulm Med. 2020;20(1):138. doi:10.1186/s12890-020-1172-4
48. Jnawali HN, Hwang SC, Park YK, et al. Characterization of mutations in multi- and extensive drug resistance among strains of Mycobacterium tuberculosis clinical isolates in Republic of Korea. Diagn Microbiol Infect Dis. 2013;76(2):187–196. doi:10.1016/j.diagmicrobio.2013.02.035
49. Ajbani K, Nikam C, Kazi M, et al. Evaluation of genotype MTBDRsl assay to detect drug resistance associated with fluoroquinolones, aminoglycosides and ethambutol on clinical sediments. PLoS One. 2012;7(11):e49433. doi:10.1371/journal.pone.0049433
50. Singhal R, Reynolds PR, Marola JL, et al. Sequence analysis of fluoroquinolone resistance-associated genes gyrA and gyrB in clinical Mycobacterium tuberculosis isolates from patients suspected of having multidrug-resistant tuberculosis in New Delhi, India. J Clin Microbiol. 2016;54(9):2298–2305. doi:10.1128/JCM.00670-16
51. Ford C, Yusim K, Ioerger T, et al. Mycobacterium tuberculosis – heterogeneity revealed through whole genome sequencing. Tuberculosis. 2012;92(3):194–201. doi:10.1016/j.tube.2011.11.003
52. Abakur EHA, Alnour TMS, Abuduhier F, Albalawi FMA, Alfifi KAS. Emergence of heteroresistance Mycobacterium tuberculosis in Saudi Arabia. Infect Disord Drug Targets. 2020;20(4):491–494. doi:10.2174/1871526519666190326141550
53. Liang B, Tan Y, Li Z, et al. Highly sensitive detection of isoniazid heteroresistance in Mycobacterium tuberculosis by DeepMelt assay. J Clin Microbiol. 2018;56(2):e01239. doi:10.1128/JCM.01239-17
54. Georghiou SB, Magana M, Garfein RS, Catanzaro DG, Catanzaro A, Rodwell TC. Evaluation of genetic mutations associated with Mycobacterium tuberculosis resistance to Amikacin, Kanamycin and Capreomycin: a systematic review. PLoS One. 2012;7(3):12. doi:10.1371/journal.pone.0033275
55. World Health Organization. Meeting Report of the WHO Expert Consultation on the Definition of Extensively Drug-Resistant Tuberculosis. World Health Organization; 2021. Available from: https://apps.who.int/iris/handle/10665/338776.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
