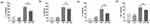Back to Journals » Drug Design, Development and Therapy » Volume 17
Probiotics Alleviate Chemotherapy-Associated Intestinal Mucosal Injury via the TLR4–NFκB Signaling Pathway
Authors Li X, Hu B, Zheng J, Pan Z, Cai Y, Zhao M, Jin X, Li ZQ
Received 13 January 2023
Accepted for publication 17 July 2023
Published 25 July 2023 Volume 2023:17 Pages 2183—2192
DOI https://doi.org/10.2147/DDDT.S403087
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Xiaochong Li,1,* Bowen Hu,1,* Jiachen Zheng,2,3 Zhiyong Pan,1 Yuxiang Cai,4 Mingjuan Zhao,1 Xiaoqing Jin,2 Zhi-Qiang Li1
1Department of Neurosurgery, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, 430071, People’s Republic of China; 2Emergency Center, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, 430071, People’s Republic of China; 3The Second Clinical School, Wuhan University, Wuhan, Hubei, 430071, People’s Republic of China; 4Department of Pathology, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, 430071, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoqing Jin; Zhi-Qiang Li, Email [email protected]; [email protected]
Introduction: Temozolomide (TMZ) induces intestinal mucosa injury that cannot be fully counteracted by supportive treatment. Probiotics regulate gut microbial composition and the host immune system and may alleviate this side effect. We aimed to investigate the potential and mechanism of Lactobacillus rhamnosus GG (LGG) in relieving intestinal mucosal injury induced by TMZ.
Methods: Glioblastoma mice were divided into four groups: CON (control), LGG (109 CFU/mL, treated for 7 days), TMZ (50 mg/kg·d, treated for 5 days), LGG+TMZ (LGG for 7 days and TMZ subsequently for 5 days). Body weight, food intake, and fecal pH were recorded. Intestinal tissue samples were collected 1 day after the end of TMZ treatment. Degree of damage to intestine, expression of IL1β, IL6, TNFα, and IL10 in jejunum were determined. Levels of tight-junction proteins (ZO1, occludin), TLR4, IKKβ, IκBα, and P65 with their phosphorylation in jejunum were measured.
Results: Decreases in body weight, food intake, spleen index in the TMZ group were mitigated in the LGG+TMZ group, and the degree of intestinal shortening and damage to jejunum villus were also alleviated. The expression of tight-junction proteins in the LGG+TMZ group was significantly greater than that in the TMZ group. IκBα in intestinal tissue significantly decreased in the TMZ group, phos–IKKβ and phos-P65 increased compared to the CON group, and LGG reversed such changes in IκBα and phos-P65 in the LGG+TMZ group. Intestinal inflammatory cytokines were significantly increased in the TMZ group, but lower in the LGG+TMZ group. Moreover, expression of TLR4 in LGG group was significantly lower than that in the CON group. LGG inhibited the rise of TLR4 after TMZ in the LGG+TMZ group compared to the TMZ group.
Conclusion: LGG inhibits the activation of the TLR4–NFκB pathway and alleviates intestinal mucosal inflammation induced by TMZ, thereby protect the jejunum villi and mucosal physical barrier.
Keywords: Lactobacillus rhamnosus GG, temozolomide, intestinal mucosal injury, TLR4, NFκB
Introduction
Intestinal mucosal injury can accompany the entire cycle of chemotherapy. The incidence of enteral mucositis is 50%–100% of patients receiving chemotherapy, depending on the type and regimen of chemotherapy drugs.1 Temozolomide (TMZ) is a first-line chemotherapeutic agent for the treatment of malignant intracranial tumors2 that induces the alkylation of DNA, and thus the autophagy or apoptosis of tumor cells. However, it also induces the apoptosis of intestinal mucosal epithelial cells, thus destroying the intestinal mucosal barrier and triggering inflammation.3 Briefly, chemotherapy-associated intestinal mucositis occurs in five pathological stages: initiation, primary damage response, signal amplification, ulceration, and healing.4
Activation of the NFκB signaling pathway and the synthesis of proinflammatory cytokines are thought to be key to the initiation and signal-amplification stage.5,6 The TLR4–NFκB signaling pathway can be continuously activated by various damage-associated molecular patterns produced by dead or injured intestinal epithelial cells, including HMGB1, ROS, and LPS from the enteric cavity, which can induce the gene transcription of proinflammatory cytokines, such as TNFα, IL1β, and IL6, thus promoting the inflammatory cascade and disrupting the intestinal mucosa.7,8 Intestinal mucositis induced by TMZ can interfere with patients’ treatment adherence to avoid unbearable symptoms, including nausea, vomiting, anorexia, stomachache, diarrhea, and constipation.9 Current supportive therapy (such as with 5-hydroxytryptamine receptor antagonists, glucocorticoids, agents affecting gastrointestinal motility, and antibiotics),10 however, can only relieve the symptoms to a moderate degree. Some 60% and 37% patients, respectively, still suffer from nausea or vomiting, indicating that these drugs cannot necessarily alleviate the underlying mucositis and damage to the mucosal barrier.11
Probiotics have recently drawn attention in the field of intestinal mucosal protection due to their health benefits.12,13 Lactobacillus rhamnosus GG (LGG) is a facultative anaerobic probiotic bacterium that belongs to the phylum Firmicutes. It has greater viability than other probiotic strains and can colonize the entire digestive tract.14 Previous studies have showed its beneficial effects on alleviating metabolic disturbance and diseases induced by environment pollutants and toxicities.15–18 Its capacity to modulate local or systemic immunoresponse has also been verified.19,20 LGG manifests its functions on intestinal microecology by producing antimicrobial substances and short-chain fatty acids, reducing intestinal pH, competing with pathogenic bacteria for nutrients, and inhibiting their adhesion to intestinal mucosa.21 It also promotes the growth of mucosal epithelium, consolidates the functions of the intestinal barrier, and regulates the intestinal immune system.22–24 A recent study revealed the significant anti-inflammatory effects of LGG, which were believed to be related to immunoregulation mediated by the TLR4 signaling pathway.25,26 Overall, LGG has become a hot spot of research on the treatment of a variety of intestinal diseases.27
However, the alleviating functions of LGG in TMZ-induced intestinal mucositis has not been confirmed and the clinical application of LGG is still unknown. In this study with a glioblastoma mouse model, we explored the effects of LGG applied before TMZ treatment, in order to confirm its benefits in alleviating intestinal mucosal injury induced by TMZ and the potential mechanisms. Our study may shed new light on combination treatment of cancer in patients suffering from the adverse effects of chemotherapy.
Methods
Study Design
Specific pathogen-free male C57BL/6 mice (n=36, 8–10 weeks old) were purchased from SPF Biotechnology (Beijing, China). All mice were housed in the Animal Experimental Center of Zhongnan Hospital at Wuhan University, where the temperature was maintained at 25°C with a 12 h light/dark cycle and free access to food and water. After adaptive feeding for 7 days, the mice were randomly allocated to four groups: control group (CON), TMZ group, LGG group, and LGG+TMZ group, with different treatments described below. Each group comprised nine mice.
Glioblastoma Mouse Model Construction
After the first collection of fecal samples following a 7-day adaptive feeding regime (day 1), the allogeneic orthotopic glioblastoma model was constructed by implanting GL261 cells into the left putamen, as described previously.28 Briefly, after anesthesia by intraperitoneal injection of 2% chloral hydrate (0.2 mL/10 g), the mice were fixed in a stereotaxic apparatus (RWD Life Science, Shenzhen, China). A burr hole was made in the left parietal bone 0.1 mm posterior to the bregma and 2.3 mm lateral to the midline. GL261 cells (3×105 cells in 2 μL PBS) were administered stereotactically through the burr hole into the left putamen to a depth of 2.3 mm from the brain surface. The cell suspension was delivered slowly for 2–3 min. The injection needle was left in place for an additional 2 min and then slowly withdrawn. The burr hole was sealed with bone wax and the incision sutured. The mouse glioblastoma cell line GL261 was obtained from the American Type Culture Collection (Rockville, MD) and cultured in DMEM supplemented with 10% FBS in a humidified 5% CO2 cell incubator at 37°C. DMEM and FBS were obtained from Gibco (CA, USA). Chloral hydrate was from Molbase (Shanghai, China).
Treatment with Lactobacillus rhamnosus GG and Temozolomide
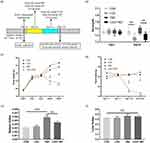
The procedures of intracranial GL261 implantation and LGG and TMZ treatment are shown in Figure 1a. LGG was purchased from American Type Culture Collection (ATCC 53103qqqq) and cultured in autoclaved de Man, Rogosa, and Sharpe (MRS) broth at 37°C for 16 h. Culture density was measured with a spectrophotometer at OD600. After washing and centrifugation, the bacterial liquid was resuspended to a density of 5×109 CFU/mL with PBS. The LGG solution was freshly prepared. LGG gavage started at 1 day after implantation (day 2) with a dosage of 109 CFU bacterial liquid for 7 days (ie, days 2–8 after cell inoculation).29 The vehicle-treated mice received sterile MRS medium by gavage. We determined the successful colonization of LGG by the decrease in pH, as it produces short-chain fatty acids and thus reduces the intestinal pH.21 TMZ and sodium carboxymethyl cellulose (SCC) were purchased from MedChemExpress (NJ, USA). TMZ was freshly dissolved in 0.5% SCC at a final concentration of 5 mg/mL. TMZ gavage started 1 day after LGG administration at a dosage of 50 mg/kg per day for 5 consecutive days (ie, days 9–13 after cell inoculation) with the vehicle-treated mice by gavage as per our previous work.30
Fecal Sample Collection and Body Reactions
As shown in Figure 1a, the mice’s stool samples were collected at two time points: day 1 (the day of glioma cell implantation) and day 14 (the 14th day after cell inoculation). All samples were collected at 8 am. After weighing, the mice were placed in sterile plastic boxes to collect fecal samples. The fresh feces of mice were weighed and fully dissolved in deionized water at a ratio of 1:9 (adding 900 µL deionized water per 100 mg of mouse feces). The residue was removed by centrifugation. The supernatant was then obtained and its pH determined. Some of the mice (n=4 per group) were killed on day 14 for tissue collection, and the remaining mice (n=5 per group) were followed in order to compare the prognostic differences in body weight and food intake. We also calculated the spleen and liver indices (the ratios of spleen or liver weight [g] to the body weight [g] of these dead mice to roughly estimate the strength of the immune system.
Hematoxylin and Eosin Staining of Intestinal Slices
Intestinal tissue from the dead mice was removed and postfixed overnight in 4% paraformaldehyde at 4°C. It was then transferred into a 30% sucrose solution in 0.1 mol/L PBS at 4°C for 72 h. Coronal section serials were sliced (15 μm) by a Cryostat (Leica Biosystems, Wetzlar, Germany) and mounted on glass slides. The sections were stained with hematoxylin and eosin, cleared in xylene, and mounted.
Enzyme-Linked Immunosorbent Assay for Inflammatory Cytokines
Inflammatory cytokines were detected by ELISA kits purchased from MultiSciences Biotech (Hangzhou, China). TNFα (EK282/4), IL1β (EK201B/3), IL6 (EK206/3), and IL10 (EK210/4) were detected according to the manufacturer’s instructions. In brief, the jejunum tissue samples (~10 mg) were homogenized in PBS on ice and then centrifuged at 5000 g at 4°C for 10 min to obtain tissue supernatant. A 96-well plate coated with capture antibody specific for each cytokine was washed and blocked before adding 100 μL supernatants and serially diluted specific standards to respective wells. Following a series of washing, the captured cytokine was detected using the specific conjugated detection antibody. The substrate reagent was added to each well and then the plate was read at 450 nm by an ELISA plate reader.
Western Blotting
The expression of junction proteins (ZO1, occludin) and the overall expression and phosphorylation level of proteins in NFκB signaling were detected by Western blotting. The frozen jejunum tissue lysed in cold radioimmunoprecipitation assay buffer containing a protease and phosphatase inhibitor cocktail (Roche, Indianapolis, IN, USA). Proteins (15 ng each) were separated by 10% SDS-PAGE and then transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). After blocking with 5% nonfat milk in Tris-buffered saline, the membranes were incubated with primary and secondary antibodies and visualized using enhanced chemiluminescence reagents (Millipore, Billerica, MA, USA) with the Viber Fusion FX7 imaging system (Viber 7 Lourmat, PAR, France). Antibodies used for ZO1 (1:500) and occludin (1:500) were obtained from Servicebio (Wuhan, China). TLR4 (1:500) antibody was purchased from Bioss (Beijing, China). IKKβ (1:1000), phos-IKKβ (1:1000), IKBα (1:1000), P65 (1:1000), phos-P65 (1:1000), and β-actin (1:1000) antibodies were purchased from Cell Signaling (Danvers, MA, USA).
Statistical Analysis
All data are expressed as means ± SD from at least three independent experiments and were analyzed by GraphPad Prism 8.0.0 (GraphPad Software, California, USA) and IBM SPSS 20.0 (IBM Corporation, New York, USA). We evaluated differences among the four groups by one-way ANOVA for normally distributed parameters or the Kruskal–Wallis test for abnormally distributed parameters with Bonferroni correction for post hoc comparison. P<0.05 was considered statistically significant.
Results
LGG Alleviated Weight Loss and Decrease in Food Intake Induced by TMZ
The experimental procedure is presented in Figure 1a. The feces pH of mice in the LGG and TMZ+LGG groups decreased significantly compared with that in the CON and TMZ groups (P<0.01, Figure 1b, Table S1), indicating that LGG had successfully colonized and metabolized short-chain fatty acids after colonization in the digestive tract. An individual’s body weight and food intake can preliminarily reflect the gastrointestinal side effects of TMZ and the intestinal protective function of LGG. The body weights of our experimental mice increased slowly before TMZ administration, with no significant differences in either weight or food intake between any groups (Figure 1c and d, Table S1). After the induction of intestinal injury by TMZ gavage, the body weights of the mice had decreased significantly in both the TMZ and TMZ+LGG groups by day 14 (P<0.01). The mice in the TMZ group had continued to lose weight on day 21 (P<0.01), whereas the weight loss of mice in the LGG+TMZ group was alleviated to a certain extent by LGG treatment (P<0.05, Figure 1c, Table S1). Food intake in the TMZ group was significantly reduced after TMZ gavage (P<0.01), but there was no significant change in TMZ+LGG group through all the 21 days before and after treatment (P>0.05, Figure 1d, Table S1). Moreover, LGG alone had no significant effect on the mice’s food intake or body weight (P>0.05). The spleen index increased after TMZ administration (P<0.001) and was significantly lower after LGG treatment (P<0.01, Figure 1e, Table S1). However, there was no significant difference in the liver index between groups (P>0.05, Figure 1f, Table S1). These results suggest that LGG can alleviate the bodily reactions induced by TMZ (Table S1).
LGG Alleviated TMZ-Induced Damage to the Intestinal Villus Structure and Barrier Function
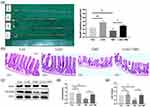
Changes in the mice’s body weight and food intake induced by LGG and TMZ may be attributed to their effects on intestinal mucosal structure and function. Figure 2 and Table S2 show the differences in intestinal structure and barrier protein expression in each group on day 14. The length of the small intestine in the TMZ group was significantly reduced after TMZ administration (P<0.05). However, LGG significantly alleviated the shortening of the small intestine in the TMZ+LGG group (P<0.05), and the small intestinal length of mice in LGG group was greater than that in the CON group (P<0.01, Figure 2a). Intestinal villi are structures for digesting and absorbing nutrients, and are important for assessing the extent of intestinal damage. The mucosal villi in the TMZ group were significantly shorter, partially broken, loosely arranged, and with deeper crypts. In contrast, the intestinal villi of mice in the TMZ+LGG group were longer and more tightly arranged (Figure 2b), indicating that LGG can significantly alleviate the TMZ-induced destruction of the intestinal microstructure.
It has been reported that TMZ ruptures the intestinal tight junctions and intestinal mucosa barrier, and thus we further detected and compared the expression levels of tight junction–related proteins (occludin, ZO1) in small intestinal tissue to study whether LGG application could improve the physical barrier. LGG applied alone did not influence the expression of these proteins (CON vs LGG group, P>0.05, Figure 2c–e). After TMZ administration, the expression of occludin (P<0.01, Figure 2c and d) and ZO1 (P<0.05, Figure 2c and e) was significantly decreased in the TMZ group, confirming the damage to the intestinal barrier. However, a decrease in occludin (P<0.05, Figure 2c and d) and ZO1 (P<0.05, Figure 2c and e) in the small intestine was counteracted in the TMZ+LGG group, indicating that LGG can effectively protect the mucosal barrier from the effects of chemotherapy.
LGG Alleviated TMZ-Induced Intestinal Mucosal Injury by Inhibiting the Expression of Inflammatory Cytokines
The expression levels of intestinal inflammatory cytokines in each group are provided in Table S3. There was no significant difference between the CON group and the LGG group (P>0.05, Figure 3a–d and Table S3). However, the expression of IL1β (P<0.001, Figure 3a), IL6 (P<0.001, Figure 3b), TNFα (P<0.001, Figure 3c), and IL10 (P<0.001, Figure 3d) significantly increased after TMZ administration, suggesting a TMZ-induced intestinal inflammatory response. In contrast, IL1β (P<0.01, Figure 3a), TNFα (P<0.01, Figure 3c), and IL10 (P<0.01, Figure 3d) in the TMZ+LGG group were significantly decreased compared with the same parameters in the TMZ group, while the level of IL6 trended insignificantly downward (P>0.05, Figure 3b). These results indicate that LGG alleviated TMZ-induced intestinal mucosal injury by inhibiting the expression of inflammatory cytokines.
LGG Exerted a Protective Effect on the Intestinal Mucosa by Suppressing TLR4–NFκB Signaling Activation
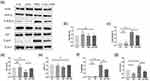
Activation of TLR4–NFκB signaling is closely related to the regulation of proinflammatory cytokine (eg, IL1β and TNFα) expression. Compared with the CON group, the expression of intestinal TLR4 protein in the LGG group decreased significantly (P<0.05, Figure 4a and g, Table S4), whereas the expression of TLR4 in the intestinal tract increased after the administration of TMZ (P<0.01). However, this rise was significantly eased by LGG in the TMZ+LGG group (P<0.001, Figure 4a and g). As shown in Figure 4, LGG alone did not affect the expression of NFκB signaling proteins (P>0.05, Figure 4, Table S4). After TMZ administration, IKBα (P<0.001, Figure 4a and d) decreased, while phos-IKKβ and phos-p65 (P<0.001, Figure 4a, c and f) increased in the intestinal tissue, indicating that TMZ activated the intestinal NFκB signaling pathway. However, there was no significant change in IKKβ or P65 protein (P>0.05, Figure 4a, b and e). Compared with the TMZ group, the level of IΚBα protein (P<0.05, Figure 4a and d) in the TMZ+LGG group increased significantly, while the level of phos-IKKβ and phos-P65 protein (P<0.001, Figure 4a, c and f) decreased, indicating that LGG may alleviate inflammation by inhibiting P65 phosphorylation. These results indicate that LGG can play a protective role in alleviating intestinal inflammation by downregulating the expression of TLR4 and inhibiting the activation of the NFκB signaling pathway.
Discussion
Intestinal mucosal injury is a limiting factor for the clinical use of chemotherapeutic drugs31 that manifests as damaged villus structure and barrier function, leading to various symptoms and increasing the risk of infection.32,33 In this study, we explored the effectiveness of LGG in minimizing TMZ-induced gastrointestinal side effects, which alleviated the body reactions, intestinal structure, and function damage by reducing the activation of TNF–NFκB signaling with decreased downstream inflammatory cytokines.
Decreased pH in intestine manifests the colonization of LGG, while LGG can also reduce the pH elevated by TMZ. A previous study has confirmed that the acidic environment of the intestine can prevent the formation of a bacterial membrane comprising pathogenic bacteria and construct a favorable microbial balance for the host.34 The fold morphology of the villus structure provides it with a huge surface area (about 200–300 m2 for adults), enabling it to absorb nutrients effectively.35 However, damage to the villus structure and shortening of the small intestine caused by chemotherapy can significantly interfere with nutrient absorption and lead to imbalance of absorption and secretion.36 Similarly to Hu’s research, we found that LGG can significantly protect the structure of the villi and reduce shortening of the small intestine, thus improving the absorption of nutrients.37 In Hu’s study, the length between the control group and LGG group was the same, while the application of LGG alone also lengthened the small intestine compared to the CON group in our study. The length of mouse intestine is attributed to various factors,38–40 and the mouse models’ conditions were not the same, as we used glioblastoma mice. The short-chain fatty acids produced by LGG may contribute to the lengthening of the intestine under disease conditions.41 However, there has been no clear report investigating the underlying mechanisms of the changes in small intestinal length under LGG application, which calls for more research. We also observed that LGG increased the food intake of mice treated with TMZ. Although the causal relationship between host appetite and enteromics is still unclear, a clinical study has confirmed that LGG can significantly accelerate the recovery of gastrointestinal motility and appetite in patients who have undergone abdominal surgery.42
The barrier function of intestinal mucus maintains the homeostasis of the internal environment by preventing the penetration of harmful exogenous agents, such as LPS and pathogens, into the lamina propria, which would further aggravate the inflammatory response. The physical intestinal barrier is composed of an orderly arrangement of various connections between mucosal epithelial cells,43,44 among which the tight and adhesion junctions are the most important, as they maintain intestinal mucosa closure. We found that TMZ significantly downregulated the expression of occludin and ZO1, which can induce the rupture of the intestinal tight junctions and increase mucosal permeability, thus damaging the physical barrier of the intestinal mucosa. However, LGG can significantly improve the expressions of ZO1 and occludin in TMZ mice and thus prevent the infiltration of LPS and the invasion of enteric pathogens, thus alleviating mucosal inflammation.45
The molecular mechanism of TMZ-induced intestinal mucosal injury attributed to the apoptosis of epithelium caused by DNA alkylation, which results in damaged villi and mucosal barrier.3 In addition, TMZ-induced inflammation further promotes intestinal mucosal injury.42 We confirmed that TMZ significantly upregulated the expression of TLR4 and the phosphorylation of IKKβ and P65 in the jejunum, indicating significant activation of the NFκB signaling pathway in the jejunum with elevated proinflammatory cytokines (IL1β, TNFα, and IL6) compared to the CON and LGG groups These inflammatory cytokines have been reported to destroy interepithelial tight junctions,46 inhibit the expression of tight-junction proteins,47 induce the shedding of epithelial cells, and promote atrophy of the villus structure.48 In this study, we found that LGG downregulated the expression of TLR4 in the intestines of mice and significantly inhibited TMZ-induced IKKβ phosphorylation and activation of the NFκB signaling pathway with decreased inflammatory cytokines in the LGG+TMZ group compared to the TMZ group. However, the expression of IL6 was not significantly decreased in the LGG+TMZ compared to the TMZ group, indicating the partial effects of LGG in reversing the TMZ-induced inflammation through TLR4–NFκB signaling. In addition, considering that LGG has no significant effect on the total expression of IKKβ, IKBα, and P65 in the intestine of non-TMZ–treated mice, we suggest that the anti-inflammatory effect of LGG may be mainly achieved by downregulating the expression of TLR4 protein.
Previous work by Li et al confirmed that LGG cand alleviate Salmonella typhimurium-induced intestinal inflammation by exopolysaccharides inhibiting the TLR4–NFκB–MAPK pathway.49 Another recent study has also shown that the secretory protein HM0539 of LGG can downregulate the expression of TLR4 in intestinal epithelial cells and therefore inhibit the TLR4–MDY88–NFκB signaling pathway–mediated inflammatory cascade.50 Overall, our work adds new evidence that LGG can alleviate intestinal mucositis in TMZ-treated glioblastoma mice by inhibiting the TLR4–NFκB signaling pathway, suggesting its potential and benefits for chemotherapy-induced side effects.
There are limitations in our study. Gastrointestinal reactions to TMZ are not only caused by intestinal mucosal injury but may also be due to other factors (eg, excitation of the vomiting center by 5-hydroxytryptamine), which needs further study. In addition, we did not further analyze the specific effective constituent of LGG or the influence of exogenous LGG on the microbial composition of the intestine, which also requires further research.
Conclusion
Patients treated with TMZ are often troubled by intestinal side effects. We found that exogenous LGG can be protective by suppressing the activation of the TLR4–NFκB pathway and thus inhibiting inflammatory cytokines. Therefore, LGG can help to preserve the intestinal structure and its barrier function, which would otherwise be damaged by TMZ. Our study provides a new idea for the treatment of chemotherapy-related gastrointestinal reactions, as well as of diseases related to intestinal structural and functional impairment.
Data Sharing
The original data presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Ethics
This study was carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Institutional Animal Care and Ethics Committee of Wuhan University (2018117).
Funding
This research was supported by the Translational Medicine Research Fund of Zhongnan Hospital of Wuhan University (ZLYNXM202011) and the National Health Commission of China (2018ZX-07S-011).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Gibson RJ, Stringer AM. Chemotherapy-induced diarrhoea. Curr Opin Support Palliat Care. 2009;3(1):31–35.
2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996.
3. Toft NJ, Winton DJ, Kelly J, et al. Msh2 status modulates both apoptosis and mutation frequency in the murine small intestine. Proc Natl Acad Sci U S A. 1999;96(7):3911–3915.
4. Scully C, Epstein J, Sonis S. Oral mucositis: a challenging complication of radiotherapy, chemotherapy, and radiochemotherapy: part 1, pathogenesis and prophylaxis of mucositis. Head Neck. 2003;25(12):1057–1070.
5. Cario E. Toll-like receptors in the pathogenesis of chemotherapy-induced gastrointestinal toxicity. Curr Opin Support Palliat Care. 2016;10(2):157–164.
6. Thorpe DW, Stringer AM, Gibson RJ. Chemotherapy-induced mucositis: the role of the gastrointestinal microbiome and toll-like receptors. Exp Biol Med. 2013;238(1):1–6.
7. Kim J. Poly(ADP-ribose) polymerase activation induces high mobility group box 1 release from proximal tubular cells during cisplatin nephrotoxicity. Physiol Res. 2016;65(2):333–340.
8. Xiao YT, Yan WH, Cao Y, Yan JK, Cai W. Neutralization of IL-6 and TNF-α ameliorates intestinal permeability in DSS-induced colitis. Cytokine. 2016;83:189–192.
9. Ribeiro RA, Wanderley CW, Wong DV, et al. Irinotecan- and 5-fluorouracil-induced intestinal mucositis: insights into pathogenesis and therapeutic perspectives. Cancer Chemother Pharmacol. 2016;78(5):881–893.
10. Herrstedt J. Antiemetics: an update and the MASCC guidelines applied in clinical practice. Nat Clin Pract Oncol. 2008;5(1):32–43.
11. Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24(27):4472–4478.
12. Badgeley A, Anwar H, Modi K, Murphy P, Lakshmikuttyamma A. Effect of probiotics and gut microbiota on anti-cancer drugs: mechanistic perspectives. Biochimica Et Biophysica Acta Rev Cancer. 2021;1875(1):188494.
13. FAO/WHO. Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk and Live Lactic Acid Bacteria. Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO); 2001.
14. Tuomola E, Crittenden R, Playne M, Isolauri E, Salminen S. Quality assurance criteria for probiotic bacteria. Am J Clin Nutr. 2001;73(2 Suppl):393s–8s.
15. Chen L. Gut microbiota manipulation to mitigate the detrimental effects of environmental pollutants. Toxics. 2021;9(6):56.
16. Hu C, Liu M, Tang L, Liu H, Sun B, Chen L. Probiotic intervention mitigates the metabolic disturbances of perfluorobutanesulfonate along the gut-liver axis of zebrafish. Chemosphere. 2021;284:131374.
17. Bai Y, Meng Q, Wang C, et al. Gut Microbiota Mediates Lactobacillus rhamnosus GG Alleviation of Deoxynivalenol-Induced Anorexia. J Agric Food Chem. 2023;71(21):8164–8181.
18. Panpetch W, Visitchanakun P, Saisorn W, et al. Lactobacillus rhamnosus attenuates Thai chili extracts induced gut inflammation and dysbiosis despite capsaicin bactericidal effect against the probiotics, a possible toxicity of high dose capsaicin. PLoS One. 2021;16(12):e0261189.
19. Shonyela SM, Feng B, Yang W, Yang G, Wang C. The regulatory effect of Lactobacillus rhamnosus GG on T lymphocyte and the development of intestinal villi in piglets of different periods. AMB Express. 2020;10(1):76.
20. Bai Y, Ma K, Li J, Li J, Bi C, Shan A. Deoxynivalenol exposure induces liver damage in mice: inflammation and immune responses, oxidative stress, and protective effects of Lactobacillus rhamnosus GG. Food Chem Toxicol. 2021;156:112514.
21. Lu R, Fasano S, Madayiputhiya N, Morin NP, Nataro J, Fasano A. Isolation, identification, and characterization of small bioactive peptides from Lactobacillus GG conditional media that exert both anti-Gram-negative and Gram-positive bactericidal activity. J Pediatr Gastroenterol Nutr. 2009;49(1):23–30.
22. Ciorba MA, Riehl TE, Rao MS, et al. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut. 2012;61(6):829–838.
23. Braat H, van den Brande J, van Tol E, Hommes D, Peppelenbosch M, van Deventer S. Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4+ T cells via modulation of dendritic cell function. Am J Clin Nutr. 2004;80(6):1618–1625.
24. Isolauri E, Majamaa H, Arvola T, Rantala I, Virtanen E, Arvilommi H. Lactobacillus casei strain GG reverses increased intestinal permeability induced by cow milk in suckling rats. Gastroenterology. 1993;105(6):1643–1650.
25. Gao K, Wang C, Liu L, et al. Immunomodulation and signaling mechanism of Lactobacillus rhamnosus GG and its components on porcine intestinal epithelial cells stimulated by lipopolysaccharide. J Microbiol Immunol Infection. 2017;50(5):700–713.
26. Qi SR, Cui YJ, Liu JX, Luo X, Wang HF. Lactobacillus rhamnosus GG components, SLP, gDNA and CpG, exert protective effects on mouse macrophages upon lipopolysaccharide challenge. Lett Appl Microbiol. 2020;70(2):118–127.
27. Capurso L. Thirty Years of Lactobacillus rhamnosus GG: a Review. J Clin Gastroenterol. 2019;53(1):S1–s41.
28. Feldman LA, Fabre MS, Grasso C, et al. Perfluorocarbon emulsions radiosensitise brain tumors in carbogen breathing mice with orthotopic GL261 gliomas. PLoS One. 2017;12(9):e0184250.
29. Orlando A, Linsalata M, Bianco G, et al. Lactobacillus rhamnosus GG Protects the Epithelial Barrier of Wistar Rats from the Pepsin-Trypsin-Digested Gliadin (PTG)-Induced Enteropathy. Nutrients. 2018;10(11):867.
30. Li XC, Wu BS, Jiang Y, et al. Temozolomide-Induced Changes in Gut Microbial Composition in a Mouse Model of Brain Glioma. Drug Des Devel Ther. 2021;15:1641–1652.
31. Pearce A, Haas M, Viney R, et al. Incidence and severity of self-reported chemotherapy side effects in routine care: a prospective cohort study. PLoS One. 2017;12(10):e0184360.
32. Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, Rubenstein EB. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer. 2003;98(7):1531–1539.
33. Anderson PM, Lalla RV. Glutamine for Amelioration of Radiation and Chemotherapy Associated Mucositis during Cancer Therapy. Nutrients. 2020;12(6):38.
34. Petrova MI, Imholz NC, Verhoeven TL, et al. Lectin-Like Molecules of Lactobacillus rhamnosus GG Inhibit Pathogenic Escherichia coli and Salmonella Biofilm Formation. PLoS One. 2016;11(8):e0161337.
35. Helander HF, Fändriks L. Surface area of the digestive tract - revisited. Scand J Gastroenterol. 2014;49(6):681–689.
36. Andersen MCE, Johansen MW, Nissen T, et al. FIBCD1 ameliorates weight loss in chemotherapy-induced murine mucositis. Support Care Cancer. 2021;29(5):2415–2421.
37. Hu M, Wu X, Luo M, Wei H, Xu D, Xu F. Lactobacillus rhamnosus FLRH93 protects against intestinal damage in mice induced by 5-fluorouracil. J Dairy Sci. 2020;103(6):5003–5018.
38. Simrén M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62(1):159–176.
39. Bikker P, Dirkzwager A, Fledderus J, et al. The effect of dietary protein and fermentable carbohydrates levels on growth performance and intestinal characteristics in newly weaned piglets. J Anim Sci. 2006;84(12):3337–3345.
40. De Angelis M, Montemurno E, Vannini L, et al. Effect of Whole-Grain Barley on the Human Fecal Microbiota and Metabolome. Appl Environ Microbiol. 2015;81(22):7945–7956.
41. Tappenden KA, Drozdowski LA, Thomson AB, McBurney MI. Short-chain fatty acid-supplemented total parenteral nutrition alters intestinal structure, glucose transporter 2 (GLUT2) mRNA and protein, and proglucagon mRNA abundance in normal rats. Am J Clin Nutr. 1998;68(1):118–125.
42. Spencer NJ. Motility patterns in mouse colon: gastrointestinal dysfunction induced by anticancer chemotherapy. Neurogastroenterol Motil. 2016;28(12):1759–1764.
43. Wells JM, Brummer RJ, Derrien M, et al. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol. 2017;312(3):G171–g93.
44. Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141(5):769–776.
45. Gao J, Li Y, Wan Y, et al. A Novel Postbiotic From Lactobacillus rhamnosus GG With a Beneficial Effect on Intestinal Barrier Function. Front Microbiol. 2019;10:477.
46. Coccia M, Harrison OJ, Schiering C, et al. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med. 2012;209(9):1595–1609.
47. Ma TY, Iwamoto GK, Hoa NT, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286(3):G367–76.
48. Jung H, Leal-Ekman JS, Lu Q, Stappenbeck TS. Atg14 protects the intestinal epithelium from TNF-triggered villus atrophy. Autophagy. 2019;15(11):1990–2001.
49. Li J, Li Q, Wu Q, et al. Exopolysaccharides of Lactobacillus rhamnosus GG ameliorate Salmonella typhimurium-induced intestinal inflammation via the TLR4/NF-κB/MAPK pathway. J Anim Sci Biotechnol. 2023;14(1):23.
50. Li Y, Yang S, Lun J, et al. Inhibitory Effects of the Lactobacillus rhamnosus GG Effector Protein HM0539 on Inflammatory Response Through the TLR4/MyD88/NF-кB Axis. Front Immunol. 2020;11:551449.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.