Back to Journals » Cancer Management and Research » Volume 8
Proactive management strategies for potential gastrointestinal adverse reactions with ceritinib in patients with advanced ALK-positive non-small-cell lung cancer
Authors Schaefer ES, Baik C
Received 16 September 2015
Accepted for publication 18 January 2016
Published 24 March 2016 Volume 2016:8 Pages 33—38
DOI https://doi.org/10.2147/CMAR.S96471
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kenan Onel
Video abstract presented by Eric S Schaefer.
Views: 284
Eric S Schaefer,1 Christina Baik2
1Section of Medical Oncology, Highlands Oncology Group, Fayetteville, AR, 2Department of Medicine, Medical Oncology Division, University of Washington School of Medicine, Seattle, WA, USA
Abstract: Anaplastic lymphoma kinase (ALK) gene fusions occur in 3%–7% of non-small-cell lung cancer (NSCLC) cases. Ceritinib, a once-daily, oral ALK inhibitor, has activity against crizotinib-resistant and crizotinib-naïve NSCLC, including brain metastases. Ceritinib (Zykadia™) was granted accelerated approval by the US Food and Drug Administration in 2014 for treating crizotinib-resistant ALK-positive NSCLC. Adverse events (AEs), particularly gastrointestinal (GI) AEs, are commonly experienced at the recommended dose of 750 mg/d and ~38% of patients require dose interruption or reduction for GI AEs. This case study details our experience with the use of proactive GI AE management regimens in patients treated with ceritinib (750 mg/d) across two study sites. Proactive Regimens A and B were implemented in patients with metastatic ALK-positive NSCLC treated with ceritinib to manage drug-related GI AEs. Regimen A comprised ondansetron and diphenoxylate/atropine or loperamide, taken 30 minutes prior to ceritinib dose. Regimen B included dicyclomine (taken with the first ceritinib dose), ondansetron (taken 30 minutes prior to ceritinib dose for the first seven doses), and loperamide (taken as needed with the onset of diarrhea). The proactive medications were tapered off depending on patient tolerability to ceritinib. Nine patient cases are presented. Starting Regimens A or B before the first dose of ceritinib, or as soon as GI symptoms were encountered, prevented the need for dose reduction due to GI toxicity in eight of the nine patients. Using these regimens, 78% of patients were able to remain on 750 mg/d fasting. Two patients received 23 months and 16 months of therapy and remain on ceritinib 750 mg/d and 600 mg/d, respectively. Although not currently recommended or implemented in clinical studies, based on the patients evaluated here, upfront or proactive treatment plans that address AEs early on can allow the majority of patients to remain on the approved 750 mg/d ceritinib dose.
Keywords: case studies, tolerability, antidiarrheal, antiemetic, GI symptoms
Introduction
Anaplastic lymphoma kinase (ALK) gene fusions and mutations are implicated in the pathogenesis of several human cancers.1 The fusion of echinoderm microtubule-associated protein-like 4 (EML4) with ALK is the most frequent of known ALK rearrangements resulting in constitutive oncogenic ALK activation. ALK gene rearrangement occurs in 3%–7% of non-small-cell lung cancer (NSCLC) cases,2,3 with higher prevalence in younger patients with adenocarcinoma who are never- or light smokers.4
The ALK protein is thus a therapeutic target for the treatment of ALK-rearranged (ALK-positive) NSCLC, and a number of ALK inhibitors are currently under clinical development. Crizotinib (XALKORI®), an oral first-in-class dual ALK and c-MET inhibitor, was approved by the US Food and Drug Administration (FDA) in 2011 to treat metastatic ALK-positive NSCLC in any line, having demonstrated clinical efficacy.5 In clinical trials, crizotinib achieved an overall response rate (ORR) of 60% and a median progression-free survival (PFS) of 8–10 months.6,7 However, patients ultimately acquire resistance within 1–2 years of therapy, resulting in disease progression, thus limiting its long-term application.8,9
Next-generation ALK inhibitors that overcome crizotinib resistance have been developed. Ceritinib (ZYKADIA™) is a once-daily oral, small-molecule, ATP-competitive ALK inhibitor that is 20 times more potent against ALK than crizotinib in preclinical studies, with activity against common secondary ALK mutations that confer resistance to crizotinib.10 Ceritinib was granted accelerated approval by the US FDA in 2014 for treating metastatic, ALK-positive NSCLC that has progressed on or is intolerant to crizotinib, based on response rate and duration of response.11 In a Phase I study, 246 ALK inhibitor-treated and ALK inhibitor-naïve patients with NSCLC achieved ORRs of 56% and 72%, respectively, with a median duration of response of 9.7 months. Median PFS was 6.9 months in prior ALK inhibitor-treated patients and 18.4 months in ALK inhibitor-naïve patients.12 Ceritinib dosed at 750 mg/d also demonstrated antitumor activity in patients with ALK-positive NSCLC, including brain metastases, with durable intracranial responses (overall intracranial response rate [OIRR]: 34.5%) observed in either previously untreated (OIRR: 60.0%) or crizotinib-treated patients (OIRR: 29.2%).13
Ceritinib is recommended at a dose of 750 mg/d, administered orally on an empty stomach (not within 2 hours of a meal).14 A food effect study14 in healthy volunteers found that dosing with a high-fat meal increases ceritinib systemic exposure (peak plasma levels) by 43%, compared with the fasted state. Adverse events (AEs) are commonly experienced at this dose level and ~60% of patients initiating treatment at 750 mg/d require at least one dose reduction, with a median time to first dose reduction of 7 weeks. The majority of patients (96%; 14% severe cases) in the Phase I study experienced gastrointestinal (GI) AEs, including diarrhea, nausea, vomiting, and abdominal pain, when taking the recommended ceritinib dose. In clinical studies, GI AEs are one of the most common reasons for dose modification (38% of patients).14
Current recommendations for the management of GI AEs are reactive and include 1) symptomatic treatment with antiemetic or antidiarrheal therapy or 2) treatment interruption and dose reduction in severe cases.14 Maintaining the prescribed dose is important for preserving efficacious systemic drug exposure. During crizotinib therapy, most relapses in patients with ALK-positive NSCLC occur in the brain15,16; therefore, the dose of ceritinib – and consequent systemic and intracranial exposure – may affect the central nervous system (CNS) concentration, which is critical for the control of CNS metastases. As such, upfront proactive treatment regimens to address nausea (eg, ondansetron [Zofran]), diarrhea (eg, loperamide [Imodium]), and abdominal pain (eg, dicyclomine [Bentyl]) could be implemented in clinics to help ensure optimum ceritinib exposure and efficacy. Here, we report a case series of nine patients treated with ceritinib (750 mg/d) for whom proactive GI AE management strategies were implemented.
Patients and methods
All patients had metastatic ALK positive NSCLC and were participants in ceritinib clinical trials. The study protocols allowed investigators to use both reactive and proactive medications to help deal with the normal GI side effects (NCT01283516, NCT01947608, NCT01685060, and NCT01828112). All clinical studies involved were conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonization. All protocols were approved by relevant Institutional Review Boards and Ethics Committees. Written informed consent was obtained from all the patients before screening. All patients were initially dosed orally with ceritinib (750 mg/d) fasting. Patients were encouraged to maintain dosage at the same time of day for the duration of the study.
Proactive regimens
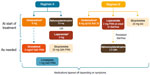
Between the two study sites, two proactive regimens were implemented in patients with metastatic ALK-positive NSCLC treated with ceritinib to manage drug-related GI AEs (Figure 1). The use of these regimens was consistent with the protocol recommendations. Regimen A comprised ondansetron 8 mg, along with either diphenoxylate and atropine 2.5 mg or loperamide 2 mg, to be taken orally 30 minutes prior to the ceritinib dose. Additional agents were administered for persistent symptoms. Regimen B included dicyclomine 20 mg twice daily (to be taken orally starting with the first ceritinib dose), ondansetron 8 mg (to be taken orally 30 minutes prior to ceritinib dose for the first seven doses), and loperamide 2 mg (to be taken orally as needed with the onset of diarrhea; two tablets at onset and one tablet with every loose stool). If diarrhea persisted with loperamide, then diphenoxylate and atropine (Lomotil) 2.5 mg was prescribed, to be taken every 6 hours as needed. For both regimens, the proactive medications were tapered off, depending on clinically judged patient tolerability to ceritinib. For instance, for Regimen A, if patients reported lack of nausea but ongoing diarrhea, prophylactic antidiarrheal medication was continued, while antinausea medication use was changed from prophylactic to as-needed (PRN) basis. For Regimen B, patients remained on the prophylactic regimen for two cycles. After two cycles, the following were recommended:
- Stop the dicyclomine at Week 1 of Cycle 3. If Grade 1 cramping occurs, use PRN, and restart dose if Grade 2 or greater.
- Stop the ondansetron at Week 2 of Cycle 3. If Grade 1 nausea or vomiting is seen, use PRN, and restart dose if Grade 2 or greater.
- Stop the loperamide at Week 3 of Cycle 3. If Grade 1 diarrhea occurs, use PRN, and restart if Grade 2 or greater.
Safety and tolerability of ceritinib in patients were monitored according to the Common Terminology Criteria for Adverse Events v4.03.
Results
Patient characteristics and treatment
A total of nine patients were included in this series. These patients were enrolled into ceritinib clinical trials across two sites and were treated by the authors, between October 2012 and March 2014. Table 1 provides an overview of patient characteristics, treatment history, and proactive management strategies. Ceritinib was the second to fifth line of treatment for these patients. Eight of nine patients had previously received crizotinib and had discontinued due to disease progression or intolerance. All patients were dosed at 750 mg/d and provided upfront with a GI AE treatment regimen to proactively address the AEs that are experienced by the majority of patients taking ceritinib. Preventive regimens were also used to potentially avoid remote AE management for patients traveling long distances to clinical study sites. Patients 1–4 were started on the two-drug proactive Regimen A, while Patients 5–9 received the three-drug Regimen B. Preventive treatment was initiated at the start of ceritinib therapy.
Treatment outcome
In eight of the nine patients starting Regimens A and B prior to initiation of ceritinib therapy, dose reductions due to GI toxicity were not required (Table 1). Patient 3 required dose reduction to 600 mg/d due to grade 3 transaminitis. Patient 4 discontinued therapy due to GI toxicities despite receiving proactive treatment. Patient 5 had a 1-week ceritinib dose interruption due to elevated creatinine levels but resumed treatment at the recommended 750 mg/d dose. In a Phase I study,12 31% of patients experienced grade 1–2 constipation while on ceritinib 750 mg/d. Two patients on Regimen A experienced intermittent constipation, suspected to be due to the use of antidiarrheal medication. Proactive regimens were tapered off in two patients on Regimen A (Patients 1 and 3) and three patients on Regimen B (Patients 5, 7, and 8) as tolerability to ceritinib increased. Proactive treatment was continued in patients who experienced persistence of diarrhea/nausea in either regimen.
Using these proactive regimens, seven of the nine (78%) patients were able to remain on ceritinib 750 mg/d fasting, with a dose reduction rate for GI side effects of 12.5% (one out of the eight patients) when accounting for the one patient whose dose was reduced due to transaminitis. Patients 3 and 7 received 16 months and 23 months of therapy and remain on ceritinib to date at 600 mg/d and 750 mg/d, respectively. All other patients discontinued ceritinib due to progressive disease, comorbidity, or intolerance.
Discussion
Ceritinib is recommended at an oral, once-daily dose of 750 mg/d based on clinical efficacy results from the Phase I dose-escalation study.11 The current prescribing information recommends ceritinib dose interruption and modification for patients experiencing intolerable nausea, vomiting, or diarrhea despite optimal antiemetic or antidiarrheal therapy.14 In the case series presented herein, only one patient discontinued ceritinib treatment due to GI-related toxicity. A majority of patients were able to remain on ceritinib at 750 mg/d within the prescribing guidelines (ie, without food) and without the need for dose reductions or interruptions due to GI AEs.
Preclinical studies have shown that ceritinib crosses the blood–brain barrier in non-tumor-bearing rats, and in the Phase I study, patients with ALK-positive NSCLC and documented brain metastases who received ceritinib 750 mg/d had an intracranial antitumor response rate of 34.5%.13
This study was limited by the relatively small number of patients treated at our institutions and a lack of comparator patients who did not receive the proactive regimen. Nonetheless, considering that 38% of patients on ceritinib require dose modification due to GI AEs,14 on the basis of the nine patients evaluated here, implementation of proactive drug treatment plans, such as Regimens A and B, may significantly increase the number of patients who remain on 750 mg daily.
GI AEs experienced on tyrosine kinase inhibitor therapy can be distressing for patients and if persistent, may affect their quality of life.17 In our experience, the initial 1–2 weeks of treatment are often the most difficult time for patients, and therefore addressing potential AEs at this stage may help prevent deviations from the recommended 750 mg daily dose. Adherence to therapy is a pertinent issue for cancer patients, and it is estimated that up to 80% of patients do not take oral antineoplastic agents according to their prescription.18 These two regimens allow for the initiation of supportive care with the first dose and are then tapered off based upon symptoms, to minimize the duration of needed treatment. Patient education has been reported to increase patient adherence to oral anticancer agents.18 Therefore, preparing patients and physicians to expect adverse reactions is valuable; dispensing the supportive medication prior to leaving the office and taking the first dose is essential; and presenting proactive AE treatment options upfront as part of a treatment package could help maximize patient drug exposure as they start a new therapy.
Acknowledgments
This study was supported by Novartis Pharmaceuticals. We thank the participating patients, their families, research coordinators, and nurses, as well as thanking Maria Alfaradhi from Articulate Science for providing editorial assistance.
Disclosure
CB has received institutional research support from Novartis Pharmaceuticals Corporation. CB and ESS have participated as advisory board members for Novartis Pharmaceuticals Corporation. The authors report no other conflicts of interest in this work.
References
Barreca A, Lasorsa E, Riera L, et al; European T-Cell Lymphoma Study Group. Anaplastic lymphoma kinase in human cancer. J Mol Endocrinol. 2011;47(1):R11–R23. | |
Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. | |
Wong DW, Leung EL, So KK, et al; University of Hong Kong Lung Cancer Study Group. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115(8):1723–1733. | |
Shaw AT, Solomon B. Targeting anaplastic lymphoma kinase in lung cancer. Clin Cancer Res. 2011;17(8):2081–2086. | |
Xalkori®. New York, NY: Pfizer Labs, Pfizer Inc.; FDA-approved prescribing information; LAB-0441-7.0. 2015. Available from:http://labeling.pfizer.com/showlabeling.aspx?id=676. Accessed February 25, 2016. | |
Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013; 368(25):2385–2394. | |
Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011–1019. | |
Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4(120):120ra17. | |
Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18(5):1472–1482. | |
Li N, Michellys PY, Kim S, et al. Activity of a potent and selective phase I ALK inhibitor LDK378 in naive and crizotinib-resistant preclinical tumor models. Poster presented at: AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics; November 12–16, 2011; San Francisco, CA. Abstract B232. | |
Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370(13):1189–1197. | |
Felip E, Kim D, Mehra R, et al. Efficacy and safety of ceritinib in patients with advanced anaplastic lymphoma kinase (ALK)-rearranged (ALK+) non-small cell lung cancer (NSCLC): an update of ASCEND-1. Poster presented at: European Society for Medical Oncology Congress; September 26–30, 2014; Madrid, Spain. Abstract 1295P. | |
Shaw AT, Felip E, Vansteenkiste JF, et al. Evaluation of ceritinib-treated patients (pts) with anaplastic lymphoma kinase rearranged (ALK+) non-small cell lung cancer (NSCLC) and brain metastases in the ASCEND-1 study. Poster presented at: European Society for Medical Oncology Congress; September 26–30, 2014; Madrid, Spain. (Abstract 1293P). | |
Novartis Pharmaceuticals Corporation: Zykadia™. FDA-approved prescribing information; T2015-114/T2015-115; 2015. Available from: http://www.pharma.us.novartis.com/product/pi/pdf/zykadia.pdf. Accessed February 10, 2016. | |
Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7(12):1807–1814. | |
Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12(11):1004–1012. | |
Califano R, Tariq N, Compton S, et al. Expert consensus on the management of adverse events from EGFR tyrosine kinase inhibitors in the UK. Drugs. 2015;75(12):1335–1348. | |
Partridge AH, Avorn J, Wang PS, Winer EP. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94(9):652–661. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.